Abstract
As yet there is no evidence of the potential antiosteoporotic effect of Urocortin-1 (UCN), a corticotropin releasing factor related peptide, in vivo. In this study, and for the first time, we investigated the effect of UCN in a rat osteopenia model. Sixty female Sprague–Dawley rats were divided into 5 groups: (1) sham-operated, (2) untreated ovariectomized (OVX) rats, (3) and (4) OVX animals treated for 5 weeks with daily subcutaneous low-dose UCN (3 μg/kg of BW) or high-dose UCN (30 μg/kg of BW) 8 weeks after ovariectomy, and (5) OVX rats treated with daily estrogen (0.2 mg/kg of BW p.o) 8 weeks after ovariectomy for 5 weeks (E). After sacrifice, the femurs were reserved for biomechanical, histomorphometric and ash testing. In the biomechanical test, the high-dose UCN rats showed significantly improved mechanical stiffness (341.6 N/mm) compared with the untreated OVX animals (275.9 N/mm). In the histomorphometric evaluation, the high-dose UCN rats demonstrated an improved trabecular microarchitecture especially and significantly at the distal femur (distal femur Tb.Ar = 41.4 % and N.Nd/mm2 = 26.8, proximal femur Tb.Ar = 71.8 % and N.Nd/mm2 = 28.7) compared with untreated OVX rats (distal femur Tb.Ar = 23.3 % and N.Nd/mm2 = 11.7, proximal femur Tb.Ar = 60.2 % and N.Nd/mm2 = 25.2). Our results show that short-term treatment with UCN seems to have a positive effect on the metaphyseal bone structure and strength of the femur in ovariectomized rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urocortin-1 (UCN) belongs to the corticotropin-releasing factor (CRF) peptide family [1]. UCN has been found to be expressed in many tissues, including the brain, adipose tissue, and muscle tissue in humans and other vertebrates, and it appears to play an important role in many organ functions, especially in cell proliferation and the regulation of stress, nutrition, and appetite, among others [2–8]. In the last decade, the positive inotropic effect of UCN on the heart has led researchers to seriously focus on developing drugs with this peptide [3]. In a previous in vitro study, we showed for the first time that UCN was produced during the differentiation of mesenchymal stem cells (MSC) into osteoblasts (osteoprogenitor cells) and was differentially regulated by differentiation factors and cytokines such as bone morphogenetic protein-2, transforming growth factor-beta-1 (TGF-beta-1) -beta-1 and dexamethasone [9]. Another study from Combs et al., reported the regulatory effect of UCN on osteoclast differentiation and function in vitro [10]. These studies suggested new perspectives on the role of urocortin in human skeletal tissue. Nevertheless, to the best of our knowledge, the real in vivo effect of UCN on vertebrate bones is still unknown. Postmenopausal osteoporosis is one of the most common systemic bone diseases with a dysbalance between bone resorption and bone formation. Many agents and conditions would be able to have a positive or negative influence on bone formation, modeling or remodeling, if they would take changes in this regulatory circuit. Many agents and drugs often show their osteoprotective or osteodestructive effect first in pathologic bone conditions like osteoporosis.
The ovariectomized (OVX) rat is an established animal model for osteoporosis research. Rats develop manifest osteopenia within 4–6 weeks after ovariectomy. In addition to the proximal tibia metaphysis and vertebral body, the proximal and distal metaphysis of the femur are further important areas in osteoporosis research in both humans and rodents [11].
In the present study, and for the first time, we investigated changes in femoral strength, trabecular microarchitecture and mineral content of the femoral metaphysis after 5 weeks administration of UCN at 2 different dosages in ovariectomized rats.
Materials and methods
Animals and treatments
All of the procedures were approved by the Institutional Animal Care and Use Committee (Oldenburg, 33.9-42502-04-10/0246). All of the chemicals were obtained from Sigma–Aldrich Chemie GmbH, unless otherwise indicated.
In our study, we used 60 3-month-old female Sprague–Dawley rats, randomized by weight and divided into 5 groups (n = 12 per group):
-
(1)
sham-operated.
-
(2)
Untreated ovariectomized (OVX).
-
(3)
Treated subcutaneously 8 weeks after ovariectomy with daily low-dose human UCN (Bachem, Germany) for 5 weeks (UCN-low = 3 μg/kg of BW).
-
(4)
Treated subcutaneously 8 weeks after ovariectomy with daily high-dose UCN for 5 weeks (UCN-high = 30 μg/kg of BW).
-
(5)
Treated 8 weeks after ovariectomy with daily 17-ß-estradiol per os (0.2 mg/kg of BW/day by food intake) for 5 weeks (E).
UCN was applied daily dissolved in 200 μl of solvent (0.9 % NaCl) subcutaneously. The SHAM, OVX and estrogen-treated rats were injected s.c. with solvent alone. Rats received soy free diet (Ssniff special diet GmbH, Soest, Germany) throughout the experiment. Body weight and food intake were recorded on a weekly basis.
The animals were allowed to move freely in their cages throughout the experiment. At the end of the experiment, the CO2-anesthetized animals were decapitated, and the femurs were dissected and prepared for testing. During the 5-week treatments, the rats were injected with the following fluorescence agents [12]: xylenol orange (90 mg/kg) on day 13, calcein green (10 mg/kg) on day 18, alizarin red (30 mg/kg) on day 24/26 and tetracycline (25 mg/kg) on day 35. Tetracycline was administered 2 h before the animals were decapitated.
Biomechanical test
The femurs were stored after cleaning the remaining soft tissues in tubes at −20 °C until use. The left femurs were prepared for biomechanical analysis as previously described [12]. The femoral shaft was positioned laterally. Force was applied vertically to the trochanter tertius using a ZWICK-testing machine, type 145660 Z020/TND (Zwick/Roell, Ulm, Germany). The bones were broken in a range between 2 N and 500 N by the same investigator. The speed of the feed motion was 50 mm/min. The load-deformation curves were saved (PC connected to the ZWICK machine, and the machine registered the applied load and the displacement), from which values for a number of the biomechanical variables could be calculated. Using the breaking curve, we calculated the maximal load and stiffness (elasticity) of the femurs (the slope of the curve) [12].
Mineral content analysis
To analyze the mineral content of the femurs, we performed an ash test after the biomechanical test. This procedure was performed in a muffle furnace at a temperature of approximately 750 °C for 1 h. The bones were weighed to the nearest 10−5 g before and after the ash procedure. The bone inorganic weight (mineral content) was expressed as a percentage of the initial organic weight [12].
Histomorphometry analysis of proximal and distal femurs
The contralateral femurs (right side) were used for histomorphometry analysis as previously described [12]. The measured area analyzed by histomorphometry at the proximal femur was between the epiphyseal line of the femoral head and intertrochanteric line (2 mm distally, concluded the head -without epiphysis-, neck and trochanteric region). For the distal end of the femurs, the analyzed region was the area between the epiphyseal line and a line 2 mm proximal to the distal metaphysis. The primary aim of this test was to analyze the trabecular microarchitecture and content of the femurs in the areas described above. To do so, we measured the percentage of trabecular area (Tb.Ar), trabecular width (Tb.Wi), number of trabecular nodes (N.Nd), and density of the trabecular nodes (connectivity = N.Nd/mm2) [13, 14].
In this study, we also measured the ratio between the bone’s outer diameter and the inner diameter of the marrow (B.Dm and Ma.Dm), using cross-sections 11 mm distal to the femoral head in the subtrochanteric region. We first measured the B.Dm of the cross-sections in the ventro-dorsal direction (in the middle of the section) and then, in a second step and on the same line, we measured the Ma.Dm. This procedure allowed the possibility of avoiding many systemic and operator-dependent errors, and it was easy to perform. The B.Dm/Ma.Dm ratio helped us to evaluate the relative changes in the cortex of the subtrochanteric region of the rat femur as previously described [12, 13].
Serum analysis
About 5 ml of blood samples were collected from the decapitated animals, allowed to clot and centrifuged at 3000 g for 10 min. Serum was removed and stored at −20 °C. Serum beta-Crosslaps level was assessed using ELISA kits (USCN, Life Science Inc. Houston, USA). Alkaline phosphatase (AP) and calcium were measured on Architect c16000 analyzer (Abbott, Wiesbaden, Germany) at the Department of Clinical Chemistry, University of Goettingen. AP activity was measured by para-nitrophenyl phosphate method at 404 nm, calcium was determined by Arsenazo III dye at 660 nm using commercially available reagents according to the manufacturer’s instructions (Abbott).
Statistics
Using one-way ANOVA with a Tukey–Kramer post hoc test (Prism TM 4.0, Graph Pad, San Diego, USA), we assessed the differences between the treatment groups. P values <0.05 were considered significant.
Results
Body weight
At the beginning of the study, there were no significant differences between the body weights of the rats in all 5 groups. At the end of the experiment, the mean weight of the sham-operated (BW = 324.8 g) and E (BW = 324.3 g) groups was significantly different in comparison with the untreated OVX animals (BW = 396.8 g) (Table 1). Both the UCN-high (388.3 g) and UCN-low (BW = 375.2) groups experienced significantly higher weight gain compared with the sham-operated and E rats. There were no significant differences among the UCN-treated groups and the untreated OVX animals.
Biomechanical test
The maximal load (F max) and stiffness were lower in the untreated OVX-rats (F max = 200 N; stiffness = 275.9 N/mm) compared with the sham-operated animals (F max = 224.6 N, stiffness = 336.6 N/mm). The results for stiffness were statistically significant (P < 0.05).
After UCN therapy, higher F max and stiffness values were observed in the UCN-high animals (stiffness = 341.6 N/mm, P < 0.05) compared with the untreated OVX group. The biomechanical properties in the UCN-high animals reached approximately the level of the sham-operated rats. There were significant differences between UCN high and low groups concerning F max and stiffness (Table 1).
Mineral content measurement
Mineral content analysis showed significantly lower values in the untreated OVX-rats (42.6 %) in comparison with the sham-operated animals (46.1 %). After the treatment, the UCN-high (43.5 %), but not the UCN-low animals (42.4 %), showed a slightly higher mineral content in comparison with untreated OVX rats; however, the results were not statistically significant (Table 1).
Histomorphometry analysis of the distal femur
The differences in the Tb.Ar, Tb.Wi and connectivity measurement results for the distal ends of the femurs between the untreated OVX group (Tb.Ar = 23.3 %; Tb.Wi = 59 μm; connectivity = 11.7 N.Nd/mm2) and sham-operated group (Tb.Ar = 60.6 %; Tb.Wi = 69 μm; connectivity = 42.9 N.Nd/mm2) were statistically significant.
The results of the Tb.Ar and connectivity measurements were significantly higher in both the UCN-high (Tb.Ar = 41.1 %; connectivity = 26.8 N.Nd/mm2) and UCN-low (Tb.Ar = 40.6 %; connectivity = 22.0 N.Nd/mm2) groups compared with the untreated OVX group. Only the UCN-low rats showed significantly higher Tb.Wi (Tb.Wi = 69 μm) values compared with the untreated OVX animals (Table 2).
Histomorphometry analysis of the proximal femur
The Tb.Ar and connectivity measurement values in the untreated OVX group (Tb.Ar = 60.2 %; connectivity = 25.2 N.Nd/mm2) were lower compared with those of the sham-operated animals (Tb.Ar = 74.3 %; connectivity = 38.6 N.Nd/mm2, P < 0.05).
The Tb.Ar and connectivity measurement values in both the UCN-high (Tb.Ar = 71.8 %; connectivity = 28.7 N.Nd/mm2) and UCN-low (Tb.Ar = 63.3 %; connectivity = 27.2 N.Nd/mm2) groups were higher compared with those in the untreated OVX group, but only the Tb.Ar difference in the UCN-high animals was statistically significant. The Tb.Ar in the UCN-high and UCN-low animals reached approximately the level of the E rats (Tb.Ar = 63.1 %; Tb.Wi = 88 μm; connectivity = 25.8 N.Nd/mm2) (Fig. 1). With respect to the Tb.Wi, there were no significant differences between the groups (Table 2).
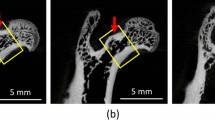
Upper row a the figures show the microradiographs of longitudinal sections of diaphysis of distal rat femurs. After treatment with UCN high dose (UCN high), we observed an improvement in trabecular content and microarchitecture in comparison to the ovariectomized animals. b The intensity of the fluorescence uptakes in UCN high rats appear weaker, although the trabecular content was significantly improved compared with the OVX animals. Lower row c the improvement in the trabecular content and architecture of proximal part of femurs seem after the UCN treatments to be less than in the distal part. d Because of the inhomogeneity of the fluorescence uptakes, it remains difficult here to interpret the finding
Cortical changes in the proximal femur
After measuring the B.Dm and Ma.Dm, we did not observe any significant differences at the cortical side of the proximal femurs. The mean values of the B.Dm and Ma.Dm and the B.Dm/Ma.Dm ratio in both of the UCN-treated groups showed approximately the same changes as did the same values in the untreated OVX, E and sham-operated animals (Table 3).
Qualitative analysis of fluorescence bands
Especially at the distal side of femurs, we observed higher uptake of fluorochrome agents in both sham and E rats in comparison with the UCN animals. The intensity of the fluorescence agents was in the UCN high animals weaker compared to other groups.
At the proximal side of femurs, we observed a similar behavior like the distal femoral area described ahead. However, the changes and differences concerning fluorescence activities at the proximal side between all groups remained more difficult to interpret (Fig. 1).
Serum analysis
The highest level of AP was clearly observed in the UCN high treated group (170.3 U/l), the results compared to sham and UCN low animals were statistically significant. There were no significant differences concerning β-crosslaps and calcium concentration between all groups (Table 4).
Discussion
The treatment of postmenopausal osteoporosis is still a challenge. There have been some experiences with different drug treatments. There are numbers of good anti-resorptives and even a bone formation stimulating agent now available. However, many of the recommended drugs have only a limited effect or they also have side effects. Research into additional therapy regimens is continuing steadily.
Urocortin I (UCN), a member of the corticotropin-related peptide family, was first discovered in the central nervous system (CNS) of rats, humans and other vertebrates, and it appears to also have different roles in the biology and physiology of many organs and organ functions, both in the CNS and in peripheral tissues such as heart, skeletal muscle, fat, placenta, and bowel [1, 15–20].
In a previous in vitro investigation, we observed differentiation and time-dependent expression of UCN mRNA and peptide in mesenchymal stem cells directed to an osteoblastic phenotype (osteoprogenitor cells) [9]. Furthermore, we observed the regulation of UCN gene expression by important growth factors such as transforming growth factor-beta-1 (TGF-beta-1) and bone morphogenetic protein-2 (BMP-2) during osteoprogenitor development [9].
Although most of the known effects of UCN and its related peptides, such as CRF, occur via two main receptors (CRFRs I and II), the pathway and mechanisms of UCN influence in osteoblastic differentiation and proliferation are still unknown. In our previously described in vitro study, we did not find any CRFRs in osteoprogenitor cells [9].
Combs et al. [10] investigated the role of UCN in osteoclast maturation and function in culture. These authors showed that UCN enabled reduced expression of several osteoclast markers and inhibited osteoclast motility. According to their results, both pre-osteoclasts and osteoclasts expressed the CRFR 2ß subtype. The authors also noted that the effect described above was caused by the inhibition of a canonical transient receptor potential 1-like cation channel [10].
Furthermore, some studies have shown an interaction between UCN, leptin and neuropeptide Y (NPY). In the last several years, the involvement of leptin and NPY in bone biology has been reported [9, 10]. According to all of these data, UCN appears to play an important role in bone biology, and it can be assumed that a regulatory cycle between osteoblasts and osteoclasts exists via UCN and its CRFRs both in health and in disease.
To date, there have been no in vivo investigations that are able to answer the question of whether UCN has any effects on pathologic conditions and bone diseases. If we start with the assumption that UCN plays a role in the bone biology, then it is well possible that UCN is also able to influence systemic bone diseases like osteoporosis. For this reason, we examined in the present study the influence of UCN on the femur of ovariectomized Sprague–Dawley rats, which are used as an established osteoporosis model.
We decided to choose two relatively lower concentrations of UCN because there are not any experiences with this new neuropeptide concerning its effect on bone tissue. The selected low dose of UCN (3 μg/kg of BW), used in our study, is lower in comparison to the experiences with this neuropeptide in other rat tissues reported in the literature.
We observed in the UCN high animals a clear advantage in trabecular content and architecture, although the intensity of the fluorochrome uptakes in the same group remain weaker compared to the other groups like sham and OVX. Such behavior can be seen most likely after treatment with some antiresorptive agents. Although the results of the serum analysis show a slightly anabolic effect of UCN (higher AP values, positive bone formation), according to the data of Coombs et al., and our in vivo fluorescence results, however, we can assume that UCN seems to have also an additional antiresorptive behavior, but further quantitative tests are necessary to confirm this finding. On the other side, it is also still unknown whether this appearance is dose dependent. It is also important to know, that the quantitative serum analysis in rat is very vulnerable and has a limited validity.
The histomorphometric analysis of the trabecular area especially at the distal end of the femurs showed advantages with respect to the trabecular content and connectivity. These trabecular improvements were reflected in the significantly higher stiffness during the breaking tests of the femurs. Although we observed a significant deference between UCN low and high in the biomechanical test, the data of histomorphometry analysis however, could not significantly confirm this finding. At the prox. femur, higher dose of UCN seems to improve the bone quality rather than bone mass. Therefore, the question of dose dependence of UCN effect on different bone and skeletal areas remains still open.
At the cortical side of the femoral diaphysis, we did not find any significant changes in the bone and marrow diameters. It is important here to note that even after a long period of osteoporosis, the changes in the cortical bone of the diaphyses can still be difficult to measure. This difficulty is due to significantly slower remodeling on the cortical side compared with the trabecular area of cancellous (metaphyseal) bone, even under osteoporotic conditions [12]. Nevertheless, it is possible that cortical bone fragility could occur through intracortical resorption. MicroCT and pQCT would help in this aim in further investigations in the future.
Although we observed a positive influence of UCN on osteopenic rat bone, the relatively high animal weights at the end of the study in both of the UCN-treated groups verified that UCN at the present dosages was not able to prevent the weight gain conditioned by ovariectomy. Using pharmacological tools and genetically modified animals, Wang et al. [21] clearly showed that peripheral injection of UCN induced a rapid and long-lasting suppression of food intake in mice. This inhibitory effect of UCN was mediated by peripheral activation of CRF2 receptors (CRFR2) [20, 21]. In our opinion, the significant weight gain of the animals during our study may have been caused by the relatively low dosages of UCN used. It is quite possible that higher dosages of UCN would prevent the weight gain. It is also possible that the inhibitory effect of UCN concerning food intake has been reversed after ovariectomy.
It is important here to mention that the influence and role of other factors like weight gain, lipid metabolism and activity of the epiphyseal line on our results are still unknown. These open questions must be answered in further investigations.
In conclusion, our data suggest that UCN appears to have some positive influence on the bone strength and trabecular microarchitecture especially at distal part of rat femur after ovariectomy. To what extent UCN is involved in the osteoblastic–osteoclastic interaction and in which parts of skeleton UCN is able significantly to influence the osteoporosis processes, is still unknown. Further studies are necessary to elucidate the role of UCN and its receptors in bone biology and diseases.
References
Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C et al (1995) Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 378:287–292
Boorse GC, Denver RJ (2006) Widespread tissue distribution and diverse functions of corticotropin-releasing factor and related peptides. Gen Comp Endocrinol 146:9–18
Bale TL, Hoshijima M, Gu Y, Dalton N, Anderson KR, Lee KF, Rivier J, Chien KR, Vale WW, Peterson KL (2004) The cardiovascular physiologic actions of urocortin II: acute effects in murine heart failure. Proc Natl Acad Sci USA 101:3697–3702
Fekete EM, Zorrilla EP (2007) Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol 28:1–27
Chatzaki E, Euthymiadis C, Kyriaki S, Lambropoulou M, Tsaroucha A, Laftsidis P, Simopoulos K (2005) Urocortin and corticotropin-releasing hormone receptor type 2 expression in the human gallbladder. Neuroendocrinology 82:177–184
Slominski A, Wortsman J (2000) Neuroendocrinology of the skin. Endocr Rev 21:457–487
Henry B, Vale W, Markou A (2006) The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J Neurosci 26:9142–9152
Fatima A, Andrabi S, Wolf G, Engelmann M, Spina MG (2013) Urocortin 1 administered into the hypothalamic supraoptic nucleus inhibits food intake in freely fed and food-deprived rats. Amino acids 44:879–885
Tezval M, Tezval H, Dresing K, Stuermer EK, Blaschke M, Stuermer KM, Siggelkow H (2009) Differentiation dependent expression of urocortin’s mRNA and peptide in human osteoprogenitor cells: influence of BMP-2, TGF-beta-1 and dexamethasone. J Mol Histol 40:331–341
Combs CE, Fuller K, Kumar H, Albert AP, Pirianov G, McCormick J, Locke IC, Chambers TJ, Lawrence KM (2012) Urocortin is a novel regulator of osteoclast differentiation and function through inhibition of a canonical transient receptor potential 1-like cation channel. J Endocrinol 212:187–197
Bagi CM, Berryman E, Moalli MR (2011) Comparative bone anatomy of commonly used laboratory animals: implications for drug discovery. Comp Med 61:76–85
Tezval M, Stuermer EK, Sehmisch S, Rack T, Stary A, Stebener M, Konietschke F, Stuermer KM (2010) Improvement of trochanteric bone quality in an osteoporosis model after short-term treatment with parathyroid hormone: a new mechanical test for trochanteric region of rat femur. Osteoporosis Int J Establ Result Coop Eur Foundation Osteoporos Natl Osteoporos Foundation USA 21:251–261
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. 1:2–17 J Bone Miner Res
Nazarloo HP, Buttrick PM, Saadat H, Dunn AJ (2006) The roles of corticotropin-releasing factor-related peptides and their receptors in the cardiovascular system. Curr Protein Pept Sci 7:229–239
Moffatt JD, Lever R, Page CP (2006) Activation of corticotropin-releasing factor receptor-2 causes bronchorelaxation and inhibits pulmonary inflammation in mice. Faseb J 20:1877–1879
Lawrence KM, Latchman DS (2006) The Urocortins: mechanisms of cardioprotection and therapeutic potential. Mini Rev Med Chem 6:1119–1126
Latchman DS (2002) Urocortin. Int J Biochem Cell Biol 34:907–910
Kageyama K, Hanada K, Nigawara T, Moriyama T, Terui K, Sakihara S, Suda T (2006) Urocortin induces interleukin-6 gene expression via cyclooxygenase-2 activity in aortic smooth muscle cells. Endocrinology 147:4454–4462
Wang L, Stengel A, Goebel-Stengel M, Shaikh A, Yuan PQ, Tache Y (2013) Intravenous injection of urocortin 1 induces a CRF(2) mediated increase in circulating ghrelin and glucose levels through distinct mechanisms in rats. Peptides 39:164–170
Wang L, Stengel A, Goebel M, Martinez V, Gourcerol G, Rivier J, Tache Y (2011) Peripheral activation of corticotropin-releasing factor receptor 2 inhibits food intake and alters meal structures in mice. Peptides 32:51–59
Acknowledgments
The authors thank R. Castro and A. Witt for their support of the animal trial.
Conflict of interest
All authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tezval, M., Hansen, S., Schmelz, U. et al. Effect of Urocortin on strength and microarchitecture of osteopenic rat femur. J Bone Miner Metab 33, 154–160 (2015). https://doi.org/10.1007/s00774-014-0578-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-014-0578-6