Abstract
This study compared the effects of ONO-5334, a cathepsin K inhibitor, with those of alendronate on bone mass and strength in ovariectomized rats. Ovariectomy resulted in significant elevation in urinary deoxypyridinoline and plasma C-terminal cross-linking telopeptide of type I collagen (CTX) 8 weeks after surgery. Peripheral quantitative computed tomography analysis showed that total, trabecular, and cortical bone mineral content (BMC) decreased in the proximal tibia, which was paralleled with a significant decline in bone strength. Treatment with ONO-5334 (0.12, 0.6, 3 or 15 mg/kg) once daily for 8 weeks dose-dependently restored the decrease in total BMC and bone mineral density (BMD) in the proximal tibia and suppressed urinary deoxypyridinoline and plasma CTX levels. Alendronate (1 mg/kg, once daily) also fully restored these bone mass parameters. Separate analysis of trabecular and cortical bones, however, showed that ONO-5334 only partially restored trabecular BMD and BMC at 15 mg/kg, whereas alendronate fully restored these parameters. On the other hand, ONO-5334 increased both cortical BMD and BMC with an effect more potent than that of alendronate. Bone geometric analysis indicated that ONO-5334 at 15 mg/kg decreased endosteal circumference without affecting periosteal circumference, resulting in marked increase in cortical thickness. Interestingly, the effects of ONO-5334 on bone strength parameters were more prominent than those of alendronate, although the two test compounds had a similar effect on total BMC. Taken together, our results indicate that ONO-5334 has pharmacological characteristics different from those of alendronate and may offer a unique therapy for patients with osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cathepsin K, a member of the papain cysteine protease superfamily, is specifically and abundantly expressed in osteoclasts [1–4]. Under the acidic microenvironment sealed by osteoclasts, cathepsin K is secreted into bone resorption lacunae, where it degrades bone organic matrix, which is mainly composed of type I collagen. In humans, loss-of-function mutations in the cathepsin K gene cause pycnodysostosis, a condition characterized by elevated bone density (osteosclerosis), bone fragility, short stature, acro-osteolysis, and skull deformities [5, 6]. Cathepsin K-deficient mice exhibit osteopetrosis and have increased trabecular and cortical bone mass due to impaired bone resorption [7–9]. Although these mice maintain or have higher osteoclast level, their osteoclasts are unable to resorb organic bone matrix. It has recently been reported that agents that can inhibit osteoclastic bone resorption without affecting bone formation would be useful for patients with osteoporosis [10]. In fact, recent preclinical studies have shown that genetic or pharmacological inhibition of cathepsin K results in maintained or even increased bone formation [11]. Put together, these findings indicate that cathepsin K is a promising target for the development of novel treatments for metabolic bone diseases.
The ovariectomized (OVX) rat is a well-established animal model of postmenopausal osteoporosis and is commonly used for evaluation of many anti-osteoporotic drugs, such as bisphosphonates, selective estrogen receptor modulators (SERMs), and parathyroid hormone (PTH) [12–16]. However, most cathepsin K inhibitors are significantly less potent against rat cathepsin K than against those of other species, including human [17]. In addition, information on the effects of cathepsin K inhibitors in OVX rats is very limited. Although it is known that cathepsin K inhibitors suppress OVX-induced bone loss in rats, the effects of these compounds on bone quality and strength remain to be evaluated [18, 19].
ONO-5334 is an orally-active low-molecular-weight inhibitor of cathepsin K from a range of animal species, including human and rat (K i values in the sub-nanomolar range). In a previous study, we have shown that ONO-5334 suppresses bone resorption in rats [20]. In this study, we compared the effects of ONO-5334 on bone turnover markers, bone mass, geometry and bone strength with bisphosphonate (alendronate) using the OVX rat model of osteoporosis.
Materials and methods
Materials
ONO-5334, N-((1S)-3-{(2Z)-2-[(4R)-3,4-dimethyl-1,3-thiazolidin-2-ylidene]hydrazino}-2,3-dioxo-1-(tetrahydro-2H-pyran-4-yl) propyl)cyclopentane carboxamide, was synthesized in our laboratories (ONO Pharmaceutical Co., Ltd., Osaka, Japan). Alendronate sodium trihydrate (alendronate) was purchased from Dr. Reddy’s Laboratories (India).
Animal experiments
All animal procedures complied with the Guidelines for Animal Experiments established by our Research Headquarters. A total of 103 female F344/DuCrlCrlj rats, aged 10 weeks, were obtained from Charles River Laboratories Japan, Inc. (Kanagawa, Japan) and housed in an animal room maintained at a temperature of 24 ± 2 °C and a relative humidity of 55 ± 15 %, under a 12-h light/dark cycle. The animals were fed standard laboratory chow (CRF-1, Oriental Yeast, Tokyo, Japan) containing 1.25 % calcium, 0.90 % phosphorus, and 6.18 IU/g vitamin D3, and were given free access to tap water. Food was provided ad libitum during the 8-day acclimation period, but was limited to 4 pellets of food (approximately 13 g) per day during the treatment period. The animals were fasted for at least 2 h before and 2 h after test compound administration.
Rats were assigned to one of the following 7 groups: sham, ovariectomy (OVX), ONO-5334 0.12, 0.6, 3 or 15 mg/kg, or alendronate 1 mg/kg (n = 14 in each group), based on body weight measured on the last day of the acclimation period. Bilateral ovariectomy or sham operation was performed at 11 weeks of age under anesthesia with an intraperitoneal injection (0.10–0.15 mL/body) of sodium pentobarbital (6.48 mg/mL, Kyoritsu Seiyaku Corporation, Tokyo, Japan). Viccillin S500 for injection (ampicillin–cloxacillin combination, Meiji Seika Kaisha Ltd., Tokyo, Japan) was applied at a dose of 0.1 mL/body to the incision site to prevent infection.
Test compound administration
ONO-5334 was suspended in 0.5 % methylcellulose (MC) solution. As positive control, alendronate was first dissolved in 0.02 mol/L sodium hydroxide solution and then diluted with 0.5 % MC. Test animals were orally administered ONO-5334, alendronate or vehicle (0.5 % MC) once daily for 8 weeks starting on the day following OVX surgery. Sham-operated animals received the vehicle instead of test compound. The dose level of alendronate was chosen based on a previous report [12].
Blood and urine sample collection
Twenty-four-hour cumulative urine samples were collected on the day before the last dosing, and blood samples were collected from the cervical vein 3 h after the last dosing to prepare plasma. Plasma samples were filtered with Ultrafree-MC (30,000 NMWL, Millipore, USA) to measure C-terminal cross-linking telopeptide of type I collagen (CTX). On the day following the last dosing, blood samples were once again collected from the abdominal aorta under anesthesia to prepare serum samples, and the rats were killed by exsanguination. The uterus and left tibia of each animal were isolated, and the uterus was weighed.
Bone turnover markers
Urinary deoxypyridinoline (DPD) concentration was measured using Osteolinks-DPD (Sumitomo Seiyaku Biomedical Co., Ltd., Osaka, Japan), and urinary DPD data were normalized to creatinine concentration. Plasma CTX concentration was measured using RatLaps ELISA (Nordic Bioscience, Herlev, Denmark), and serum osteocalcin (OC) was measured using a Rat Osteocalcin ELISA System (Amersham Pharmacia Biotech K. K., Tokyo, Japan).
Peripheral quantitative computed tomography analysis
Peripheral quantitative computed tomography (pQCT, XCT Research SA+, software version 5.40, Stratec Medizintechnik GmbH) was used to measure bone mineral density (BMD), bone mineral content (BMC), and geometric parameters including cortical thickness and endosteal and periosteal circumferences at 4 mm from the proximal end of the left tibia. The trabecular region was separated using contour mode 2, peel mode 20 with a trabecular area of 35 %, and the cortical region was separated using cortmode 1 with a threshold of 690 mg/cm3.
Mechanical tests
The bone strength of the left proximal tibia was measured as described by Hogan et al. [21]. Compression testing was performed using a Universal Material Testing Machine (Type 5544, software: Merlin version 5.11, Instron Japan Co., Ltd.). The left tibia was cut at 3 and 5 mm from the proximal end with a precision saw (ISOMET, Buehler, Lake Bluff, IL, USA) to prepare a 2-mm-thick bone specimen. The bone specimen was placed between two platens, and a load was applied at a rate of 0.5 mm/min until failure. The load–displacement curve was generated using instrument software, and maximum load, stiffness, and energy absorption were calculated.
Statistical analyses
Data are summarized as mean and standard error (SE). Statistical analyses were performed using SAS System Release 8.2 (SAS Institute, Japan). For group comparisons, the sham and alendronate groups were compared to the OVX group using Student’s t test. The OVX and ONO-5334 groups were compared using Dunnett’s test. Correlation analysis of bone mass (total BMC and BMD) and bone strength (maximum load) was performed using Pearson’s correlation. Differences were considered significant at p < 0.05. Bone strength data from one animal in the sham group were excluded because of damage to the bone specimen during sample preparation.
Results
Changes in body and uterus weights
The mean body weight in the OVX group was significantly higher than that in the sham group at the end of the treatment period (Table 1). There was no significant difference in body weight between the OVX group and any of the other groups. The weight of the uterus of each animal in the OVX, ONO-5334 and alendronate groups was much lower than that of any animal in the sham group, indicating the success of OVX surgery (data not shown). No adverse effect was observed in any of the animals that received ONO-5334 or alendronate.
Changes in bone turnover markers
Ovariectomy resulted in significant elevation (73 % vs. Sham) in urinary DPD in the vehicle-treated animals 8 weeks after surgery (Table 2). This increase was associated with a slight increase (23 % vs. Sham) in plasma CTX, but no change in serum osteocalcin level. Treatment with ONO-5334 at the dose of 15 mg/kg once daily for 8 weeks significantly suppressed the OVX-induced increase in urinary DPD, while the effect of alendronate resulted in a urinary DPD level lower than that in the sham-operated animals. Although both ONO-5334 at all test doses and alendronate decreased the OVX-induced increase in plasma CTX level below that in the sham group, this decrease was more prominent in the animals treated with ONO-5334 at doses higher than 0.12 mg/kg. There was no significant change in serum osteocalcin level following treatment with ONO-5334 or alendronate.
Effects of test compounds on bone mass and geometry as assessed by pQCT
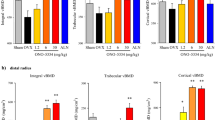
Ovariectomy decreased both total and trabecular bone mass (BMD and BMC) in the proximal tibia in the vehicle-treated animals (Fig. 1a–d). However, in the cortical region, while BMC decreased by 10 %, BMD rather increased by 3 % after surgery (Fig. 1e, f). ONO-5334 inhibited the OVX-induced decrease in total BMD and BMC in a dose-dependent manner, with total BMD and BMC in the animals treated with ONO-5334 at 15 mg/kg and in those treated with alendronate roughly comparable to those in the sham group. In the trabecular region, although ONO-5334 partially suppressed the OVX-induced decrease in both BMD and BMC, this effect was less evident than that of alendronate, which prevented the OVX-induced decrease in both parameters. On the other hand, in the cortical region, ONO-5334 at 15 mg/kg strongly increased both BMD and BMC by 5 and 24 %, respectively, compared to the levels in the OVX rats. Such an increasing effect was stronger than that of alendronate.
Effects of ONO-5334 on BMD and BMC in the proximal tibia. Rats orally received ONO-5334 (0.12, 0.6, 3 or 15 mg/kg), alendronate (ALN, 1 mg/kg) or vehicle (Veh) for 8 weeks. Total BMD (a) and BMC (b), trabecular BMD (c) and BMC (d), cortical BMD (e) and BMC (f) were measured by pQCT. Data are presented as mean + SE (n = 14). ## p < 0.01 vs. sham group (Student’s t test). * , **p < 0.05, 0.01 vs. OVX group, respectively (Dunnett’s test). $$ p < 0.01 vs. OVX group (Student’s t test)
As for cortical bone geometry, cortical thickness was significantly decreased in the OVX group (Fig. 2a). This decrease was associated with a decrease in periosteal circumference, but no change in endosteal circumference (Fig. 2b, c). ONO-5334 at the high doses prevented the decrease in cortical thickness and even increased this parameter above the level in the sham group at the highest dose. This increase was accompanied by significant decrease in endosteal circumference, but no change in periosteal circumference. Alendronate prevented the decrease in cortical thickness and periosteal circumference without affecting endosteal circumference.
Effects of ONO-5334 on cortical bone geometry in the proximal tibia. Rats orally received ONO-5334 (0.12, 0.6, 3 or 15 mg/kg), alendronate (ALN, 1 mg/kg) or vehicle (Veh) for 8 weeks. Cortical thickness (a), periosteal circumference (b), and endosteal circumference (c) were measured by pQCT. Data are presented as mean + SE (n = 14). ## p < 0.01 vs. sham group (Student’s t test). **p < 0.01 vs. OVX group (Dunnett’s test). $$ p < 0.01 vs. OVX group (Student’s t test)
Effects of test compounds on bone strength in the proximal tibia
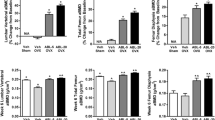
OVX significantly decreased both maximum load and energy absorption in the proximal tibia (Fig. 3a, c). ONO-5334 prevented the decrease in both parameters at the dose of 3 mg/kg and even increased them above the level in the sham group at 15 mg/kg. Treatment with alendronate, on the other hand, only maintained maximum load and energy absorption at the level of the sham group. There was no significant change in stiffness following OVX, or treatment with ONO-5334 or alendronate (Fig. 3b).
Effects of ONO-5334 on bone mechanical strength in the proximal tibia. Rats orally received ONO-5334 (0.12, 0.6, 3 or 15 mg/kg), alendronate (ALN, 1 mg/kg) or vehicle (Veh) for 8 weeks. Maximum load (a), energy absorption (b) and stiffness (c) were obtained by compression test. Data are presented as mean + SE (n = 13–14). ## p < 0.01 vs. sham group (Student’s t test). *, **p < 0.05, 0.01 vs. OVX group, respectively (Dunnett’s test). $, $$ p < 0.05, 0.01 vs. OVX group (Student’s t test)
Samples of the proximal tibia showed a significant positive linear relationship between total BMC and maximum load (Fig. 4). Pearson’s correlation coefficient (r) was 0.71 (p < 0.001), 0.71 (p < 0.001), and 0.40 (p < 0.05) in the sham and OVX groups; the OVX and ONO-5334 groups; and the OVX and alendronate groups, respectively. A significant positive correlation was also observed between total BMD and maximum load in these groups (data not shown).
Correlation between bone mass and bone strength in the proximal tibia. Pearson correlation analysis between bone mass (total BMC) and bone strength (maximum load) was conducted. A significant positive correlation was noted between the sham and OVX groups (a), OVX and ONO-5334 groups (b), and OVX and alendronate groups (c). r correlation coefficient
Discussion
The effects of cathepsin K inhibitors on bone mass and strength have not been fully elucidated in the generally used rat model of osteoporosis. In particular, there is no evidence of the effectiveness of cathepsin K inhibitors on bone strength in this model. The current study shows that ONO-5334, a novel cathepsin K inhibitor, not only prevents decrease in bone mass, but also preserves bone strength in OVX rats as a model of osteoporosis. The effects of ONO-5334 were more evident in the cortical bone than in the trabecular bone, reflecting the different mode of action of this cathepsin K inhibitor compared to alendronate.
In OVX rats, total bone mass and bone strength were significantly decreased in the proximal tibia (Figs. 1, 3), confirming the development of osteoporosis. However, in the cortical bone, OVX rather increased cortical BMD despite a decrease in cortical BMC. This suggests that cortical bone tissue volume decreased to a higher degree than BMC. Such change is also supported by narrowing of cortical thickness associated with preferential decrease in periosteal circumference.
High bone turnover characterized by increase in both bone resorption and formation is another typical change associated with postmenopausal osteoporosis. In the current study, OVX rats showed significant increase in bone resorption marker, urinary DPD level, but no change in bone formation marker serum osteocalcin level (Table 2). These findings are consistent with those of another study that used F344/DuCrlCrlj rats [22], a strain similar to that used in the current study. However, in that study the bone formation rate (BFR/BS and BFR/BV) consistently increased in the lumbar vertebral body. It is therefore likely that F344/DuCrlCrlj rats have a high bone formation rate even after OVX, although this is not reflected by changes in serum osteocalcin level.
Although both ONO-5334 and alendronate significantly decreased bone resorption markers urinary DPD and plasma CTX level (Table 2), they showed different efficacy in the suppression of bone resorption markers. The effect of ONO-5334 on plasma CTX level was more potent than that on urinary DPD, while alendronate showed a similar effect on both markers. It is not surprising, though, that plasma CTX level was strongly decreased by ONO-5334 to a level below that in the sham and alendronate groups, as CTX is directly produced by cathepsin K from type I collagen [23]. On the other hand, measurement of the free form of urinary DPD in the current study may not reflect the actual bone resorption state caused by cathepsin K inhibition. This may be due to the fact that urinary DPD contains both free and peptide-bound DPD and that peptide-bound DPD can be further degraded by other enzymes, such as matrix metalloproteinases.
ONO-5334 prevented the OVX-induced decrease in proximal tibia total BMD and BMC to the same extent as alendronate (Fig. 1a, b). Interestingly, separate analysis of trabecular and cortical bones by pQCT revealed that the increasing effect of ONO-5334 on BMD/BMC in these two bones was different from that of alendronate (Fig. 1c–f); that is, the effect of ONO-5334 was milder than that of alendronate in the trabecular bone, but stronger than that of alendronate in the cortical bone. Analysis of cortical bone geometry in the proximal tibia also supports this difference in efficacy (Fig. 2). ONO-5334 at doses of 3 and 15 mg/kg significantly decreased the endosteal circumference in the proximal tibia without affecting the periosteal circumference, resulting in a marked increase in cortical thickness. Such changes were, however, not evident in the alendronate group. These results indicate that ONO-5334 potently expanded the cross-sectional area of the cortical bone, which potentially affected mechanical bone strength.
As expected, bone strength in the proximal tibia was increased by treatment with ONO-5334 (Fig. 3). The maximum load in the ONO-5334 (15 mg/kg) group was higher than that in the alendronate group, even though total BMC was comparable in the two groups. Bone strength is known to be associated with cortical bone mass more than with trabecular bone mass. Since ONO-5334 at the dose of 15 mg/kg markedly increased cortical BMC rather than trabecular BMC, such increase in bone strength might be due to increase in cortical bone mass as reflected by increase in cortical BMD/BMC and cortical thickness in the proximal tibia.
To assess the influence of ONO-5334 on bone quality, we investigated the correlation between bone mass and bone strength in the proximal tibia (Fig. 4). In the OVX and ONO-5334 groups, BMC highly correlated with maximum load, suggesting that ONO-5334 maintained normal bone biomechanical properties. Although not shown, similar results were also obtained by BMD analysis. It is interesting to note here that the coefficient for the curve Maximum load vs. Total BMC was steeper than that in the case of alendronate, as this may reflect bone structural differences between animals treated with ONO-5334 and those treated with alendronate.
The current study highlights the difference in efficacy on bone parameters between ONO-5334 and alendronate, especially in the cortical and trabecular bones. The reason for this difference in efficacy is not clear, but may include differences in pharmacokinetics and mode of action. As bisphosphonates are likely to be distributed at the high turnover site of the bone, it is believed that alendronate concentration in the trabecular bone is higher than that in the cortical bone [24]. On the other hand, ONO-5334 is considered to be equally distributed in the trabecular and cortical bones. This difference in distribution may contribute to difference in efficacy. However, the high efficacy of ONO-5334 on cortical bone parameters cannot be solely explained by such a difference in distribution, as ONO-5334 increased cortical BMC and thickness to a level higher than that in the sham group.
Bisphosphonates, including alendronate, inhibit bone resorption by impairing osteoclast function or inducing osteoclast apoptosis [25, 26]. It is known that such disruption of osteoclast activity leads to secondary decrease in osteoblast activity and consequently suppression of bone formation. In contrast, ONO-5334 reduces bone resorption by inhibiting cathepsin K-mediated collagen degradation without affecting osteoclast viability [20]. It has recently been reported that non-resorbing osteoclasts are a source of anabolic signals for osteoblasts [10]. It is therefore important to maintain osteoclast viability. In fact, cathepsin K-deficient mice exhibit decreased bone resorption, but increased osteoclast number and bone formation activity [8, 9]. Thus, the potent efficacy of ONO-5334 on cortical bone may have been due to sustained or enhanced bone formation in addition to suppression of bone resorption. Other cathepsin K inhibitors, such as balicatib and odanacatib, also preserve endosteal bone formation and stimulate periosteal bone formation in the cortical bone of OVX monkeys [27, 28]. These findings as well as ours support the notion that cathepsin K inhibitors are able to potently reduce bone resorption, while promoting bone formation activity by not interfering with osteoclast viability. The results of clinical trials with these cathepsin K inhibitors are awaited.
In conclusion, we have shown in this study that ONO-5334, a cathepsin K inhibitor, prevents decreases in bone mass and strength without deteriorating bone biomechanical properties in OVX rats. ONO-5334 preferentially increased cortical bone mass as compared to trabecular bone mass, suggesting the therapeutic potential of cathepsin K inhibitors for patients with osteoporosis.
References
Yasuda Y, Kaleta J, Brömme D (2005) The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv Drug Deliv Rev 57:973–993
Inaoka T, Bilbe G, Ishibashi O, Tezuka K, Kumegawa M, Kokubo T (1995) Molecular cloning of human cDNA for cathepsin K: novel cysteine proteinase predominantly expressed in bone. Biochem Biophys Res Commun 206:89–96
Drake FH, Dodds RA, James IE, Connor JR, Debouck C, Richardson S, Lee-Rykaczewski E, Coleman L, Rieman D, Barthlow R, Hastings G, Gowen M (1996) Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J Biol Chem 271:12511–12516
Garnero P, Borel O, Byrjalsen I, Ferreras M, Drake FH, McQueney MS, Foged NT, Delmas PD, Delaissé JM (1998) The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem 273:32347–32352
Gelb BD, Shi GP, Chapman HA, Desnick RJ (1996) Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science 273:1236–1238
Johnson MR, Polymeropoulos MH, Vos HL, Ortiz de Luna RI, Francomano CA (1996) A nonsense mutation in the cathepsin K gene observed in a family with pycnodysostosis. Genome Res 6:1050–1055
Gowen M, Lazner F, Dodds R, Kapadia R, Feild J, Tavaria M, Bertoncello I, Drake F, Zavarselk S, Tellis I, Hertzog P, Debouck C, Kola I (1999) Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res 14:1654–1663
Li CY, Jepsen KJ, Majeska RJ, Zhang J, Ni R, Gelb BD, Schaffler MB (2006) Mice lacking cathepsin K maintain bone remodeling but develop bone fragility despite high bone mass. J Bone Miner Res 21:865–875
Pennypacker B, Shea M, Liu Q, Masarachia P, Saftig P, Rodan S, Rodan G, Kimmel D (2009) Bone density, strength, and formation in adult cathepsin K (−/−) mice. Bone 44:199–207
Karsdal MA, Martin TJ, Bollerslev J, Christiansen C, Henriksen K (2007) Are nonresorbing osteoclasts sources of bone anabolic activity? J Bone Miner Res 22:487–494
Duong le T (2012) Therapeutic inhibition of cathepsin K-reducing bone resorption while maintaining bone formation. BoneKEy Reports 1: article number 67. doi:10.1038/bonekey.2012.67
Azuma Y, Oue Y, Kanatani H, Ohta T, Kiyoki M, Komoriya K (1998) Effects of continuous alendronate treatment on bone mass and mechanical properties in ovariectomized rats: comparison with pamidronate and etidronate in growing rats. J Pharmacol Exp Ther 286:128–135
Gasser JA, Ingold P, Venturiere A, Shen V, Green JR (2008) Long-term protective effects of zoledronic acid on cancellous and cortical bone in the ovariectomized rat. J Bone Miner Res 23:544–551
Tanaka M, Mori H, Kayasuga R, Ochi Y, Kawada N, Yamada H, Kishikawa K (2008) Long-term minodronic acid (ONO-5920/YM529) treatment suppresses increased bone turnover, plus prevents reduction in bone mass and bone strength in ovariectomized rats with established osteopenia. Bone 43:840–848
Sato M, Bryant HU, Iversen P, Helterbrand J, Smietana F, Bemis K, Higgs R, Turner CH, Owan I, Takano Y, Burr DB (1996) Advantages of raloxifene over alendronate or estrogen on nonreproductive and reproductive tissues in the long-term dosing of ovariectomized rats. J Pharmacol Exp Ther 279:298–305
Sato M, Zeng GQ, Turner CH (1997) Biosynthetic human parathyroid hormone (1–34) effects on bone quality in aged ovariectomized rats. Endocrinology 138:4330–4337
Stroup GB, Lark MW, Veber DF, Bhattacharyya A, Blake S, Dare LC, Erhard KF, Hoffman SJ, James IE, Marquis RW, Ru Y, Vasko-Moser JA, Smith BR, Tomaszek T, Gowen M (2001) Potent and selective inhibition of human cathepsin K leads to inhibition of bone resorption in vivo in a nonhuman primate. J Bone Miner Res 16:1739–1746
Lark MW, Stroup GB, James IE, Dodds RA, Hwang SM, Blake SM, Lechowska BA, Hoffman SJ, Smith BR, Kapadia R, Liang X, Erhard K, Ru Y, Dong X, Marquis RW, Veber D, Gowen M (2002) A potent small molecule, nonpeptide inhibitor of cathepsin K (SB 331750) prevents bone matrix resorption in the ovariectomized rat. Bone 30:746–753
Kim MK, Kim HD, Park JH, Lim JI, Yang JS, Kwak WY, Sung SY, Kim HJ, Kim SH, Lee CH, Shim JY, Bae MH, Shin YA, Huh Y, Han TD, Chong W, Choi H, Ahn BN, Yang SO, Son MH (2006) An orally active cathepsin K inhibitor, furan-2-carboxylic acid, 1-{1-[4-fluoro-2-(2-oxo-pyrrolidin-1-yl)-phenyl]-3-oxo-piperidin-4-ylcarbamoyl}-cyclohexyl)-amide (OST-4077), inhibits osteoclast activity in vitro and bone loss in ovariectomized rats. J Pharmacol Exp Ther 318:555–562
Ochi Y, Yamada H, Mori H, Nakanishi Y, Nishikawa S, Kayasuga R, Kawada N, Kunishige A, Hashimoto Y, Tanaka M, Sugitani M, Kawabata K (2011) Effects of ONO-5334, a novel orally-active inhibitor of cathepsin K, on bone metabolism. Bone 49:1351–1356
Hogan HA, Ruhmann SP, Sampson HW (2000) The mechanical properties of cancellous bone in the proximal tibia of ovariectomized rats. J Bone Miner Res 15:284–292
Kimoto A, Tanaka M, Nozaki K, Mori M, Fukushima S, Mori H, Shiroya T, Nakamura T (2013) Intermittent minodronic acid treatment with sufficient bone resorption inhibition prevents reduction in bone mass and strength in ovariectomized rats with established osteopenia comparable with daily treatment. Bone 55:189–197
Nishi Y, Atley L, Eyre DE, Edelson JG, Superti-Furga A, Yasuda T, Desnick RJ, Gelb BD (1999) Determination of bone markers in pycnodysostosis: effects of cathepsin K deficiency on bone matrix degradation. J Bone Miner Res 14:1902–1908
Azuma Y, Sato H, Oue Y, Okabe K, Ohta T, Tsuchimoto M, Kiyoki M (1995) Alendronate distributed on bone surfaces inhibits osteoclastic bone resorption in vitro and in experimental hypercalcemia models. Bone 16:235–245
Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF (1995) Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res 10:1478–1487
Reszka AA, Halasy-Nagy JM, Masarachia PJ, Rodan GA (1999) Bisphosphonates act directly on the osteoclast to induce caspase cleavage of mst1 kinase during apoptosis. A link between inhibition of the mevalonate pathway and regulation of an apoptosis-promoting kinase. J Biol Chem 274:34967–34973
Jerome C, Missbach M, Gamse R (2011) Balicatib, a cathepsin K inhibitor, stimulates periosteal bone formation in monkeys. Osteoporos Int 22:3001–3011
Cusick T, Chen CM, Pennypacker BL, Pickarski M, Kimmel DB, Scott BB, le Duong T (2012) Odanacatib treatment increases hip bone mass and cortical thickness by preserving endocortical bone formation and stimulating periosteal bone formation in the ovariectomized adult rhesus monkey. J Bone Miner Res 27:524–537
Acknowledgments
We thank Yoko Kishida for technical support in animal care and assessments. We also thank Akiko Kunishige, Satoshi Nishikawa, Yasuaki Hashimoto, Masafumi Sugitani, and Katsuya Kishikawa (Ono Pharmaceutical Co., Ltd.) for their helpful comments and discussions on this manuscript.
Conflict of interest
All authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ochi, Y., Yamada, H., Mori, H. et al. ONO-5334, a cathepsin K inhibitor, improves bone strength by preferentially increasing cortical bone mass in ovariectomized rats. J Bone Miner Metab 32, 645–652 (2014). https://doi.org/10.1007/s00774-013-0542-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-013-0542-x