Abstract
Osteoporosis is one of the major complications of glucocorticoid therapy. Osteoporosis is usually defined by the levels of bone mineral density (BMD) assessed by dual energy X-ray absorptiometry (DEXA); however, glucocorticoids often induce fractures in patients with normal BMD. Thus, novel diagnostic approaches are required. In this study, we examined whether multidetector-row computed tomography (MDCT) is useful to assess the bone status in glucocorticoid-induced osteoporosis (GIO). Because bisphosphonates have been proven to prevent bone fracture in GIO, we tried to detect the therapeutic effects of bisphosphonates in GIO by MDCT. Fifteen Japanese patients with immunoglobulin A nephropathy who had normal renal function were enrolled in this open-label randomized trial. Patients were randomly divided into three groups—calcitriol (VD), menatetrenone (VK), or bisphosphonate (Bis). Bone conditions were analyzed twice by three different methods—bone turnover markers, DEXA, and MDCT—at the start and 6 months after the start of therapy. Both bone markers and DEXA could not detect significant differences among the therapeutic groups; however, MDCT-based analyses detected the preventive effects of bisphosphonates in GIO. Compared to VD, Bis improved structural indices, such as bone volume fraction, trabecular separation, marrow star volume, and structure model index whereas the difference between VD and VK was not significant. Finite element analysis revealed that simulated fracture load in the Bis group was significantly improved. These findings suggested that MDCT-based assessment is superior to bone markers and/or DEXA in assessing the therapeutic effect of bisphosphonates on GIO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucocorticoids are one of the most commonly prescribed drugs and have both beneficial and adverse effects [1, 2]. Glucocorticoid therapy has resulted in marked improvement in outcomes of many auto-immune or inflammatory diseases, including glomerulonephritis, but a variety of adverse effects limits its clinical utility [1, 2]. Among several adverse effects, glucocorticoid-induced osteoporosis (GIO) is one of the most important complications [3]. The standard diagnostic approach for osteoporosis is to assess bone mineral density (BMD) by dual energy X-ray absorptiometry (DEXA) [4, 5]. However, DEXA is insufficient to assess the osteoporotic status in patients with glucocorticoid therapy, because bone fractures occur even at normal BMD status in these patients [6]. Thus, novel diagnostic approaches to evaluate the osteoporotic status in GIO are required.
Bone quality, in addition to BMD, is a determinant of bone strength [7]. Bone quality encompasses several bone characteristics, such as apatite crystallization, property of collagen and/or its crosslink, and trabecular microarchitecture [7]. Although these characteristics could not be examined non-invasively until recently, advances in diagnostic imaging now allow non-invasive evaluation of trabecular microarchitecture [ 8, 9 ]. Ito et al. demonstrated that multidetector-row computed tomography (MDCT)-based three-dimensional (3D) assessment of trabecular microarchitecture was useful to evaluate the fracture risk in postmenopausal women [8]. However, it remains uncertain whether MDCT-based analysis is also useful in the assessment of GIO.
Three lines of medications are recommended for the treatment or prevention of GIO in Japan [10]. Bisphosphonates are first-line drugs and vitamin D and vitamin K are second-line drugs [10]. The powerful preventive effects of bisphosphonates on GIO have been revealed in previous prospective randomized controlled trials [11–15]. Moreover, meta-analysis of clinical trials on GIO showed that bisphosphonates are superior to vitamin D in decreasing fracture risk [16]. Because Carbonare et al. [17] demonstrated that risedronate prevented 3D trabecular microarchitecture in animal GIO, the preventive effects of bisphosphonates in human GIO seem to be attributable, at least partly, to the protection of trabecular microarchitecture. From this point of view, MDCT might be a suitable tool for the assessment of bone status in human GIO.
Here, we examined bone turnover markers, BMD, and trabecular microarchitecture in patients with immunoglobulin A nephropathy (IgAN) who received high-dose glucocorticoid therapy. We obtained 3D microarchitecture of their lumbar vertebrae non-invasively by MDCT. The aim of this study was to evaluate the usability of MDCT in comparison with conventional analyses, DEXA and bone turnover markers, to assess the bone status in glucocorticoid-treated patients.
Materials and methods
Study population
In this prospective open-label randomized trial, we enrolled 15 Japanese patients who met criteria for poor or relatively poor prognostic IgAN from March 2007 [18]. IgAN patients who were categorized as having a good or relatively good prognosis were excluded because clinical guidelines for IgAN in Japan do not recommend adrenocorticosteroid therapy for these patients [18]. All patients (10 males, mean age 28.6 years; range 17–48 years) had normal renal function [estimated glomerular filtration rate (eGFR) ≥60 ml/min/1.73 m2) and had never received steroids, bisphosphonates, menatetrenone, native or active vitamin D, or raloxifene. To evaluate changes derived from glucocorticoid therapy but not from menopause, we excluded postmenopausal women. We also excluded patients with fracture history or with systemic diseases, such as diabetes mellitus, systemic lupus erythematosus, rheumatoid arthritis, and Henoch–Schönlein purpura. All patients underwent tonsillectomy, two courses of methylprednisolone pulse therapy, and subsequent oral prednisolone therapy (Fig. 1a). The conditions of the bone at pre- and post-intervention were evaluated by three different methods—bone turnover markers, areal BMD by DEXA, and trabecular microarchitecture by MDCT. Post-intervention data were collected 6 months after the start of glucocorticoid therapy. Two patients withdrew before the post-intervention tests (Fig. 1b). The thirteen patients, who received the post-intervention tests, were subjected to per-protocol analysis (Fig. 1b). The baseline characteristics of the 13 patients are shown in Table 1. We adhered to the Declaration of Helsinki throughout this study. The Ethics Committee of Osaka University Medical Hospital approved the study protocol (approval number 06122) and all patients provided written informed consent to participate in all aspects of this study. This trial is registered as the Preventive Risedronate Intervention for those Undergoing Steroid therapy with IgA nephropathy (PRIUS-IgA Study) (the UMIN clinical trials registry, number UMIN000002474). (https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=confirm&action=brows&recptno=R000003035&key=1002nTDQIN2vlSyUCjXrAEOu).
Flow of the participants in the trial. a Treatment protocol for the patients with IgAN and b flow of the participants are schematically shown. a All patients received tonsillectomy, two courses of methylprednisolone pulse therapy, and subsequent oral prednisolone therapy. Blood tests, DEXA and MDCT were performed both at the start and at 6 months after the start of the therapy. b Based on anti-osteoporotic medication, patients were randomly divided into three groups—active vitamin D (VD) group (calcitriol 0.5 μg/day), vitamin K2 (VK) group (menatetrenone 45 mg/day), or bisphosphonate (Bis) group (risedronate 2.5 mg/day). Data are mean ± SD
Treatment protocol
Treatment protocol is summarized in Fig. 1a. One course of steroid pulse therapy consists of 500 mg/day of methylprednisolone dripped infusion for three sequential days. The interval between the two pulse therapies was covered by oral prednisolone at a dose of 1 mg/kg/day (maximally 50 mg/day). After the two pulse therapies, all patients received oral prednisolone at a dose of 30 mg/day. The doses of oral prednisolone were gradually reduced (5 mg/1–2 months).
Based on anti-osteoporotic medication, enrolled patients were randomly divided into three groups (active vitamin D, vitamin K, or bisphosphonate group) by envelope method (Fig. 1b). Patients in the bisphospohante group received risedronate (2.5 mg/day) first and were allowed to change their medication to alendronate (35 mg/week) in case the patients preferred weekly formulation. Weekly formulation of risedronate was not available in several pharmacies in Japan in 2007. Calcitriol (0.5 μg/day) and menatetrenone (45 mg/day) was prescribed to patients in the vitamin D and vitamin K group, respectively.
Measured variable
Evaluation of blood and urinary samples
Blood and urinary samples were obtained from patients after an overnight fast. Clotted blood samples were centrifuged for serum separation. Obtained serum was frozen at −80 °C until analyses. Serum chemistry parameters (creatinine, albumin, calcium, and inorganic phosphate) were measured by standard automated techniques. Estimated GFR was calculated according to Japanese coefficient-modified CKD-EPI equation [19]. Serum levels of intact parathyroid hormone (PTH) and 1,25-dihydroxy vitamin D [1,25(OH)2D] were measured by Elecsys intact PTH ECLIA reagent (Cobas; Roche Diagnostics, Basel, Switzerland) and 1,25-hydroxyvitamin D RIA kit ‘TFB’ (Immunodiagnostic Systems, Boldon, UK), respectively. Bone turnover was estimated by serum BAP (Osteolinks® BAP; DS Pharma Biomedical, Osaka, Japan) and serum NTX (Osteomark® NTx; Inverness Medical Innovations, NJ, USA).
Measurement of areal BMD by DEXA
Areal BMD and its Z score of the lumbar spine (L 2–L 4) were determined by DEXA (Hologic Discovery™ Dual Energy X-ray Absorptiometry System; Hologic, Bedford, MA, USA). Results were expressed as BMD (g/cm2). BMD Z scores represent standard deviations from an average person of the same age and sex.
Measurement of volumetric BMD and trabecular architecture by MDCT
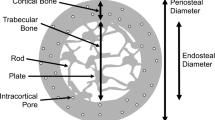
We evaluated trabecular microarchitecture of the third lumbar spine using MDCT systems (16-slice technique; Light Speed Ultra 16; GE Yokokawa Medical Systems, Tokyo, Japan). Patients were in the supine position for horizontal scanning of the vertebral body. The MDCT imaging parameters were scan kVp, 120 kVp; scan mAs, 300 mAs; table speed, 9.37 mm/s; image reconstruction interval, 0.2 mm; scan length, 45.2 mm; field of view (FOV), 100 mm; pixel matrix, 512 × 512 pixels, and pixel size, 0.2 mm. The central part of the vertebra was measured at a 10 mm height. Structural indices of trabecular bone were calculated by a 3D image analysis system (TRI/3D-BON; RATOC System Engineering, Tokyo, Japan). Parameters were calculated in 3D as follows: volumetric BMD (vBMD) was determined using a reference phantom (B-MAS200; KYOTO KAGAKU, Kyoto, Japan). Using a vBMD value for trabecular bone, >150 mg/cm3 within the bone marrow was extracted. Bone volume (BV) was calculated using tetrahedrons corresponding to the enclosed volume of the triangulated surface. Total tissue volume (TV) analyzed was the entire marrow area volume including trabecular bone. Trabecular bone volume fraction (BV/TV) was calculated from these values. Trabecular thickness (Tb.Th) and trabecular separation (Tb.Sp) were determined by filling maximal spheres into the structure according to the method described by Hildebrand and Rügsegger [20]. Trabecular number (Tb.N) was estimated as a trabecular bone number crossing the line perpendicular to the growing direction of vertebrae based on the plate model [21]. Marrow star volume was defined as the mean volume of the marrow cavity surveyed from a designated point in all directions without obstruction by the trabecula [22]. By displaying the surface of the structure to an infinitesimal amount, the structure model index (SMI) was calculated according to the method described by Hildebrand and Rügsegger [20]. The SMI quantifies the plate versus rod characteristics of trabecular bone. An SMI of 0 reflects a purely plate-shaped bone and an SMI of 3 indicates a purely rod-like bone.
Calculation of the fracture load by using finite element analysis
In fracture load analysis, von Mises stress >4.2 MPa was defined as the stress that induces trabecular fracture. Using finite element analysis (FEA) software (TRI/3D-FEM; RATOC System Engineering) [23, 24], fracture load was determined to the level that induces fractures in 1 % of trabecular bone.
An FEA model was created using a 0.2-mm isometric hexagonal mesh from pixels which constitute the trabecular bone [25]. For trabecular bone, Young’s modulus was determined using Carter’s equation and vBMD value [26]. Poisson’s ratio is set as 0.3 for all trabecular bone. The distal part of vertebral trabecular bone was fixed and compressed simulation was applied at a load of 500 N from the proximal section.
Statistical analyses
With regard to postmenopausal osteoporosis, Borah et al. [27] demonstrated that risedronate significantly improved BV/TV compared with placebo by enrolling 8 or 11 women in each group. Therefore, we hypothesized that approximately 10 patients in each group (30 in total) might be needed in our study. We assumed that the difference in %change in BV/TV for 6 months between calcitriol and risedronate user was 5.5 % based on this study. As an adaptive design, we recalculated the number of the patients needed in this study when we finished studying 15 patients (half of predefined 30), employing the obtained data of standard deviation (SD) of %change in BV/TV.
Baseline characteristics were compared using the Kruskal–Wallis test (Table 1). The changes in bone turnover markers, BMD, and structural indices of trabecular bone were analyzed by the Dunnett multiple comparisons test with the vitamin D group as a reference (Figs. 2, 3, 5, 6, 7). The intra-group analysis between before and after each treatment was performed by paired t test (Table 2). All data were shown as mean ± SD (Figs. 2, 3, 5, 6 7; Tables 1, 2). All statistical tests were two-sided and P < 0.05 was considered significant. All data were analyzed using JMP version 8.0.1 for Windows (SAS institute Inc., Cary, NC, USA).
Changes in serum markers for bone turnover. The changes in a serum bone alkaline phosphatase (BAP) and b serum cross-linked N-telopeptide of type I collagen (NTX) were not different among the three groups (NS not significant; a P = 0.677 for VK and P = 0.936 for Bis; b P = 0.695 for VK and P = 0.986 for Bis). Data are mean ± SD
Results
Characteristics of enrolled subjects
Because SD of %change in BV/TV after studying 15 patients was found to be far smaller than we had expected, the power of our study to detect the predefined effect of bisphosphonate was 0.8664 when setting up a type 1 error of 5 %. Therefore, we decided not to enroll any more patients. All 15 patients received glucocorticoid therapy in accordance with the clinical guidelines for IgAN in Japan [18]. Treatment protocol consisted of tonsillectomy, two courses of methylprednisolone pulse therapy, and subsequent oral prednisolone therapy (Fig. 1a) [2, 18]. Based on anti-osteoporotic medications, enrolled patients were randomly divided into three groups—active vitamin D (VD), vitamin K2 (VK), or bisphosphonate (Bis) group (Fig. 1b) [10]. Patients in each group received 0.5 μg/day of calcitriol, 45 mg/day of menatetrenone, or 2.5 mg/day of risedronate, respectively.
We could not follow one patient in the VD group because he moved out of the area (Fig. 1b). One patient in the Bis group withdrew due to a pyrosis caused by risedronate (Fig. 1b). These two patients were not included in the following analyses. Another patient in the Bis group who changed the anti-osteoporotic medication from risedronate to alendronate was included in the per-protocol efficacy analysis (see “Materials and methods”). The baseline characteristics of the 13 patients are shown in Table 1. There were no statistically significant differences among the three groups (Table 1).
Conventional assessments of the bone could not detect significant differences among the three groups
Glucocorticoids can regulate cells both in osteoblastic and osteoclastic lineage [28]. However, the most significant event that leads to bone loss in GIO is the suppression of bone formation [28]. To evaluate the bone turnover status of the enrolled patients, we examined changes in serum bone alkaline phosphatase (BAP) and serum cross-linked N-telopeptide of type I collagen (NTX) (Fig. 2). Reflecting the suppressed bone formation in GIO, serum BAP decreased to similar levels in all groups (Fig. 2a). Although bisphosphonate, but not menatetrenone, is a potent inhibitor of bone resorption, changes in serum NTX were also at similar levels in these groups (Fig. 2b). In addition to bone turnover markers, two parameters obtained by DEXA also showed no statistically significant differences among the three groups (Fig. 3). These findings suggest that bone turnover markers and DEXA were insufficient to detect therapeutic differences among these anti-osteoporotic medications, at least in our study conditions.
MDCT-based analyses detected the preventive effect of bisphosphonates in GIO
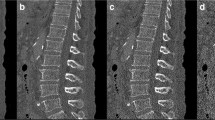
We further analyzed whether MDCT-based assessment of trabecular microarchitecture can detect the preventive effect of bisphosphonate in our study conditions. Figure 4 shows a representative reconstructed 3D microarchitecture of the third lumbar vertebra. Microarchitecture of trabecular bone was well reconstructed in our MDCT-based analysis (Fig. 4). Using reconstructed microarchitecture, we analyzed structural indices of trabecular bone (Figs. 5, 6, 7).
MDCT-based analyses of Tb.N, Tb.Th, Tb.Sp, and marrow star volume. a Tb.N and b Tb.Th were not different among the three groups, whereas c Tb.Sp and d star volume significantly decreased in the Bis group (NS not significant; a P = 0.120 for VK and P = 0.992 for Bis; b P = 0.345 for VK and P = 0.920 for Bis; c P = 0.698 for VK and *P = 0.015 for Bis; d P = 0.892 for VK and *P = 0.013 for Bis). Data are mean ± SD
MDCT-based analyses of SMI and fracture load. a SMI significantly decreased in the Bis group. b Reflecting the improved trabecular microarchitecture, the Bis group tolerated higher load than two vitamin groups. a P = 0.762 for VK and *P = 0.009 for Bis; b P = 0.978 for VK and *P = 0.034 for Bis). Data are mean ± SD
Consistent with the results in areal BMD by DEXA (Fig. 3b), values in vBMD obtained by MDCT were not different among the three groups (Fig. 5a). In addition to vBMD, all structural indices demonstrated that there were no statistically significant differences between the VD group and the VK group (Figs. 5b, 6, 7). However, MDCT detected the therapeutic effects of bisphosphonates in several structural indices. Bisphosphonates significantly improved BV/TV in comparison with vitamin D therapy (Fig. 5b). The improvement of BV/TV was associated with decreased Tb.Sp but not with Tb.N or Tb.Th (Fig. 6a–c). Tb.N increased and Tb.Th decreased to similar levels in all groups (Fig. 6a, b). In accordance with the decreased Tb.Sp, marrow star volume declined in the Bis group (Fig. 6d). MDCT also revealed that bisphosphonates were superior to vitamin D in decreasing SMI value (Fig. 7a). As a result of protected microarchitecture in the Bis group, fracture load calculated by FEA in the Bis group was significantly higher than that of the VD group (Fig. 7b). Because glucocorticoids affect the growth plate during growing years [29, 30], the effect of glucocorticoids in adolescent patients might be different from that in adult patients. Therefore, we performed a sensitivity analysis excluding one patient <20 years. Consequently, similar statistically significant results were obtained (data not shown). These findings indicated that MDCT is useful to assess the therapeutic effect of bisphosphonates in GIO. MDCT-based assessment of trabecular microarchitecture detected the preventive effects of bisphosphonates, even in the absence of statistically significant improvement of the bone turnover markers and the parameters in DEXA.
Intra-group analysis in each group showed similar results (Table 2). In the VD and VK groups, glucocorticoids significantly deteriorated BV/TV, while no significant changes were observed in the Bis group, suggesting that risedronate prevented glucocorticoid-induced deterioration of bone volume and trabecular architecture.
Discussion
In this study, we examined trabecular microarchitecture in IgAN patients who received high-dose glucocorticoid therapy. Non-invasive MDCT-based analyses detected the therapeutic effects of bisphosphonates in GIO, even in the absence of statistically significant improvements in bone turnover markers (Fig. 2), BMD Z score (Fig. 3a), and areal BMD (Fig. 3b).
3D microarchitecture of trabecular bone in postmenopausal women has been investigated under several conditions. Using bone biopsy samples, Recker et al. [27] and Borah et al. [31] reported that bisphosphonates protect 3D trabecular microarchitecture in postmenopausal women. Recker et al. [31] revealed that zoledronic acid improved BV/TV, Tb.N, Tb.Sp, and connectivity density. Borah et al. [27] demonstrated that another bisphosphonate, risedronate, improved structural indices, such as BV/TV, Tb.Th, marrow star volume, and SMI. Here, we showed that bisphosphonates improved BV/TV, Tb.Sp, marrow star volume, and SMI in glucocorticoid-treated patients (Figs. 5, 6, 7) without performing bone biopsy. Our results indicated that the assessment of trabecular microarchitecture is useful to detect the therapeutic effect of bisphosphonates not only in postmenopausal women but also in patients treated with glucocorticoids.
High bone remodeling rate has been reported to be associated with fracture risk in postmenopausal women. Both the Epidemiologie de l’Ostéoporose (EPIDOS) and Os des Femmes de Lyon (OFELY) studies showed that postmenopausal women with increased levels of bone resorption markers had high fracture risk independent of bone density and physical performance [32, 33]. Moreover, reduction in bone turnover has been revealed to be an independent predictor of therapeutic effects on fracture risk reduction in postmenopausal women [34–36]. Although previous prospective randomized controlled trials have shown that bisphosphonates, a potent inhibitor of bone resorption, prevent bone fracture in patients treated with glucocorticoids, the relationship between bone turnover markers and the fracture risk in GIO remains controversial [11–15]. Indeed, our result showed that bisphosphonates prevented structural decay of trabecular microarchitecture by glucocorticoids even in the absence of significant improvement in serum BAP and NTX (Fig. 2). The controversy on turnover markers in GIO may be derived from the regimens of glucocorticoid therapy. Kuroki et al. [37] demonstrated that the changes in serum NTX differed among the regimens. In contrast to a persistently increasing trend of serum NTX in low-dose glucocorticoid therapy, pulse therapy showed a transient increase of serum NTX [37]. These finding indicate that two factors, the regimen of glucocorticoid therapy and the timing of sampling, affect bone resorption markers. Thus, it seems difficult to accurately evaluate the therapeutic effects of bisphosphonates in GIO by bone turnover markers. The direct assessment of bone structure, i.e., MDCT-based assessment, is more suitable for GIO.
There are two major limitations to the current study. First, we did not set up a placebo-treated control group. Because the patients enrolled in our study were treated with high doses of glucocorticoids, it was not ethically allowed to set up a placebo group. Thus, we compared the difference among the three anti-osteoporotic agents. Second, we did not evaluate the actual bone fracture in our subjects. Thus, we could not conclude that the improvement of trabecular microarchitecture in glucocorticoid-treated patients really prevented bone fracture. In postmenopausal osteoporosis, Ito et al. [8] demonstrated that structural indices assessed by MDCT, such as low BV/TV, fewer Tb.N, higher SMI, and lower connectivity, were associated with fracture risk. Further study is required to determine whether MDCT-based assessment is predictive of bone fracture not only in postmenopausal women but also in patients treated with glucocorticoids.
Iliac bone biopsy samples have been used in the majority of previous clinical studies that have investigated structural indices of trabecular bone [28, 31]. In these studies, 3D microarchitecture has been assessed by μCT [28, 31]. Here, we employed MDCT to analyze the microarchitecture of lumbar spine. Because both μCT-based and MDCT-based assessments have their own intrinsic advantages and disadvantages, it is difficult to determine which is a superior analytical method. A disadvantage of MDCT-based assessment is radiation exposure. However, the amount of radiation in our MDCT examination did not exceed that in usual CT examinations. Average volume CT dose index (CTDIvol) and dose-length product (DLP) of our MDCT protocol were 72 and 417.78 mGy, respectively. Dosimetry metrics of normal thin-slice brain CT using the same CT machine are approximately 70 mGy in CTDIvol and 800–1000 mGy in DLP. In normal abdominal CT scans, CTDIvol and DLP are approximately 20 and 500 mGy, respectively. Moreover, we scanned only the third lumbar vertebra to avoid radiation exposure to organs that have a high effective dose/DLP (ED/DLP) ratio [38]. Although radiation exposure is a potential disadvantage, this point is also linked to two advantages of MDCT-based assessment. First, MDCT is a non-invasive method. MDCT does not require invasive bone biopsy, whereas μCT-based assessment cannot be carried out without bone biopsy. Second, MDCT can directly assess the osteoporotic status of clinically relevant sites, i.e., vertebrae. GIO often results in vertebral and/or femoral neck fracture but not iliac fracture. The potential limitation of μCT-based assessment is that the iliac crest is not a good representative of clinically relevant sites susceptible to glucocorticoids.
In conclusion, non-invasive MDCT-based analyses detected the therapeutic effects of bisphosphonates in GIO, even in the absence of any significant differences in conventional analytical modalities. Our results suggest that MDCT-based assessment of trabecular microarchitecture is useful to detect therapeutic effects of bisphosphonates in GIO.
References
Bertsias GK, Boumpas DT (2010) Pathogenesis, diagnosis and management of neuropsychiatric SLE manifestations. Nat Rev Rheumatol 6:358–367
Hotta O, Miyazaki M, Furuta T, Tomioka S, Chiba S, Horigome I, Abe K, Taguma Y (2001) Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am J Kidney Dis 38:736–743
Compston J (2010) Management of glucocorticoid-induced osteoporosis. Nat Rev Rheumatol 6:82–88
Brown JP, Josse RG (2002) Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ 167:S1–S34
Nakamura T (2007) Japanese guidelines for the prevention and treatment of osteoporosis (2006 edition) and its significance. Nippon Rinsho 65:29–34
Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton LJ III, Tenenhouse A, Reeve J, Silman AJ, Pols HA, Eisman JA, McCloskey EV, Mellstrom D (2004) A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res 19:893–899
Benhamou CL (2007) Effects of osteoporosis medications on bone quality. Joint Bone Spine 74:39–47
Ito M, Ikeda K, Nishiguchi M, Shindo H, Uetani M, Hosoi T, Orimo H (2005) Multi-detector row CT imaging of vertebral microstructure for evaluation of fracture risk. J Bone Miner Res 20:1828–1836
Ito M (2011) Recent progress in bone imaging for osteoporosis research. J Bone Miner Metab 29:131–140
Nawata H, Soen S, Takayanagi R, Tanaka I, Takaoka K, Fukunaga M, Matsumoto T, Suzuki Y, Tanaka H, Fujiwara S, Miki T, Sagawa A, Nishizawa Y, Seino Y (2005) Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research (2004). J Bone Miner Metab 23:105–109
Adachi JD, Bensen WG, Brown J, Hanley D, Hodsman A, Josse R, Kendler DL, Lentle B, Olszynski W, Ste-Marie LG, Tenenhouse A, Chines AA (1997) Intermittent etidronate therapy to prevent corticosteroid-induced osteoporosis. N Engl J Med 337:382–387
Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, Thamsborg G, Liberman UA, Delmas PD, Malice MP, Czachur M, Daifotis AG (1998) Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med 339:292–299
Cohen S, Levy RM, Keller M, Boling E, Emkey RD, Greenwald M, Zizic TM, Wallach S, Sewell KL, Lukert BP, Axelrod DW, Chines AA (1999) Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 42:2309–2318
Reid DM, Hughes RA, Laan RF, Sacco-Gibson NA, Wenderoth DH, Adami S, Eusebio RA, Devogelaer JP (2000) Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res 15:1006–1013
Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD et al (2001) Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum 44:202–211
de Nijs RN, Jacobs JW, Algra A, Lems WF, Bijlsma JW (2004) Prevention and treatment of glucocorticoid-induced osteoporosis with active vitamin D3 analogues: a review with meta-analysis of randomized controlled trials including organ transplantation studies. Osteoporos Int 15:589–602
Dalle Carbonare L, Bertoldo F, Valenti MT, Zordan S, Sella S, Fassina A, Turco G, Realdi G, Lo Cascio V, Giannini S (2007) Risedronate prevents the loss of microarchitecture in glucocorticoid-induced osteoporosis in rats. J Endocrinol Invest 30:739–746
Tomino Y, Sakai H (2003) Clinical guidelines for immunoglobulin A (IgA) nephropathy in Japan, second version. Clin Exp Nephrol 7:93–97
Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S (2010) Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis 56:32–38
Hildebrand T, Rügsegger P (1997) A new method for the model-independent assessment of thickness in three-dimensional images. J Microsci 185:67–75
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Vesterby A, Gundersen HJ, Melsen F (1989) Star volume of marrow space and trabeculae of the first lumbar vertebra: sampling efficiency and biological variation. Bone 10:7–13
Zienkiewicz OC, Taylor RL (1996) The finite element method, 4th edn, vol 1. McGraw-Hill Book Company (UK) Limited, New York
Stegaroiu R et al (2006) Peri-implant stress analysis in simulation model with or without trabecular bone stress. Int J Prosthdontics 19:40–42
Ulrich D, Riebergen B, Laib A, Ruegsegger P (1998) Mechanical analysis of bone and its microarchitecture based on invivo voxel images. Technol Health Care 6:421–427
Carter DR, Hayes WC (1977) The compressive behavior of bone as a two-phase porous structure. J Bone Joint Surg Am 59:954–962
Borah B, Dufresne TE, Chmielewski PA, Johnson TD, Chines A, Manhart MD (2004) Risedronate preserves bone architecture in postmenopausal women with osteoporosis as measured by three-dimensional microcomputed tomography. Bone 34:736–746
Canalis E, Bilezikian JP, Angeli A, Giustina A (2004) Perspectives on glucocorticoid-induced osteoporosis. Bone 34:593–598
Allen DB (1996) Growth suppression by glucocorticoid therapy. Endcrinol Metab Clin North Am 25:699–717
Baron J, Klein KO, Colli MJ, Yanovski JA, Novosad JA, Bacher JD, Cutler GB Jr (1994) Catch-up growth after glucocorticoid excess: a mechanism intrinsic to growth plate. Endocrinology 135:1367–1371
Recker RR, Delmas PD, Halse J, Reid IR, Boonen S, Garcia-Hernandez PA, Supronik J, Lewiecki EM, Ochoa L, Miller P, Hu H, Mesenbrink P, Hartl F, Gasser J, Eriksen EF (2008) Effects of intravenous zoledronic acid once yearly on bone remodeling and bone structure. J Bone Miner Res 23:6–16
Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD (2000) Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 15:1526–1536
Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, Cormier C, Breart G, Meunier PJ, Delmas PD (1996) Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res 11:1531–1538
Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD (2003) Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 18:1051–1056
Reginster JY, Adami S, Lakatos P, Greenwald M, Stepan JJ, Silverman SL, Christiansen C, Rowell L, Mairon N, Bonvoisin B, Drezner MK, Emkey R, Felsenberg D, Cooper C, Delmas PD, Miller PD (2006) Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis 65:654–661
Bauer DC, Garnero P, Hochberg MC, Santora A, Delmas P, Ewing SK, Black DM (2006) Pretreatment levels of bone turnover and the antifracture efficacy of alendronate: the fracture intervention trial. J Bone Miner Res 21:292–299
Kuroki Y, Kaji H, Kawano S, Kanda F, Takai Y, Kajikawa M, Sugimoto T (2008) Short-term effects of glucocorticoid therapy on biochemical markers of bone metabolism in Japanese patients: a prospective study. J Bone Miner Metab 26:271–278
Huda W, Mettler FA (2010) Volume CT dose index and dose-length product displayed during CT: what good are they? Radiology 258:436−442
Acknowledgments
This research was supported by a grant from The Kidney Foundation Japan (JKFB08-7), ROD-21 research foundation (to T Hamano), and Eisai Co., Ltd. (Tokyo, Japan).
Conflict of interest
This research was supported by a grant from Eisai Co., Ltd. (Tokyo, Japan), which sells risedronate in Japan. We have full control of all primary data. We agree to allow the journal to review our primary data if requested. This research was planned by T. Hamano, K. Tomida, S. Mikami, and N. Fujii. All data were analyzed by K. Inoue, N. Nango, K Tomida, and S. Mikami. This manuscript was prepared by K. Inoue, N. Nango, I. Matsui, and T. Hamano. All the procedures were supervised by H. Rakugi, Y. Isaka, and Y. Tsubakihara.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Inoue, K., Hamano, T., Nango, N. et al. Multidetector-row computed tomography is useful to evaluate the therapeutic effects of bisphosphonates in glucocorticoid-induced osteoporosis. J Bone Miner Metab 32, 271–280 (2014). https://doi.org/10.1007/s00774-013-0485-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-013-0485-2