Abstract
The purpose of the study was to investigate bone mineral density (BMD) in children with type 1 diabetes (DM1) and to establish the relationships between BMD, physical activity, glycemic control, and markers of systemic oxidative stress and inflammation. We studied 30 children with DM1, aged 4.7–18.6 years, and 30 healthy subjects, matched by sex, age, and body mass index (BMI). Mean duration of DM1 was 5.4 ± 3.4 years and mean glycosylated hemoglobin (HbA1c) level over 12 months was 9.8 ± 1.5%. Lumbar and total bone mineral density (BMD, g/cm2) were measured by dual-energy X-ray absorptiometry (DXA). We calculated the apparent volumetric lumbar BMD (BMDvol, g/cm3) and total mineral content adjusted for age and height (BMCadj), and measured plasma intercellular adhesion molecule-1 (ICAM-1), high sensitivity C-reactive protein (hs-CRP), and urinary 8-iso-prostaglandin F2a (F2-IsoPs). Calcium (Ca) intake was assessed by questionnaire and physical activity by questionnaire and accelerometer (ActiGraph, count/h). Total BMCadj and lumbar BMDvol were significantly lower in children with DM1 than in controls (101.8 ± 7.7 vs. 107 ± 5.7%, P = 0.005; 0.32 ± 0.08 vs. 0.36 ± 0.09 g/cm3, P = 0.05, respectively). These differences were mostly caused by the differences in boys. Plasma ICAM-1 and hs-CRP levels were significantly higher in the DM1 group compared to the controls. Ca intake and urine F2-IsoPs levels were similar between the groups. Diabetic boys were less active than controls (18231 ± 6613 vs. 24145 ± 7449 count/h, P = 0.04). In the DM1 group, lumbar BMDvol correlated inversely with urinary F2-IsoPs (r = −0.5; P = 0.005) and plasma ICAM-1 levels (r = −0.4; P = 0.02), and also with HbA1c levels after adjustment for age (r = −0.45; P < 0.05). Total BMCadj correlated inversely with HbA1c levels (r = −0.4; P = 0.02). We conclude that children with DM1, particularly boys, have lower BMD. Poor glycemic control, elevated markers of oxidative stress, and inflammation are associated with lower BMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with type 1 diabetes (DM1) are at risk of decreased bone mineral density (BMD) [1]. However, the potential effects of DM1 on bone metabolism in children remain a quite controversial issue. Both normal and decreased BMD have been reported in children with DM1 [2–9]. Moreover, recently it has been shown that patients who manifest DM1 at an early age may have only transiently impaired bone development [10].
The mechanisms behind impaired bone metabolism in DM1 are not clear. A recent meta-analysis by Vestergaard from 2007 [1] showed that the increase in fracture risk was higher and BMD lower in patients who showed vascular complications of diabetes. It has been suggested that lower BMD is linked to poor metabolic control [6] and low insulin-like growth factor 1 levels [3]. Patients with DM1 have elevated oxidative stress [11] and inflammatory markers [12]. It has been recently shown, in the Diabetes Control and Complications Trial, that higher plasma intercellular adhesion molecule-1 (ICAM-1) levels predict an increased risk of progressive nephropathy in DM1 and may represent an early risk marker that reflects the important role of vascular endothelial dysfunction in this long-term complication [13]. The possible links between DM1, BMD, oxidative stress, and inflammation in children remain to be elucidated. Recently we established that children with DM1 are characterized by increased myeloperoxidase activity (a marker of inflammation), arterial stiffness, and carotid intima-media thickness [14]. Thus, it seems reasonable to investigate inflammation-, oxidative stress-, and blood vessel wall-related aspects concerning BMD in children with DM1 using the corresponding markers [high sensitivity C-reactive protein (hs-CRP), urinary 8-iso-prostaglandin F2a (F2-IsoPs), ICAM-1]. Therefore, the purpose of the study was to investigate BMD in children with DM1 and to investigate the relationships between BMD, physical activity, the glycemic control of diabetes, and markers of oxidative stress and inflammation.
Materials and methods
Patients and controls
This cross-sectional study included 30 Caucasian children with DM1 [mean age, 13.1 ± 3.6 years (range 4.7–18.6 years); 19 boys, 11 girls] attending the regional Diabetes Clinic and 30 healthy Caucasian control subjects, matched by age (±2 years), sex, and body mass index (BMI) (±3 kg/m2). Patients with DMI were divided by Tanner pubertal stage as follows: 6 boys and 1 girl in stage 1, 4 boys and 4 girls in stages 2 or 3, and 9 boys and 6 girls in stages 4 or 5. Control subjects divided by pubertal stage comprised 7 boys and 1 girl in stage 1, 3 boys and 3 girls in stage 2 or 3, and 9 boys and 7 girls in stage 4 or 5. All patients with diabetes had no clinical evidence of vascular complications. The mean duration of diabetes was 5.4 ± 3.4 years (range 1.0–14.6 years). The patients were treated with a multiple insulin injection regimen (n = 21) or two daily injections of a mixture of short- and long-acting insulin (n = 9). The mean daily insulin dose was 0.9 ± 0.2 units/kg per day. Inclusion criteria for both children with diabetes and control subjects were age between 3 and 20 years and lack of acute infection. Children with diabetes were included only if at least 1 year had passed since the diagnosis. The diagnosis of DM1 was based on the American Diabetes Association criteria. Two children with diabetes also had celiac disease. Informed consent was obtained from each subject and/or their parents. The local ethical committee approved the protocol.
Methods
Bone mineral density (BMD)
Total body and lumbar (L1–L4) bone mineral content (BMC) and bone area (BA) were measured by dual-energy X-ray absorptiometry (DXA) (GE Lunar DPX-IQ; USA). BMD was calculated by BMC/BA. The BMD data obtained from DXA are expressed as grams per centimeter squared, not as true volumetric density, and therefore are influenced greatly by bone size. To minimize the effect of bone size on BMD values, total bone mineral content (BMC, g) was adjusted for age and height (BMCadj, %) by Warner et al. [15], and we calculated the apparent volumetric lumbar BMD (BMDvol, g/cm3) using the Kröger formula [16].
Physical activity
Accelerometer
Physical activity was measured by a uniaxial accelerometer (ActiGraph, USA). Subjects were asked to wear the accelerometer through the day (not during the night) on 3 consecutive days. Each 3-day period included 2 days in school and 1 day of a weekend. Data were analyzed by a specific software program provided by the manufacturer (ActiSoft Analysis Software version 3.2.; USA). Mean activity count per hour over the 3-day period was used in the analysis.
Questionnaire
Children over 12 years of age and parents of those under 12 years completed a validated modified questionnaire [17] about the child’s exercise level approximately 1 week before their clinic appointment. Subjects were asked to indicate how many times they participate in different forms of exercise for more than 15 min during their free time in an average week. Exercise was classified into three categories: strenuous exercise (heart beats rapidly), moderate (not exhausting), and mild exercise (minimal effort). A total score was generated (exercise in an average week) by summing the reported frequencies for each of the three categories after multiplying each category with the corresponding metabolic equivalents (MET) score (9, 5, or 3, respectively).
Calcium (Ca) intake
A questionnaire was used to assess daily milk (ml) and weekly dairy product consumption (milk, yogurt, cheese, and ice cream). Patients were divided subsequently into three groups: low, none or only one dairy product once (or less) per week; moderate, at least two dairy products two to three times a week or daily milk consumption of 125–250 ml; and high, two dairy products six to seven times a week or four to five times a week and daily milk consumption >250 ml, or daily milk consumption >500 ml.
Clinical and laboratory investigations
Blood and urine samples were taken after overnight fasting between 8:00 and 9:00 AM; for subjects with diabetes, samples were taken before insulin administration. The urinary content of 8-iso-prostaglandin F2a (F2-IsoPs) was analyzed by a competitive enzyme-linked immunosorbent assay (ELISA) (BIOXYTECH 8-Isoprostane Assay; OxisResearch, USA). Serum intercellular adhesion molecule-1 (ICAM-1) was assessed using human soluble ICAM-1 CD54 immunoassay (R&D Systems Europe, UK), and high sensitivity C-reactive protein (hs-CRP) was determined in serum using a chemiluminescence enzyme immunometric assay on an Immulite immunoassay analyzer (DPC, USA), according to the manufacturer’s instructions. The urinary concentrations of F2-IsoPs were corrected by urinary creatinine concentrations to account for the differences in renal function. Subjects above the upper limit of the normal range of hs-CRP (>5 mg/l) were excluded in the comparison of hs-CRP between diabetes and control groups (n = 3 in the diabetes group and n = 1 in the control group), as having apparently some infection or inflammatory disorder. The glycosylated hemoglobin (HbA1c) was measured by latex immunoagglutination inhibition using a DCA 2000+ Analyzer (Bayer Diagnostics Europe, Ireland).
Height was measured on a wall-mounted stadiometer and weight with a digital scale. Pubertal stage was assessed according to Tanner. Daily insulin doses and mean HbA1c during the 12 months before the study were obtained from the registry of the Outpatient Diabetes Clinic.
Statistical analysis
All data were tested for normality using the Kolmogorov–Smirnov test. Continuous data are presented as mean values with standard deviation (SD). Comparisons between the groups were performed using the Student’s two-tailed t test. Bivariate correlations were analyzed by the Pearson correlation test. In addition, partial correlation analysis adjusted for age and/or gender was used to analyze the relationships between HbA1c and lumbar BMDvol or total BMCadj. Stepwise multiple linear regression analysis was used to determine the best set of predictors of BMD using age, pubertal stage, gender, physical activity, and biochemical markers as possible determinant factors. All P values were two-sided, and differences were considered statistically significant at P < 0.05. All statistical calculations were performed using the software R, version 2.4.1 for Windows.
Results
The clinical characteristics of the study groups are shown in Table 1. The two groups did not differ in age, gender, BMI, height, weight, and pubertal stage. Bone and physical activity characteristics are shown in Table 2.
Bone measurements
BMC, BA, and areal BMD of total body and lumbar region were not different between the diabetes group and the control group. The mean lumbar BMDvol and total BMCadj were significantly lower in the diabetes group than in their sex- and age-matched controls (Table 2). Differences remained significant even after the two children with celiac disease were excluded from the analysis. The differences in these two parameters were mostly the results of the differences in boys in the two groups (total BMCadj 99.7 ± 8.0 vs. 105.8 ± 6.2, P = 0.01; lumbar BMDvol 0.29 ± 0.06 vs. 0.33 ± 0.07, P = 0.07), whereas in girls total BMCadj and lumbar BMDvol did not differ significantly. According to stepwise regression analysis in which all 60 subjects were included, the most significant determinants of total BMCadj were the presence of diabetes and male gender (both negatively) (R 2 adj = 0.19; P = 0.001), whereas the most important determinants of lumbar BMDvol were age (positively), male gender (negatively), and the presence of diabetes (negatively) (R 2 adj = 0.58; P < 0.0001), but not physical activity. There was no significant difference in BMD characteristics in either group regarding their calcium intake.
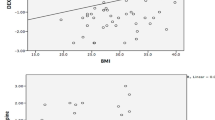
There was a negative correlation between total BMCadj and HbA1c level (r = −0.4; P = 0.02) (Fig. 1). The correlation remained significant after adjustment for gender in partial correlation analysis. Children with a HbA1c level > 10.6% had statistically significantly lower total BMCadj than those with HbA1c levels below this cutoff value (103.28 ± 7.6 vs. 96.75 ± 6.3; P = 0.05). Lumbar BMDvol is highly age dependent and therefore must be adjusted for age. Using partial correlation analysis, lumbar BMDvol was significantly correlated with HbA1c when adjusted for age (r = −0.45; P < 0.05), as well as when adjusted for age and gender (r = −0.39; P < 0.05). In multivariate regression analysis, HbA1c, age, and gender were the most important determinants of lumbar BMDvol in the diabetes group, explaining 58% of its variability (R 2 adj = 0.58; P < 0.0001).
Physical activity
Weekly activity score and physical activity measured by accelerometer were not different between the diabetes and the control group. However, boys with diabetes were less active than the control boys measured by accelerometer (18231 ± 6613 vs. 24145 ± 7449 count/h, P = 0.04) (Table 2). We did not find any correlations between physical activity and BMD parameters.
Laboratory data
Plasma ICAM-1 and hs-CRP level were significantly higher in the DM1 group compared to the control group (see Table 1). Urinary F2-IsoPs levels were not statistically different between the groups (Table 1). In the diabetes group, lumbar BMDvol, but not total BMCadj, was negatively correlated with plasma ICAM-1 levels (r = −0.4; P = 0.02) (Fig. 2) and urinary F2-IsoPs levels (r = −0.5; P = 0.005) (Fig. 3). We did not find any correlations between hs-CRP and BMD parameters. We also did not find any significant correlation between BMD parameters and physical activity, ICAM-1, F2-IsoPs, HbA1, or hs-CRP levels, if examined separately in boys or girls with diabetes.
Discussion
The most important finding of this study is that lower BMD characteristics in children with type 1 diabetes are linked to poor glycemic control and higher serum ICAM-1 and urinary F2-IsoPs levels.
Patients with DM1 have increased risk of osteopenia and fractures [1]. Bone loss can begin in childhood [2–6], but there are reports of children with DM1 who do not exhibit bone loss [7–9]. BMD and BMC have been measured in children using different methods. Especially in children, the DXA method has limitations because of the two-dimensional measurement and therefore its dependence on height. To minimize the effect of bone size on BMD values, we adjusted total BMC for age and height and calculated the apparent volumetric lumbar BMD, which corrects the areal BMD value with the width of the vertebra. The total BMCadj and lumbar BMDvol were significantly lower in children with DM1 than in controls. Some data indicate that a decrease in osteoblast number and/or osteoblast differentiation contributes to reduced bone formation [18].
We found that male gender was associated with lower BMD in children with DM1, similar to the study by Heap et al. [6]. In children with diabetes, a larger bone mass deficit has been described in girls compared to boys [3], whereas some other studies did not find any sex-specific effect on BMD [7, 8]. Our patients and controls were quite well matched by pubertal stage, but there were more prepubertal boys than girls. Puberty is known to be an important time interval in terms of peak bone mass gain, a time in which bone formation occurs at a higher rate compared to that at other stages of life [19]. Although the mean age of boys at pubertal stages 2 or 3 in our study did not differ between the patients (n = 4) and the controls (n = 3), delayed onset of puberty can be still one reason why the boys with diabetes had lower BMD. More than a decade ago in a much bigger study, Estonian boys with DM1 had a tendency to have delayed pubertal development with slightly reduced testicular volume in relationship to age [20]. A recent study by Rohrer et al. [21] in a large German cohort of more than 2400 pediatric DM1 patients found that male, and not female, gender was a significant determinant of the delayed pubertal onset. The same study found that elevated HbA1c level was associated with significantly delayed pubertal onset. In our study, male patients had a slightly higher mean HbA1c value than females (10.1 vs. 9.5%), but the difference was not statistically significant. However, our girls with diabetes had also lower BMD parameters compared to healthy girls, but these differences were statistically not significant. The small sample size (n = 11) may be also one reason why we were not able to show significant differences between the girls in regard to BMD characteristics.
Our study group had relatively poorly controlled diabetes, with large variations in mean HbA1c levels. The children with higher HbA1c levels had lower total BMCadj. HbA1c levels above 9.8% have been found to be a risk factor for osteopenia, defined as BMD Z-scores between −l and −2.5, and HbA1c above 12.1% for osteoporosis [22]. We did not use the Z-score because it does not take into account the size of the bones; i.e., in shorter children a negative BMD Z-score is actually not “as negative” as it should be in children with average height. However, relatively high Hba1c values indicate that our subjects with DM1 are very likely to be osteopenic. On the other hand, many studies have found no correlation between BMD and HbA1c [1, 3, 4]. Therefore, there should be other parameters in addition to poor glycemic control that might have an impact on BMD.
Physical activity is an important determinant of BMD. Children with many chronic diseases, such as acute lymphoblastic leukemia (ALL) [23], have been shown to be less active than healthy children. Therefore, we also studied physical activity using two methods. Physical activity assessed by questionnaire did not show any difference between diabetes patients and healthy children. We found that boys with diabetes had lower BMD and were less physically active compared to healthy boys, when activity was measured with a more direct method, i.e., with the accelerometer. However we were unable to detect a significant correlation between physical activity and BMD parameters. In multiple regression analysis, the presence of diabetes and male gender, but not low physical activity, were the most important determinants of BMD, indicating that low physical activity has an influence on bone density, but not as much as the disease itself. We did not find any significant correlation between BMD and physical activity, HbA1c, or markers of oxidative stress and inflammation, if examined separately in boys or girls. The number of patients in each gender was probably too small to establish statistically significant correlations in each group separately.
Hyperglycemia in diabetes is closely associated with glucose autooxidation and protein glycation; this increases the production of free radicals and oxidative stress [11]. We measured F2-IsoPs as a marker of systemic oxidative stress. F2-IsoPs are formed by free radical-catalyzed peroxidation of phospholipid-bound arachidonic acid and can be reliably measured in both plasma and urine [24, 25]. F2-IsoPs can nowadays be considered as one of the most reliable markers of oxidative stress [26]. Recent studies have shown that the formation and urinary excretion of F2-IsoPs is elevated in children with DM1 [27, 28]. We found slightly elevated F2-IsoPs levels in children with DM1 compared to controls, but this difference was not statistically significant. A higher urine F2-IsoPs level in children with DM1 correlated inversely with lumbar BMDvol. In vitro studies have shown that oxidative stress inhibits osteoblastic differentiation [29, 30] and induces osteoblast insults and apoptosis [31, 32]. Hamada et al. [33] demonstrated that streptozotocin-induced type 1 diabetic mice exhibited low-turnover osteopenia associated with increased oxidative stress. A link between increased level of urinary F2-IsoPs and reduced lumbar spine BMD has also been shown in population-based human studies [34]. According to our knowledge, this is the first study describing the correlation between the markers of oxidative stress and BMD in children with DM1.
We found higher ICAM-1 and hs-CRP levels in children with diabetes. With type 1 diabetes, hyperglycemia is known to modulate expression of cell adhesion molecules and cytokines, which through monocyte–endothelium interaction leads to the initiation and progression of vascular complications [12, 13]. We found that serum ICAM-1 concentration in children with DM1 was inversely correlated to lumbar BMDvol, which represents more trabecular bone, and not to total BMCadj, which represents more cortical bone. Trabecular bone, where bone turnover is higher than in cortical bone, is therefore potentially more susceptible to damage by factors such as inflammation and oxidative stress. Cellular interactions through adhesion molecules and their impact together with cytokines represent an important concept in bone metabolism, particularly in inflammatory diseases. It has been suggested that elevated cytokine levels can activate osteoclast bone resorption and suppress osteoblast differentiation and bone formation [35]. However, the exact mechanism of how ICAM-1 affects bone formation is not entirely understood. The fact that ICAM-1 and F2-IsoPs in our study were correlated to lumbar BMDvol and not to total BMCadj suggests that lower lumbar spine BMD in patients with DM1 is associated with increased oxidative stress and inflammation.
The present study found that children with DM1, particularly boys, have lower BMD. Poor glycemic control, elevated markers of oxidative stress, and inflammation were associated with lower BMD. These data indicate that children with diabetes who have higher risk for vascular damage also have a higher risk for osteopenia.
References
Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes: a meta-analysis. Osteoporos Int 18:427–444
Gunczler P, Lanes R, Paoli M, Martinis R, Villaroel O, Weisinger JR (2001) Decreased bone mineral density and bone formation markers shortly after diagnosis of clinical type 1 diabetes mellitus. J Pediatr Endocrinol Metab 14:525–528
Léger J, Marinovic D, Alberti C, Dorgeret S, Chevenne D, Marchal CL, Tubiana-Rufi N, Sebag G, Czernichow P (2006) Lower bone mineral content in children with type 1 diabetes mellitus is linked to female sex, low insulin-like growth factor type I levels, and high insulin requirement. J Clin Endocrinol Metab 91:3947–3953
Salvatoni A, Mancassola G, Biasoli R, Cardani R, Salvatore S, Broggini M, Nespoli L (2004) Bone mineral density in diabetic children and adolescents: a follow-up study. Bone (NY) 34:900–904
Moyer-Mileur L, Dixon S, Quick J, Askew E, Murray M (2004) Bone mineral acquisition in adolescents with type 1 diabetes. J Pediatr 145:662–669
Heap J, Murray MA, Miller SC, Jalili T, Moyer-Mileur LJ (2004) Alterations in bone characteristics associated with glycemic control in adolescents with type 1 diabetes mellitus. J Pediatr 144:56–62
Pascual J, Argente J, Lopez MB, Muñoz MB, Martinez G, Vazquez MA, Jodar E, Perez-Cano R, Hawkins F (1998) Bone mineral density in children and adolescents with diabetes mellitus type 1 of recent onset. Calcif Tissue Int 62:31–35
De Schepper J, Smitz J, Rosseneu S, Bollen P, Louis O (1998) Lumbar spine bone mineral density in diabetic children with recent onset. Horm Res 50:193–196
Liu EY, Wactawski-Wende J, Donahue RP, Dmochowski J, Hovey KM, Quattrin T (2003) Does low bone mineral density start in post-teenage years in women with type 1 diabetes? Diabetes Care 26:2365–2369
Bechtold S, Putzker S, Bonfig W, Fuchs O, Dirlenbach I, Schwarz HP (2007) Bone size normalizes with age in children and adolescents with type 1 diabetes. Diabetes Care 30:2046–2050
Brownlee M (2000) Negative consequences of glycation. Metabolism 49:9–13
Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP (2001) Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol 158:147–152
Lin J, Glynn RJ, Rifai N, Manson JE, Ridger PM, Nathan DM, Schaumberg DA (2008) Inflammation and progressive nephropathy in type 1 diabetes mellitus in the diabetes control and complications trial (DCCT). Diabetes Care 31:2338–2348
Heilman K, Zilmer M, Zilmer K, Lintrop M, Kampus P, Kals J, Tillmann V (2009) Arterial stiffness, carotid artery intima-media thickness and plasma myeloperoxidase level in children with type 1 diabetes. Diabetes Res Clin Pract (in press)
Warner JT, Cowan FJ, Dunstan FD, Evans WD, Webb DK, Gregory JW (1998) Measured and predicted bone mineral content in healthy boys and girls aged 6–18 years: adjustment for body size and puberty. Acta Paediatr 87:244–249
Kröger H, Vainio P, Nieminen J, Kotaniemi A (1995) Comparison of different models for interpreting bone mineral density measurements using DEXA and MRI technology. Bone 17:157–159
Godin G, Shepard RJ (1985) Simple methods to assess exercise behaviour in the community. Can J Appl Sport Sci 10:141–146
Krakauer J, McKenna M, Burderer N, Rao D, Whitehouse F, Pafitt A (1995) Bone loss and bone turnover in diabetes. Diabetes 44:775–782
Matkovic V, Jelic T, Wardlaw GM, Ilich JZ, Goel PK, Wright JK, Andon MB, Smith KT, Heaney RP (1994) Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J Clin Invest 93:799–808
Tillmann V, Adojaan B, Shor R, Price DA, Tuvemo T (1996) Physical development in Estonian children with type 1 diabetes. Diabet Med 13:97–101
Rohrer T, Stierkorb E, Heger S, Karges B, Raile K, Schwab KO, Holl RW, Diabetes-Patienten-Verlaufsdaten (DPV) Initiative (2007) Delayed pubertal onset and development in German children and adolescents with type 1 diabetes: cross-sectional analysis of recent data from the DPV diabetes documentation and quality management system. Eur J Endocrinol 157:647–653
Camurdan MO, Ciaz P, Bidezi A, Demirel F (2007) Role of hemoglobin A(1c), duration and puberty on bone mineral density in diabetic children. Pediatr Int 49:645–651
Tillmann V, Darlington ASE, Eiser C, Bishop NJ, Davies HA (2002) Male sex and low physical activity are associated with reduced spine bone mineral density in survivors of childhood acute lymphoblastic leukemia. J Bone Miner Res 17:1073–1080
Awad JA, Morrow JD, Takahashi K, Roberts LJ (1993) Identification of non-cyclooxygenase-derived prostanoid (F2-isoprostane) metabolites in human urine and plasma. J Biol Chem 268:4161–4169
Wang Z, Ciabattoni G, Créminon C, Lawson JA, FitzGerald GA, Patrono C, Maclouf J (1995) Immunological characterization of urinary 8-epi-PGF2 excretion in man. J Pharmacol Exp Ther 275:94–100
Morrow JD, Roberts LJ (1996) The isoprostanes: current knowledge and directions for future research. Biochem Pharmacol 51:1–9
Flores L, Rodela S, Abian J, Clària J, Esmatjes E (2004) F2 isoprostane is already increased at the onset of type 1 diabetes mellitus: effect of glycemic control. Metabolism 53:1118–1120
Davì G, Chiarelli F, Santilli F, Pomilio M, Vigneri S, Falco A, Basili S, Ciabattoni C, Patrono C (2003) Enhanced lipid peroxidation and platelet activation in the early phase of type 1 diabetes mellitus. Role of interleukin-6 and disease duration. Circulation 107:3199–3203
Mody N, Parhami F, Sarafian TA, Demer LL (2001) Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med 31:509–519
Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, Luo SQ (2004) Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun 314:197–207
Chen RM, Wu GJ, Chang HC, Chen JT, Chen TF, Lin YL, Chen TL (2005) 2, 6-Diisopropylphenol protects osteoblasts from oxidative stress-induced apoptosis through suppression of caspase-3 activation. Ann N Y Acad Sci 1042:448–459
Fatokun AA, Stone TW, Smith RA (2007) Hydrogen peroxide mediates damage by xanthine and xanthine oxidase in cerebellar granule neuronal cultures. Neurosci Lett 416:34–38
Hamada Y, Kitazawa S, Kitazawa R, Fujii H, Kasuga M, Fukagawa M (2007) Histomorphometric analysis of diabetic osteopenia in streptozotocin-induced diabetic mice: a possible role of oxidative stress. Bone (NY) 40:1408–1414
Basu S, Michaëlsson K, Olofsson H, Johansson S, Melhus H (2001) Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun 288:275–279
McCabe LR (2007) Understanding the pathology and mechanisms of type 1 diabetic bone loss. J Cell Biochem 102:1343–1357
Acknowledgments
This study was funded by Estonian Science Foundation Grant Nr. 6588, the targeted financed grants SF0182695s05 and SF0180105s08 from the Ministry of Science and Education. Support was also given by the European Union through the European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Heilman, K., Zilmer, M., Zilmer, K. et al. Lower bone mineral density in children with type 1 diabetes is associated with poor glycemic control and higher serum ICAM-1 and urinary isoprostane levels. J Bone Miner Metab 27, 598–604 (2009). https://doi.org/10.1007/s00774-009-0076-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-009-0076-4