Abstract
To clarify the significance of the osteophytes that appear during the progression of osteoarthritis (OA), we investigated the expression of inflammatory cytokines and proteases in osteoblasts from osteophytes. We also examined the influence of mechanical stress loading on osteoblasts on the expression of inflammatory cytokines and proteases. Osteoblasts were isolated from osteophytes in 19 patients diagnosed with knee OA and from subchondral bone in 4 patients diagnosed with femoral neck fracture. Messenger RNA expression and protein production of inflammatory cytokines and proteases were analyzed using real-time RT-PCR and ELISA, respectively. To examine the effects of mechanical loading, continuous hydrostatic pressure was applied to the osteoblasts. We determined the mRNA expression and protein production of IL-6, IL-8, and MMP-13, which are involved in the progression of OA, were increased in the osteophytes. Additionally, when OA pathological conditions were simulated by applying a nonphysiological mechanical stress load, the gene expression of IL-6 and IL-8 increased. Our results suggested that nonphysiological mechanical stress may induce the expression of biological factors in the osteophytes and is involved in OA progression. By controlling the expression of these genes in the osteophytes, the progression of cartilage degeneration in OA may be reduced, suggesting a new treatment strategy for OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In osteoarthritis (OA), the most important pathological changes are degeneration and destruction of articular cartilage. However, periarticular osseous changes, such as osteophyte formation and subchondral bone sclerosis, are also observed in OA. The significance of osseous changes of osteophyte and subchondral bone in OA remains controversial, but it seems that they are involved in the progression of articular cartilage degeneration [1]. An osteophyte is an outgrowth of the osseous tissue, the circumference of which is covered with cartilage. Osteophyte formation varies depending on the sites and causes: traction spurs formed at the attachment site of the ligament and tendon to the bone; inflammatory spurs formed in the vertebral body; or chondro-osteophytes protruding into the joint through the articular cartilage with bone covered with periosteum [2]. As OA progresses, chondro-osteophytes are formed [3].
OA may have multiple origins, and current evidence suggests that both mechanical and biological factors play important roles in its progression. Among the biological factors, proinflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), induce the expression of proteases and inhibit the formation of extracellular matrix [4–6]. Interleukin-8 (IL-8) is also known to be a proinflammatory cytokine and can induce cartilage degeneration [7]. Matrix metalloproteinase-13 (MMP-13) and ADAMTS-5 are the main catabolic enzymes for cartilage-specific collagen type II and aggrecan, respectively, and have been reported to play crucial roles in the initiation and progression of cartilage degeneration [8, 9]. Both articular cartilage and synovial tissue secrete inflammatory cytokines and proteases, and the expression patterns of these factors in these tissues have been investigated. However, the expression of these factors in osteophytes adjacent to articular cartilage has not been investigated in sufficient detail. If these factors are produced in osteophytes, they are likely to affect the degeneration of adjacent articular cartilage.
In addition to inflammatory cytokines and proteases, nonphysiological mechanical stress is an important factor in promoting cartilage degeneration in OA [10]. Many investigators have reported the influence of mechanical stress loading on osteoblasts on changes in the expression of genes specific to osseous tissue, such as collagen type I, alkaline phosphatase, and extracellular bone matrix proteins [11]. However, few have studied the influence of mechanical stress loading on osseous tissue on the expression of genes possibly involved in the progression of OA.
In this study, we compared the expression of inflammatory cytokines and proteases in osteoblasts isolated from osseous tissue inferior to the cartilage of osteophytes and in osteoblasts isolated from subchondral bone without OA. To clarify the significance of osteophytes that appear in the progression of OA, we also examined the influence of mechanical stress loading on the expression of inflammatory cytokines and proteases.
Materials and methods
This study was approved by the local Ethics Committee of Kyoto Prefectural University of Medicine, and written informed consent was obtained from all patients.
Clinical specimens
Osteophytes were obtained from 19 patients (8 men and 11 women) aged 55.4–83.1 years (mean, 74.3 ± 7.4 years) who were diagnosed with knee OA based on American College of Rheumatology (ACR) criteria and who underwent total knee arthroplasty (TKA) (Fig. 1A-a). The cartilage areas were classified using the International Cartilage Repair Society Grade (ICRS Grade) as described previously [12]. Grading was performed independently by two surgeons in a blinded manner. Subchondral bones were obtained from the femoral head of four patients (all women) aged 72.6–80.9 years (mean, 77.5 ± 3.7 years) who were diagnosed as having femoral neck fracture based on osteoporosis that was treated with femoral head prosthetic replacement (FHP) (Fig. 1A-c). All femoral heads were evaluated as ICRS Grade 0 (without cartilage degeneration). OA subchondral bones were also collected from lateral femoral condyles evaluated as ICRS Grade 1 (only a superficial lesion) (Fig. 1A-b). Patients who received steroids, bisphosphonate, or chondromodulators that influence bone metabolism, were excluded from the study. Posteroanterior upright radiographs of straight knees in the OA patients were classified according to the Kellgren and Lawrence system (K.L.) [13] and were divided into two groups according to femorotibial angle (FTA): Large FTA group with FTA ≥ 186° and Small FTA group with FTA < 186°.
A Radiographs of the knee diagnosed with osteoarthritis and of the hip diagnosed with femoral neck fracture. Close-up views of the osteophyte (a), the knee lateral condyle (b), and the femoral head (c). Open squares show the areas from which bone tissue was collected. B Diagram of a special continuous hydrostatic pressure (CHP) apparatus (B). This apparatus was devised to apply arbitrary CHP to cells. Hydrostatic pressure was transmitted through the gas-free culture medium, and the temperature was controlled using a thermostat
Preparation of cells
The osteoblasts were isolated as described in a previous study [14]. Briefly, the bone tissues were dissected into fragments (3–5 mm in diameter) and washed thoroughly with phosphate-buffered saline (PBS). The dissected bone fragments were placed on the bottom of a 100-mm dish such that the fragments were separated. The bone fragments were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 4.5 g/l glucose, 0.584 g/l l-glutamine, and 0.11 g/l sodium pyruvate (Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (FBS) (Trace Scientific, Melbourne, Australia), 100 units/ml penicillin, and 100 µg/ml streptomycin (Gibco-BRL, Grand Island, NY, USA) at 37°C in 5% CO2/95% humidified air (standard conditions). Upon confluence, the cells were treated with trypsin/ethylenediaminetetraacetic acid (EDTA) (Nacalai Tesque). The bone fragments were removed by filtration of the digest through a 20-μm pore membrane, and the following experiments were conducted on the isolated cells.
Alkaline phosphatase staining and Oil Red O staining
Osteoblasts isolated from the osteophytes were seeded onto collagen type I-coated 8-well chamber slides (Asahi Techno Glass, Tokyo, Japan). To evaluate the osteoblastic phenotype, alkaline phosphatase (ALP) staining was performed as described previously [15]. Briefly, the cells were washed twice with PBS and fixed with 3.7% formalin. The cells were transferred into the stain solution [0.1 mg/ml naphthol AS-MX phosphate (Sigma-Aldrich, St. Louis, MO, USA), 0.6 mg/ml Fast-blue BB salt (WAKO, Osaka, Japan), 5 μl/ml N,N-dimethylformamide, 2 mM MgCl2, and 0.1 M Tris–HCl] and incubated under standard conditions for 30 min. After cultivation, the cells were washed once with PBS and observed under differential interference contrast microscopy (Olympus, Tokyo, Japan). To evaluate the adipogenic phenotype, Oil Red O staining was performed as described previously [16]. Briefly, the cells were washed twice with PBS and fixed with 10% formalin. The cells were transferred into the stain solution consisting of 0.1% Oil Red O (Sigma-Aldrich) in 60% isopropanol and incubated under standard conditions for 20 min. After cultivation, the cells were rinsed briefly in 60% isopropanol, washed thoroughly in distilled water, and observed by differential interference contrast microscopy (Olympus). Adipose tissue-derived progenitor cells were isolated from the infrapatellar fat pad in the knees of OA patients. The isolated cells were cultured in adipogenic induction medium as described previously [16, 17]; these cells were used as a positive control for Oil Red O staining (data not shown).
RNA isolation and cDNA synthesis
Total RNA was extracted from the isolated osteoblasts following 24-h cultivation in monolayer at a density of 5 × 104 cells/cm2 in DMEM using ISOGEN (Nippon Gene, Tokyo, Japan). Reverse transcription of RNA was performed using a Gene Amp PCR System 9700 (Applied Biosystems, Foster City, CA, USA). First-strand cDNA was synthesized using an RNA PCR kit (Applied Biosystems) with random hexamers to prime cDNA synthesis as described previously [18].
RT-PCR
Reverse transcription-polymerase chain reaction (RT-PCR) was performed as described previously [19]. Specific primer sets for collagen type I, collagen type II, ALP, osteocalcin (OCN), aP2, PPARγ2, and GAPDH were designed based on the published sequence data [16, 20, 21] as follows: collagen type I, forward primer 5′-GGACACAATGGATTGCAAGG-3′ and reverse primer 5′-TAACCACTGCTCCACTCTGG-3′; collagen type II, forward primer 5′-CCGAGGCAACGATGGTCAGC-3′ and reverse primer 5′-TGGGGCCTTGTTCACCTTTGA-3′; ALP, forward primer 5′-GGGGGTGGCCGGAAATACAT-3′ and reverse primer 5′-GGGGGCCAGACCAAAGATAG-3′; OCN, forward primer 5′-ATGAGAGCCCTCACACTCCTC-3′ and reverse primer 5′-GCCGTAGAAGCGCCGATAGGC-3′; aP2, forward primer 5′-TAGAAAGAAGTAGGAGTGGGC-3′ and reverse primer 5′-CCACCACCAGTTTATCATCCTC-3′; PPARγ2, forward primer 5′-AGGAGCAGAGCAAAGAGGTG-3′ and reverse primer 5′-AGGACTCAGGGTGGTTCAGC-3′; GAPDH, forward primer 5′-CTTTTAACTCTGGTAAAGTGG-3′ and reverse primer 5′-TTTTGGCTCCCCCCTGCAAAT-3′. PCR amplification was performed under the conditions described in previous studies [16, 20, 21]. HCS-2/8 cells and MG 63 cells were used as positive controls for chondrocyte- and osteoblast-specific genes, respectively; 293 cells were used as a negative control for osteoblast-specific genes. Adipose tissue was used as a positive control for adipocyte-specific genes.
Real-time RT-PCR
Quantitative real-time RT-PCR was performed using a Biosystem 7300 Real-time PCR system (Applied Biosystems) by monitoring the increase in reporter fluorescence of each TaqMan probe for interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), tumor necrosis factor-α (TNF-α), matrix metalloproteinase-13 (MMP-13), and ADAMTS-5 during PCR. Assay IDs were as follows: Hs00174097_m1 for IL-1β, Hs00174131_m1 for IL-6, Hs00174103_m1 for IL-8, Hs00174128_m1 for TNF-α, and HS00233992_m1 for MMP-13. The PCR primers and TaqMan probe for ADAMTS-5 were designed by Assays-by-Design Service (Applied Biosystems) as follows: forward primer 5′-CAGCCACCATCACAGAATTCCT-3′, reverse primer 5′-GTGGTAGGTCCAGCAAACAGTTA-3′ and probe 5′-[FAM] CCATGGCCATCATCC [TAMRA]-3′. In a 25-μl PCR, 1 μl cDNA (100 ng) was amplified using the 50 nM forward and reverse primers, 200 nM ribosomal RNA (rRNA) probe (VIC-TAMRA labeled), and TaqMan 2× Universal PCR Master Mix (Applied Biosystems) for the internal control; and 20× mix primers and TaqMan probe (FAM-TAMRA labeled) and TaqMan 2× Universal PCR Master Mix for the target genes. Thermal cycling was performed using 40 cycles at 95°C for 15 s followed by 60°C for 1 min. For quantification of gene expression, the comparative Ct method was used to calculate the relative fold changes normalized against rRNA [22]. The amount of gene expression was calculated as the difference (ΔC T) between C T value of the sample for the target gene and mean C T value of the same sample for the endogenous control (rRNA). Relative expression was calculated as the difference (ΔΔC T) between C T values of the test sample and those of the control sample. Relative expression of the genes of interest was calculated and expressed as 2−ΔΔCT, as described previously [23].
Effect of IL-6/sIL-6R on osteoblasts
Isolated cells were cultured with DMEM supplemented with 10% FBS for 24 h. The cells were stimulated with recombinant human IL-6 and sIL-6R (R&D Systems, Minneapolis, MN, USA) in serum-free DMEM for 24 h as previously described [5, 24]. Total RNA was extracted from the cells, and culture supernatant was then harvested.
Continuous hydrostatic pressure apparatus
A specially designed apparatus was prepared for application of continuous hydrostatic pressure (CHP) to the cells (Hikari Koatsu, Hiroshima, Japan) (Fig. 1B) as described previously [19]. Polystyrene culture dishes 60 mm in diameter (Asahi Techno Glass) were placed in an impermeable, deformable Teflon pouch, which was then filled with serum-free DMEM and placed in a stainless steel pressurization vessel. The pressurization vessel was then filled with kerosene to uniformly transmit pressure to the cells through the serum-free culture medium in the packed Teflon pouch in a gas-free environment. The pouch was subjected to CHP with a minimum applied pressure of 0 MPa and a maximum pressure of 50 MPa. The temperature was maintained at 37°C using a computer-controlled thermostat.
Application of CHP
MG63 cells, which can maintain many characteristics of the osteoblast phenotype for long periods in culture, and OA subchondral bone osteoblasts (OA SBO) from lateral femoral condyles evaluated as ICRS Grade 1 were cultured in monolayer at a density of 5 × 104 cells/cm2 in DMEM in 60-mm culture dishes for 24 h. The cells were exposed to 25 or 50 MPa of CHP for 2 h. When the exposure to CHP was terminated, the dishes were removed from the pressurization apparatus and maintained at atmospheric pressure in air containing 5% CO2. Cells in dishes placed in the same apparatus under the same conditions but not exposed to CHP were used as nonpressurized controls. Total RNA was extracted from the cells 30 min after termination of exposure to CHP.
Enzyme-linked immunosorbent assay (ELISA)
Cells (5 × 104) were seeded into each well of 48-well plates (well diameter, 10 mm) (Iwaki Glass, Chiba, Japan), followed by addition of 250 µl DMEM containing 10% FBS. After culture for 24 h under standard conditions, 250 µl serum-free medium was added and the cells were cultured for an additional 24 h. The culture supernatant was then harvested and concentrations of IL-6, IL-8, and MMP-13 were measured using ELISA kits for human IL-6, IL-8, and MMP-13 with assay sensitivities of 0.156 pg/ml, 1.5 pg/ml, and 0.094 ng/ml, respectively (IL-6, IL-6 QuantiGlo ELISA Kit; IL-8, Human IL-8 Immunoassay; R&D Systems; MMP-13, Matrix Metalloproteinase-13, Human; Biotrak ELISA System; Amersham Biosciences, Buckinghamshire, UK). The concentrations of these molecules were normalized to the total amount of protein in the supernatant. The determinations were performed in duplicate for each cell culture preparation.
Statistical analyses
Comparisons between two groups with equal variance were performed using Student’s t test, and those with unequal variance were performed using the Mann–Whitney U test. Statistical significance was defined as a P value less than 0.05.
Results
Classification of radiographic findings
All subjects were classified into K.L. Grade 4 with varus deformities. The mean FTA in all subjects was 185.9° ± 6.9° (range, 175.0°–200.0°). Nine patients [3 men and 6 women, aged 66.4–83.1 years (mean, 75.2 ± 6.7 years)] were assigned to the Large FTA group, and 10 patients [5 men and 5 women, aged 55.4–81.0 years (mean, 73.5 ± 8.2 years)] were assigned to the Small FTA group.
Identification of cells
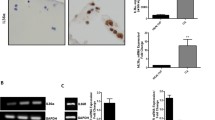
To investigate characterization of the osteoblasts from osteophytes, ALP staining and Oil Red O staining were performed. All the cells were positive for ALP staining and negative for Oil Red O staining (Fig. 2a). The mRNA expression of the osteoblast-specific genes, ALP and OCN, the chondrocyte-specific gene, collagen type II, and the adipocyte-specific genes, aP2 and PPARγ2, were analyzed by RT-PCR. The osteoblasts from osteophytes were positive for ALP and OCN but negative for collagen type II, aP2, and PPARγ2 mRNA expression (Fig. 2b).
a Characterization of osteoblasts isolated from the osteophytes. Osteoblasts from the osteophyte were seeded onto a collagen type I-coated 8-well chamber slide. Alkaline phosphatase (ALP) staining and Oil Red O staining were performed as described in “Materials and methods”. b Total RNA was extracted from the indicated cells, and mRNA expression of collagen type I, collagen type II, ALP, osteocalcin (OCN), aP2, PPARγ2, and GAPDH were analyzed by reverse transcription-polymerase chain reaction (RT-PCR) as described in “Materials and methods”. HCS HCS-2/8 cells (positive control for chondrocyte-specific gene), MG63 MG63 cells (positive control for osteoblast-specific genes), OPH osteoblasts from osteophytes, 293 293 cells (negative control for osteoblast-specific genes), adipose adipose tissue derived from fat pad in the knees of OA patients (positive control for adipocyte-specific genes)
Gene expression of inflammatory cytokines and proteases in OA osteoblasts from the osteophytes
Total RNA was extracted from the cells and analyzed identically using real-time RT-PCR. Real-time RT-PCR demonstrated that mRNA expression of IL-6, IL-8, and MMP-13 in OA osteoblasts from the osteophytes were significantly enhanced compared to those in osteoblasts from subchondral bone without OA (P < 0.01, <0.01, and <0.001, respectively) (Fig. 3a, b). However, mRNA expressions of other genes including IL-1β, TNF-α, and ADAMTS-5 showed no remarkable differences between osteophytes and subchondral bone without OA (Fig. 3a, b).
Increased gene expression of interleukin (IL)-6, IL-8, and matrix metalloproteinase (MMP)-13 in osteoblasts isolated from osteophytes of patients with osteoarthritis (OA). Total RNA was extracted from the cells, and the mRNA expression of inflammatory cytokines, including IL-1β, IL-6, IL-8, and tumor necrosis factor (TNF)-α, and proteases, including MMP-13 and ADAMTS-5, were analyzed by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) method. For quantification of gene expression, the comparative Ct method was used to calculate the relative fold changes normalized against ribosomal RNA. Relative expression of the genes was calculated as described in “Materials and methods”. The mRNA expression ratios of osteoblasts from the osteophytes compared to those from subchondral bone without OA are shown. a The relative mRNA expression of IL-1β, IL-6, IL-8, and TNF-α in OA osteoblasts from osteophytes, and in osteoblasts from subchondral bone without OA. b The relative mRNA expression of MMP-13 and ADAMTS-5 in OA osteoblasts from osteophytes, and in osteoblasts from subchondral bone without OA. Statistical significant difference was analyzed using the Mann–Whitney U test. **P < 0.01, ***P < 0.001 [osteoblasts from osteophytes (OPH), n = 19; subchondral bone osteoblasts without OA (SBO), n = 4]. The data are shown as box plots. Each box represents the 25th to 75th percentiles; the lines outside the boxes represent the 10th and 90th percentiles; and the lines inside the boxes represent the median
Concentrations of IL-6, IL-8, and MMP-13 in culture supernatant of OA osteoblasts from the osteophytes
IL-6, IL-8, and MMP-13 production in OA osteoblasts isolated from the osteophytes were measured using specific ELISA kits. IL-6, IL-8, and MMP-13 levels in the cell culture supernatant of OA osteoblasts from osteophytes were significantly higher than those of osteoblasts from subchondral bone without OA (P < 0.05, <0.05, and <0.01, respectively) (Fig. 4a–c). IL-8 and MMP-13 levels in the cell culture supernatant of osteoblasts from subchondral bone without OA were below the limits of detection (Fig. 4b, c).
Concentrations of IL-6, IL-8, and MMP-13 in culture supernatant of osteoblasts isolated from osteophytes of patients with osteoarthritis (OA). Five × 104 osteoblasts isolated from osteophytes from OA patients and subchondral bone without OA were seeded into each well of 48-well-plates, and medium containing 10% fetal bovine serum (FBS) was added. After cultivation for 24 h, the medium was removed, and serum-free medium (250 μl) was added. After further cultivation for 24 h, the culture supernatant was harvested and the concentrations of IL-6, IL-8, and MMP-13 were measured using enzyme-linked immunosorbent assay (ELISA) kits. The concentrations were normalized to the total amount of protein. The determinations were performed in duplicate for each cell culture preparation of IL-6 (a), IL-8 (b), and MMP-13 (c). Statistical significant difference was analyzed using Student’s t test. *P < 0.05, **P < 0.01 [subchondral bone osteoblasts without OA (SBO), n = 3; osteoblasts from osteophytes (OPH), n = 7). The data are shown as box plots, as described in Fig. 3
Gene expression of IL-6, IL-8, and MMP-13 in OA osteoblasts from the osteophytes in the Large FTA and Small FTA groups
In OA osteoblasts from the osteophytes, mRNA expression of IL-6 and IL-8 in the Large FTA group, which had severe joint deformation, increased approximately threefold and fourfold more than those in the Small FTA group, respectively (IL-6, 4.65 ± 0.88-fold in the Large FTA group and 1.50 ± 0.33-fold in the Small FTA group; IL-8, 6.84 ± 2.46-fold in the Large FTA group and 1.64 ± 0.80-fold in the Small FTA group) (Fig. 5a, b). The mRNA expression of MMP-13 showed no remarkable differences between the Large and Small FTA groups (Fig. 5c). Real-time RT-PCR also showed that the expression of IL-6, IL-8, and MMP-13 in both the Large FTA and Small FTA groups was significantly higher compared to subchondral bone osteoblasts without OA (SBO) (data not shown).
Increased gene expression of IL-6 and IL-8 in osteoblasts isolated from osteophytes in the large femorotibial angle (Large FTA) group. The mRNA expression of IL-6, IL-8, and MMP-13 was analyzed by real-time RT-PCR, as described in Fig. 3. The relative mRNA expression of IL-6 (a), IL-8 (b), and MMP-13 (c) in OA osteoblasts from the osteophytes in Large FTA and Small FTA groups is shown. Statistically significant difference was analyzed using the Mann–Whitney U test. *P < 0.05 (Large FTA group, n = 9; Small FTA group, n = 10). The data are shown as box plots, as described in Fig. 3
Effect of IL-6/sIL-6R on MMP-13 mRNA expression in OA osteoblasts from osteophytes (OPH) and subchondral bone osteoblasts without OA (SBO)
Real-time RT-PCR demonstrated that MMP-13 mRNA expression was significantly increased in cells treated with IL-6/sIL-6R compared to untreated cells for both OPH and SBO (Fig. 6a, b).
Effect of IL-6/sIL-6R on MMP-13 mRNA expression in OA osteoblasts from osteophytes (OPH) and subchondral bone osteoblasts without OA (SBO). Isolated cells were cultured in 60-mm culture dishes with Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS for 24 h. The cells were further cultured with or without recombinant human IL-6 and sIL-6R (R&D Systems, Minneapolis, MN, USA) at 100 ng/ml in serum-free DMEM for 24 h. Total RNA was extracted from the cells, and the mRNA expression of MMP-13 was analyzed by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) method. a mRNA expression ratios of osteoblasts from the osteophytes (OPH) treated with IL-6/sIL-6R [IL-6 (+)] compared to OPH untreated with IL-6/sIL-6R [IL-6 (−)], b mRNA expression ratios of subchondral bone osteoblasts without OA (SBO) with IL-6/sIL-6R compared to SBO untreated with IL-6/sIL-6R. Statistically significant difference was analyzed using Student’s t test. *P < 0.05, **P < 0.01 (OPH, n = 4; SBO, n = 4)
Effect of IL-6/sIL-6R on MMP-13 production in OA osteoblasts from osteophytes (OPH) and subchondral bone osteoblasts without OA (SBO)
MMP-13 production in cells treated with IL-6/sIL-6R was significantly increased compared to untreated cells with IL-6/sIL-6R for both OPH and SBO (Fig. 7a, b).
Effect of IL-6/sIL-6R on MMP-13 production in OA osteoblasts from osteophytes (OPH) and subchondral bone osteoblasts without OA (SBO). Cells (5 × 104 osteoblasts) isolated from osteophytes from OA patients and subchondral bone without OA were seeded into each well of 48-well-plates, and medium containing 10% FBS was added. After cultivation for 24 h, the medium was removed, and with or without recombinant human IL-6 and sIL-6R (R&D Systems) at 100 ng/ml in serum-free DMEM (250 μl) was added. After further cultivation for 24 h, the culture supernatant was harvested and the concentrations of MMP-13 were measured using ELISA). The concentrations were normalized to the total amount of protein. The concentrations of the cells treated with IL-6/sIL-6R [IL-6 (+)] compared to the cells untreated with IL-6/sIL-6R [IL-6 (−)] are shown. The determinations were performed in duplicate for each cell culture preparation of MMP-13 in the osteoblasts isolated from osteophytes from OA patients (OPH) (a) and subchondral bone without OA (SBO) (b). Statistically significant difference was analyzed using Student’s t test. *P < 0.05 (OPH, n = 4; SBO, n = 4)
Effects of CHP on the gene expression of IL-6, IL-8, and MMP-13 in MG 63 cells and OA subchondral bone osteoblasts (OA SBO)
After exposure to 25 or 50 MPa of CHP for 2 h, total RNA was extracted from the cells and analyzed identically using real-time RT-PCR. Real-time RT-PCR showed that the levels of mRNA expression of IL-6 and IL-8 in MG63 cells and OA SBO from lateral femoral condyles evaluated as ICRS Grade 1 following exposure to 25 or 50 MPa of CHP increased in a dose-dependent manner (Fig. 8a, b). The level of mRNA expression of MMP-13 was unaffected by exposure to either 25 or 50 MPa of CHP (Fig. 8c).
Induction of IL-6 and IL-8 gene expression in MG63 cells and OA subchondral bone osteoblasts (OA SBO) by continuous hydrostatic pressure (CHP) exposure. The cells, as indicated, were seeded into petri dishes, and 25 or 50 MPa of CHP was applied for 2 h. Thirty minutes after the termination of CHP exposure, total RNA was extracted from the cells. The mRNA expression of IL-6 (a), IL-8 (b), and MMP-13 (c) was analyzed by real-time RT-PCR, as described in Fig. 3. Statistically significant difference was analyzed using Student’s t test. *P < 0.05 versus control; **P < 0.01 versus control [MG63 cells (MG63), n = 3; OA subchondral bone osteoblasts (OA SBO), n = 3). Values are the mean ± SEM
Discussion
In this study, we investigated the expression of genes and proteins in osteoblasts derived from osteophytes formed in the medial knee joints of OA patients. We found that the mRNA expression and protein production of IL-6, IL-8, and MMP-13, factors involved in the progression of OA, were increased in osteophytes. Furthermore, when OA pathological conditions were simulated by applying a nonphysiological mechanical stress load to the osteoblasts, the gene expression of IL-6 and IL-8 increased. These results addressed the issue of the significance of osteophytes in OA patients.
It has been reported that osteophyte formation may precede the joint space narrowing attributable to degeneration and destruction of the cartilage in an animal model of OA [25] and in humans with anterior cruciate ligament rupture [26]. On the other hand, experiments in animal models have shown that osteophyte formation can occur following the severe loss of matrix macromolecules, in the absence of gross changes in the articular surface [27]. It is still controversial whether osteophyte formation precedes cartilage degeneration or vice versa.
OA treatments targeting cartilage tissues have been widely studied, but most of these studies focused on articular cartilage; few studies have focused on the osseous tissue surrounding the cartilage. With regard to the involvement of osteophytes in the progression of OA, the relationship of osteophyte formation to joint space narrowing has been reported [28, 29]. In biological analyses, the expression patterns of collagens and proteoglycans that comprise osteophytes have been studied [30, 31]. However, there have been no previous reports regarding the expression of genes that are involved in cartilage degeneration in osteophytes in clinical cases.
IL-6 and IL-8, which showed increased expression in the cartilage as OA progressed, are inflammatory cytokines involved in cartilage degeneration [32]. MMP-13 has been reported to be a factor involved in OA progression [33]. The increased expression of IL-6, IL-8, and MMP-13 in osteoblasts from osteophytes formed in the medial knee joints of OA patients may be involved in OA progression by promoting cartilage degeneration of the medial condyle, which usually shows initial changes in OA. MMP-13 also plays an important role in the remodeling of osseous tissue [34], and the overexpression of MMP-13 produces hypertrophy of chondrocytes [35]. IL-8 also induces chondrocytic hypertrophy [36]. The increased expression of MMP-13 and IL-8 in osteophytes could play a role in OA progression by inducing chondrocytic hypertrophy. Although IL-6, IL-8, and MMP-13 have been reported to exert various effects on bone tissue, the role of IL-6 as a key molecule that regulates the balance of bone resorption and bone formation in bone remodeling is of particular importance [37, 38]. However, the effects of IL-6, IL-8, and MMP-13 on osteophyte formation have not been reported in detail and remain unclear.
Knee pain in OA is reported to be possibly induced by synovial inflammation and joint fluid accumulation [39]. In addition, there is a close correlation between the formation of osteophytes in the medial condyle of the knee joints and knee pain [40]. Osteophyte formation is also related to clinical symptoms such as decreased joint function [28]. The results of the present study suggested that the induction of synovitis from increased expression of IL-6 and IL-8 might be a factor in the knee pain associated with OA.
In addition, a correlation has been reported between the degree of joint deformity and osteophyte formation [41]. Furthermore, as excision of osteophytes in the loaded area increases joint instability in the knee joint, osteophyte formation has been reported to be involved in the acquisition of joint stability [42]. Although osteophyte formation has recently been reported to occur in unloaded areas, mechanical stress is highly likely to be loaded on mature osteophytes formed on the medial femorotibial joint in the knee joint. As various types of mechanical stress exist, such as tensile force, shearing force, compressive force, and hydrostatic pressure, the details of the mechanical stress loading on osteophytes are unclear. However, as high proportions of bulk water are present in articular cartilage and bone, changes in gene expression caused by hydrostatic pressure were examined in the present study. To date, there have been no thorough investigations of the relationship between mechanical stress loading and gene expression changes in osteophytes. The local pressure applied to normal joints while walking is approximately 3–10 MPa, while that to OA joints is 20 MPa or more [43]. In OA, a mechanical stress of ≥20 MPa, which is nonphysiological for normal joints, is loaded on the medial condyle and its surroundings.
We found that the expression of IL-6 and IL-8 increased in osteoblasts in a stress magnitude-dependent manner. In the osteoblasts isolated from osteophytes, the expression of IL-6 and IL-8 increased significantly more in the Large FTA group, which had more severe joint deformation, than in the Small FTA group. The increased expression of IL-6 and IL-8 in osteophytes related to joint deformity might be involved in the induction of IL-6 and IL-8 expression caused by mechanical stress on the osseous tissue. No remarkable changes in the expression of MMP-13 were observed when loading nonphysiological pressures onto MG63 or OA SBO. Some studies have reported that IL-6 induces MMP-13 expression [5, 24, 44]. In this study, IL-6 can directly induce MMP-13 mRNA expression and production in OA osteoblasts from osteophytes and subchondral bone osteoblasts without OA. The increased induction of IL-6 resulting from mechanical stress could be involved in the increased expression of MMP-13 in osteophytes. The induction of the expression of proteases, such as MMP-3 and ADAMTS-5, has been reported to be involved in the relationship between mechanical stress applied to articular cartilage and the pathology of OA [10]. However, the present study is the first to investigate the influence of mechanical stress loading onto osseous tissue on OA progression and related factors.
Our results demonstrated that nonphysiological mechanical stress accompanying joint deformity may induce the expression of IL-6, IL-8, and MMP-13 in osteophytes, which are involved in OA progression. Factors such as IL-17, IL-18, MMP-1, -3, and -9, and TIMP-1 have also been reported to be involved in cartilage degeneration in OA [45–48], and these factors must also be examined in future studies. In addition, in the present study, we obtained data for human samples and found a large range of values for mRNA and protein expression. As we also observed a tendency toward increases in IL-1β and TNF-α mRNA expression, further studies are required to test for significant differences in larger numbers of cases. By controlling the expression of genes for these factors in osteophytes, the progression of cartilage degeneration and knee pain in OA patients could be reduced, suggesting a new treatment strategy for OA.
References
Bailey AJ, Mansell JP (1997) Do subchondral bone changes exacerbate or precede articular cartilage destruction in osteoarthritis of the elderly? Gerontology 43:296–304
Menkes CJ, Lane NE (2004) Are osteophytes good or bad? Osteoarthritis Cartilage 12(suppl A):S53–S54
Moskowitz RW, Goldberg VM (1987) Studies of osteophyte pathogenesis in experimentally induced osteoarthritis. J Rheumatol 14:311–320
Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR (2005) Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum 52:128–135
Franchimont N, Rydziel S, Delany AM, Canalis E (1997) Interleukin-6 and its soluble receptor cause a marked induction of collagenase 3 expression in rat osteoblast cultures. J Biol Chem 272:12144–12150
Legendre F, Dudhia J, Pujol JP, Bogdanowicz P (2003) JAK/STAT but not ERK1/ERK2 pathway mediates interleukin (IL)-6/soluble IL-6R down-regulation of type II collagen, aggrecan core, and link protein transcription in articular chondrocytes. Association with a down-regulation of SOX9 expression. J Biol Chem 278:2903–2912
Matsukawa A, Yoshimura T, Maeda T, Ohkawara S, Takagi K, Yoshinaga M (1995) Neutrophil accumulation and activation by homologous IL-8 in rabbits. IL-8 induces destruction of cartilage and production of IL-1 and IL-1 receptor antagonist in vivo. J Immunol 154:5418–5425
Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van Wart H, Poole AR (1997) Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest 99:1534–1545
Vankemmelbeke MN, Holen I, Wilson AG, Ilic MZ, Handley CJ, Kelner GS, Clark M, Liu C, Maki RA, Burnett D, Buttle DJ (2001) Expression and activity of ADAMTS-5 in synovium. Eur J Biochem 268:1259–1268
Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ (2005) Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum 52:2386–2395
Diehl P, Schmitt M, Schauwecker J, Eichelberg K, Gollwitzer H, Gradinger R, Goebel M, Preissner KT, Mittelmeier W, Magdolen U (2005) Effect of high hydrostatic pressure on biological properties of extracellular bone matrix proteins. Int J Mol Med 16:285–289
Kleemann RU, Krocker D, Cedraro A, Tuischer J, Duda GN (2005) Altered cartilage mechanics and histology in knee osteoarthritis: relation to clinical assessment (ICRS Grade). Osteoarthritis Cartilage 13:958–963
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16:494–502
Beresford JN, Gallagher JA, Gowen M, Couch M, Poser J, Wood DD, Russell RG (1984) The effects of monocyte-conditioned medium and interleukin 1 on the synthesis of collagenous and non-collagenous proteins by mouse bone and human bone cells in vitro. Biochim Biophys Acta 801:58–65
Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T (1999) Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci 112(pt 20):3519–3527
De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP (2001) Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 44:1928–1942
Khan WS, Adesida AB, Hardingham TE (2007) Hypoxic conditions increase hypoxia-inducible transcription factor 2-alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res Ther 9:R55
Inoue A, Takahashi KA, Arai Y, Tonomura H, Sakao K, Saito M, Fujioka M, Fujiwara H, Tabata Y, Kubo T (2006) The therapeutic effects of basic fibroblast growth factor contained in gelatin hydrogel microspheres on experimental osteoarthritis in the rabbit knee. Arthritis Rheum 54:264–270
Takahashi K, Kubo T, Arai Y, Kitajima I, Takigawa M, Imanishi J, Hirasawa Y (1998) Hydrostatic pressure induces expression of interleukin 6 and tumour necrosis factor alpha mRNAs in a chondrocyte-like cell line. Ann Rheum Dis 57:231–236
Ahn SE, Kim S, Park KH, Moon SH, Lee HJ, Kim GJ, Lee YJ, Park KH, Cha KY, Chung HM (2006) Primary bone-derived cells induce osteogenic differentiation without exogenous factors in human embryonic stem cells. Biochem Biophys Res Commun 340:403–408
Robbins JR, Thomas B, Tan L, Choy B, Arbiser JL, Berenbaum F, Goldring MB (2000) Immortalized human adult articular chondrocytes maintain cartilage-specific phenotype and responses to interleukin-1-beta. Arthritis Rheum 43:2189–2201
Bustin SA (2002) Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol 29:23–39
Sakao K, Takahashi KA, Arai Y, Inoue A, Tonomura H, Saito M, Yamamoto T, Kanamura N, Imanishi J, Mazda O, Kubo T (2008) Induction of chondrogenic phenotype in synovium-derived progenitor cells by intermittent hydrostatic pressure. Osteoarthritis Cartilage 16:805–814
Legendre F, Bogdanowicz P, Boumediene K, Pujol JP (2005) Role of interleukin 6 (IL-6)/IL-6R-induced signal tranducers and activators of transcription and mitogen-activated protein kinase/extracellular. J Rheumatol 32:1307–1316
Gilbertson EM (1975) Development of periarticular osteophytes in experimentally induced osteoarthritis in the dog. A study using microradiographic, microangiographic, and fluorescent bone-labelling techniques. Ann Rheum Dis 34:12–25
Buckland-Wright JC, Lynch JA, Dave B (2000) Early radiographic features in patients with anterior cruciate ligament rupture. Ann Rheum Dis 59:641–646
Williams JA, Thonar EJ (1989) Early osteophyte formation after chemically induced articular cartilage injury. Am J Sports Med 17:7–15
Ozdemir F, Tukenmez O, Kokino S, Turan FN (2006) How do marginal osteophytes, joint space narrowing and range of motion affect each other in patients with knee osteoarthritis. Rheumatol Int 26:516–522
Nagaosa Y, Lanyon P, Doherty M (2002) Characterisation of size and direction of osteophyte in knee osteoarthritis: a radiographic study. Ann Rheum Dis 61:319–324
Gelse K, Soder S, Eger W, Diemtar T, Aigner T (2003) Osteophyte development—molecular characterization of differentiation stages. Osteoarthritis Cartilage 11:141–148
Sweet MB, Thonar EJ, Immelman AR, Solomon L (1977) Biochemical changes in progressive osteoarthrosis. Ann Rheum Dis 36:387–398
Kaneko S, Satoh T, Chiba J, Ju C, Inoue K, Kagawa J (2000) Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther 6:71–79
Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE (1996) Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest 97:761–768
Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, Yu Y, Fosang AJ, Schorpp-Kistner M, Angel P, Werb Z (2004) Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development (Camb) 131:5883–5895
Tchetina EV, Kobayashi M, Yasuda T, Meijers T, Pidoux I, Poole AR (2007) Chondrocyte hypertrophy can be induced by a cryptic sequence of type II collagen and is accompanied by the induction of MMP-13 and collagenase activity: Implications for development and arthritis. Matrix Biol 26:247–258
Cecil DL, Rose DM, Terkeltaub R, Liu-Bryan R (2005) Role of interleukin-8 in PiT-1 expression and CXCR1-mediated inorganic phosphate uptake in chondrocytes. Arthritis Rheum 52:144–154
Poli V, Balena R, Fattori E, Markatos A, Yamamoto M, Tanaka H, Ciliberto G, Rodan GA, Costantini F (1994) Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J 13:1189–1196
Ferrari SL, Garnero P, Emond S, Montgomery H, Humphries SE, Greenspan SL (2001) A functional polymorphic variant in the interleukin-6 gene promoter associated with low bone resorption in postmenopausal women. Arthritis Rheum 44:196–201
Torres L, Dunlop DD, Peterfy C, Guermazi A, Prasad P, Hayes KW, Song J, Cahue S, Chang A, Marshall M, Sharma L (2006) The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage 14:1033–1040
Boegard T, Rudling O, Petersson IF, Jonsson K (1998) Correlation between radiographically diagnosed osteophytes and magnetic resonance detected cartilage defects in the tibiofemoral joint. Ann Rheum Dis 57:401–407
Felson DT, Gale DR, Elon Gale M, Niu J, Hunter DJ, Goggins J, Lavalley MP (2005) Osteophytes and progression of knee osteoarthritis. Rheumatology (Oxf) 44:100–104
Pottenger LA, Phillips FM, Draganich LF (1990) The effect of marginal osteophytes on reduction of varus–valgus instability in osteoarthritic knees. Arthritis Rheum 33:853–858
Hodge WA, Fijan RS, Carlson KL, Burgess RG, Harris WH, Mann RW (1986) Contact pressures in the human hip joint measured in vivo. Proc Natl Acad Sci USA 83:2879–2883
de la Torre P, Diaz-Sanjuan T, Garcia-Ruiz I, Esteban E, Canga F, Munoz-Yague T, Solis-Herruzo JA (2005) Interleukin-6 increases rat metalloproteinase-13 gene expression through Janus kinase-2-mediated inhibition of serine/threonine phosphatase-2A. Cell Signal 17:427–435
Honorati MC, Bovara M, Cattini L, Piacentini A, Facchini A (2002) Contribution of interleukin 17 to human cartilage degradation and synovial inflammation in osteoarthritis. Osteoarthritis Cartilage 10:799–807
Dai SM, Shan ZZ, Nishioka K, Yudoh K (2005) Implication of interleukin 18 in production of matrix metalloproteinases in articular chondrocytes in arthritis: direct effect on chondrocytes may not be pivotal. Ann Rheum Dis 64:735–742
Wu W, Billinghurst RC, Pidoux I, Antoniou J, Zukor D, Tanzer M, Poole AR (2002) Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum 46:2087–2094
Hulejova H, Baresova V, Klezl Z, Polanska M, Adam M, Senolt L (2007) Increased level of cytokines and matrix metalloproteinases in osteoarthritic subchondral bone. Cytokine 38:151–156
Acknowledgments
We thank Dr. Kawano and Dr. Aoshiba for supplying the clinical specimens. No research or institutional support was received for this work.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sakao, K., Takahashi, K.A., Arai, Y. et al. Osteoblasts derived from osteophytes produce interleukin-6, interleukin-8, and matrix metalloproteinase-13 in osteoarthritis. J Bone Miner Metab 27, 412–423 (2009). https://doi.org/10.1007/s00774-009-0058-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-009-0058-6