Abstract
We investigated the effect of eel calcitonin (elcatonin) on the process of fracture repair in the osteotomized femur of cynomolgus monkeys, since they possess a Haversian remodeling system similar to that of humans. Alendronate was used for comparison. Twenty female cynomolgus monkeys (Macaca fascicularis), aged 18–22 years, were allocated into five groups: control (CNT, n = 4), low-dose elcatonin group (0.5 U/kg; ELL, n = 4), high-dose elcatonin group (5 U/kg; ELH, n = 4), low-dose alendronate group (10 μg/kg; ALL, n = 4) and high-dose alendronate group (100 μg/kg; ALH, n = 4). All animals were given subcutaneous injections twice a week for 3 weeks. Then fracture was produced surgically by transversely cutting the midshaft of the right femur and fixing with stainless steel plate. After fracture, treatments were continued until sacrifice at 26 weeks after surgery. The femora were assessed by micro CT, contact microradiograph, three-point bending mechanical test and histomorphometry. Micro CT showed that callus sizes in elcatonin-treated groups were similar to CNT, whereas alendronate-treated groups had larger calluses than those in the CNT and elcatonin-treated groups. Fracture lines almost disappeared in the CNT and elcatonin-treated groups but remained clear in the alendronate-treated groups. Total area did not differ significantly between the elcatonin-treated groups and the CNT but was significantly greater in the ALH compared to the CNT and elcatonin-treated groups, due to increased callus area in the ALH group. Callus remodeling was less suppressed in the elcatonin-treated groups than in the alendronate-treated groups when compared with callus remodeling in the CNT. Although no significant differences in structural mechanical properties such as ultimate load, stiffness and work to failure were found among all groups, ultimate stress was significantly reduced in the ALH group compared with CNT and ELL groups. In conclusion, mild suppression of callus remodeling by elcatonin did not impair overall fracture healing process. In contrast, alendronate delayed structural fracture healing process by strongly suppressing callus remodeling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antiresorptive agents are used widely in the treatment of osteoporosis in postmenopausal woman [1–9]. These agents have been shown to prevent bone loss and osteoporotic fractures by suppressing the osteoclastic resorption of bone [1, 2]. Secondly, they are effective because they reduce bone remodeling by suppressing bone formation activity [1, 2, 10, 11]. Alendronate reduces activation frequency by 90% after 2 years treatment in postmenopausal women [11].
Since osteoporotic patients are prone to fracture, physicians are often confronted with the situation of fracture occurring in patients under treatment. However, few studies have focused on the effect of antiresorptive agents on the healing process after the patients sustain fractures during treatment. We have demonstrated that bisphosphonates such as incadronate and alendronate treatment resulted in strong and enlarged calluses but delayed callus remodeling in fractured femora of rats [12–16]. Thus, it is possible that strong suppression of osteoclastic bone resorption may prolong the natural fracture healing process.
Elcatonin, a synthetic derivative of eel calcitonin, consists of 31 amino acids with stable ethylene linkage as a result of substituting the intra-chain disulfide bond of calcitonin. Elcatonin is physicochemically and biologically stable, and inhibits bone resorption through direct action on the calcitonin receptors on osteoclast [17]. In Japan, this drug has been approved in clinical treatment of osteoporosis, mainly for the analgesic effect, in addition to the effect of bone loss prevention [18–21].
Because of its analgesic effect, elcatonin is frequently used for pain relief in patients with fractures, especially immediately after fracture. Based on this clinical usage, it is important to examine the effects of this agent on the fracture healing process.
In this study, we examined the effect of elcatonin on the fracture healing process compared with the effect of alendronate, using the femoral fracture model in cynomolgus monkey that possesses intracortical remodeling similar to humans.
Materials and methods
Animals
Twenty female cynomolgus monkeys (Macaca fascicularis) aged 18–22 years (Siconbrec Inc. Makati, Philippines) with normal menstrual cycle were housed in a room maintained at 22 to 30°C and a 12-h light-dark cycle, with free access to water and restricted access to standard monkey breeder pellets (Republic Flour Mills Corp., Luguna, Philippines) of 100 g/day. All animal procedures were conducted in accordance with the National Institutes of Health (NIH) guidelines, following a protocol approved by the Kagawa University Animal Study Committee.
Experimental design
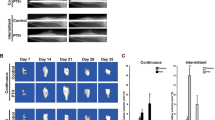
After 1-month acclimatization, all monkeys were allocated into five groups by matching body weights: control group (CNT, n = 4), low-dose elcatonin group (ELL, n = 4), high-dose elcatonin group (ELH, n = 4), low-dose alendronate group (ALL, n = 4) and high-dose alendronate group (ALH, n = 4). Animals in CNT group were given subcutaneous injection of 0.9% saline vehicle. The remaining four groups were given subcutaneous injection of elcatonin (Asahi Kasei Corp., Tokyo, Japan) at a dose of 0.5 U/kg per day (ELL group) or 5 U/kg per day (ELH group), or alendronate (Teijin Pharma Limited, Tokyo, Japan) at a dose of 10 μg/kg per day (ALL group) or 100 μg/kg per day (ALH group). Injections were given twice a week. ELL and ALL doses are clinical doses for human osteoporosis treatment, while ELH and ALH doses are 10 times the corresponding clinical doses. Treatment was initiated 3 weeks before experimental fracture, and continued after treatment until sacrifice. Body weights were measured weekly and doses were adjusted accordingly (Fig. 1).
Experimental protocol. CNT group: vehicle (0.9% saline) treatment, ELL group: treatment with elcatonin at 0.5 U/kg, ELH group: treatment with elcatonin at 5 U/kg, ALL group: treatment with alendronate at 10 μg/kg, ALH group: treatment with alendronate at 100 μg/kg, before and after fracture surgery. Subcutaneous injection was performed twice a week
Fracture surgery
Fracture was produced surgically in all animals after 3 weeks of pretreatment. Under general anesthesia with a 2:1 mixture (10 mg/kg, i.m.) of ketamine hydrochloride (Troy Laboratories Pty. Ltd., Smithfield, Australia) and xylazine hydrochloride (Troy Laboratories Pty. Ltd.), a transverse osteotomy was performed at the midshaft of the right femur using a fine-toothed circular microsaw (Kiso Power Co., Osaka, Japan). The fracture was repositioned and fixed tightly with a stainless plate and screws (Osteo-mini ACP plate, Stryker, Michigan, USA). Unrestricted ambulation was allowed after recovery from general anesthesia, and dosing was continued until sacrifice.
Evaluations
Anteroposterior and lateral radiographs of the fractured femora were taken using an Acoma portable X-ray unit (20 mA, 60 Kvp, 0.4 S; PX-20 N, Acoma X-ray Industry Co., Ltd. Tokyo, Japan) immediately after fracture surgery and repeated every 4 weeks until sacrifice to check fracture alignment and fixation. The animals were sacrificed at 26 weeks after surgery. All animals were double-labeled intravenously with calcein (8 mg/kg; Dojin Chemical Institute, Kumamoto, Japan) at 22 and 9 days before sacrifice. After necropsy, the femora were excised and dissected free of soft tissues. The stainless plate and screws were removed carefully so as not to damage the callus. Anteroposterior soft radiographs of the femora were taken and the femora were frozen at −80°C wrapped in gauze soaked in isotonic saline until micro computed tomographic (micro CT) scanning.
After thawing at room temperature, the femora were scanned by micro CT (25 μA, 70 KV, 1.8 W; NX-LCP-C80, Nittetsu Elex Co., Ltd. Tokyo, Japan). The bones were placed horizontally on the table. One hundred slices were scanned both at the proximal and distal sides of the fracture site. Then sagittal image of the fracture were reconstructed. After micro CT scanning, the femora were tested for mechanical properties by a three-point bending method using a materials testing machine (MZ500S, Maruto, Tokyo, Japan). A femur was placed on two lower support bars (40 mm apart) with the anterior surface facing upward. The fracture plane was centered at the loading point, and load was applied at a rate of 166.7 mm/min until breakage. From the load versus displacement curve, the structural mechanical properties of the fractured bone were determined as ultimate load (maximum force that the specimen sustained), stiffness (the slope of the linear portion of the load vs. deformation curve before failure) and work to failure (the area under the load vs. deformation curve before failure). These structural parameters depend on both intrinsic material properties and geometry.
The load vs. displacement data were normalized to obtain intrinsic material properties such as ultimate stress, elastic modulus, and toughness, which are independent of cross-sectional area and shape.
Ultimate stress was calculated from the ultimate load by the following formula:
where L is the length between supports, b is the width of the femur in anteroposterior direction, and I is the cross-sectional moment of inertia.
Elastic modulus was calculated as:
Toughness was calculated from energy absorption as:
Cross-sectional moment of inertia (I) was calculated with the assumption that femoral cross-section is elliptically shaped:
where a is the width in mediolateral direction, and t is the average cortical thickness.
Average cortical thickness was calculated from histomorphometric thickness measurements obtained from each of four quadrants of the femora section using a digitizing system.
After mechanical testing, the segment proximal to the fracture was fixed in 10% cold neutral buffered formalin for 3 days, decalcified in 10% EDTA at 4°C for 4 weeks, and then embedded in paraffin. Within 500 μm from the original fracture line, 5-μm cross-sections were cut for tartrate-resistant acid phosphatase (TRAP) staining. The presence of osteoclasts was determined by TRAP activity using a leukocyte acid phosphatase kit (Sigma Chemical Co., St. Louis, MO, USA).
The segment distal to the fracture was fixed in 70% ethanol, stained in Villanueva bone stain (Polysciences Inc., Warrington, PA, USA), dehydrated in graded ethanol, defatted in acetone, and embedded in methyl methacrylate. Within 500 μm from the original fracture line, 100-μm undecalcified transverse sections were cut using a diamond microtome saw (RM2065, Leica Instruments GmbH, Nussloch, Germany) for contact microradiographs (100 Kvp, 2 μA, 5 min; μFX-1000; Fujifilm, Tokyo, Japan). Then specimens were ground to 30 μm in thickness for histomorphometric measurement.
Histomorphometric analysis was performed using a semi-automated digitizing image analyzer. The system consisted of a light or epifluorescent microscope and a digitizing pad coupled with computer and histomorphometric software (System Supply CO., Nagano, Japan)
Total cross-sectional area (T.Ar; mm2), medullary area (Me.Ar; mm2), callus area (Cl.Ar; mm2), and thickness (Cl.Th; μm) were measured at 40× magnification, and bone area (B.Ar = T.Ar − Me.Ar) was calculated. Percent bone area (%B.Ar = B.Ar × 100/T.Ar; %) and percentage callus area (%Cl.Ar = Cl.Ar × 100/T.Ar; %) were also calculated. Single-labeled surface, double-labeled surface, and the interlabeling width were measured at the callus. Mineral apposition rate (MAR; μm/day), mineralizing surface (MS/BS; %) and bone formation rate (BFR/BS; mm3/mm2 per year) were calculated. Osteoclast measurements including osteoclast number (N.Oc; #) and derived parameters (N.Oc/BS; #/μm) were performed at 100× magnification at the callus.
Statistical analysis
Statistical computation of data was performed using the statistical package StatView 5.0. Differences among treatment groups were tested by one-way analysis of variance (ANOVA). If a significant difference was obtained, differences between the means of two groups were tested by Fisher’s protected least significant difference (PLSD). A P-value less than 0.05 were considered significant.
Results
General condition
The animals resumed normal activity within a few days after fracture surgery and no health problems were pointed out during the study period. Body weight of the animal was not significant among the groups during the study period.
Micro-CT
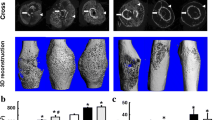
Micro-CT at the sagittal plane revealed that alendronate-treated groups had larger callus at both periosteal and endosteal surfaces than CNT or elcatonin-treated groups. Especially, ALH had the largest callus. In contrast, callus sizes were similar in elcatonin-treated groups and CNT. Fracture lines were clearly visible in the alendronate-treated groups, but were obscure in CNT and elcatonin-treated groups (Fig. 2).
Contact microradiographs
Contact microradiographs also confirmed that callus size was larger in the alendronate-treated groups compared with callus sizes in the CNT and the elcatonin-treated groups, and was the largest in ALH (Fig. 3).
Mechanical test
The ultimate load, stiffness, and work to failure in the femur did not differ significantly among the five groups. When these data were normalized by cross-sectional moment of inertia, the intrinsic material properties such as ultimate stress, elastic modulus, and toughness tended to be smaller in ELH and alendronate-treated groups. Ultimate stress was significantly (P < 0.05) lower only in ALH compared with CNT (Table 1).
Histomorphometry
Total area (T.Ar) of the femur was larger in the alendronate-treated groups compared with CNT but was similar in elcatonin-treated groups and CNT. Especially, T.Ar was significantly larger in ALH than in ELL or CNT (P < 0.05 in both). Percent bone area (%B.Ar) was also significantly larger in ALH than in ELL or CNT (P < 0.05 in both). Percent callus area (%Cl.Ar) tended to be larger in alendronate-treated groups than in CNT, and was the largest in ALH among the five groups. Similarly, ALH had the greatest callus thickness (Cl.Th).
Remodeling parameters in callus were suppressed in a dose-dependent manner following both elcatonin and alendronate treatment, but the extent of remodeling suppression tended to be less in elcatonin-treated groups. Mineral apposition rate (MAR), bone formation rate (BFR/BS) and mineralizing surface (MS/BS; %) were significantly lower in elcatonin- and alendronate-treated groups than in CNT. Especially, ALH showed the lowest values for all dynamic parameters among the five groups.
Number of osteoclast (N.Oc/Cl.Ar) was significantly less in both elcatonin- and alendronate-treated groups than in CNT (Table 2).
Discussion
Intracortical remodeling plays an important role in restoring structural and mechanical properties of fractured bone [22–26]. Therefore, a simian fracture model that possesses the Haversian remodeling system is expected to provide more precise information regarding human fracture healing process than animal models without such a system. To the best of our knowledge, this is the first study to evaluate the effect of calcitonin on fracture healing using nonhuman primate. Although the statistical power of this study may not be strong because of a limited number of animals, we believe that the results of this study provide important information regarding the effects of osteoporotic drug treatment on patients who sustained fractures during treatment.
The primary aim of this study was to examine the effect of elcatonin on the fracture healing process using a femoral osteotomy model in cynomolgus monkey that possess a Haversian intracortical remodeling system similar to humans. Our results clearly indicated that treatment with both elcatonin and alendronate at clinical doses for treating human osteoporosis in Japan suppressed callus remodeling but did not impair the mechanical or structural fracture healing process. However, our data also showed that alendronate at a dose ten times the clinical dose (ALH) decreased some intrinsic material properties of fractured femur through more severe suppression of callus remodeling, although highly increased callus volume was observed only in alendronate-treated groups. In contrast, elcatonin at a dose ten times the clinical dose (ELH) did not decrease any biomechanical properties in spite of histological suppression of callus remodeling.
Our previous study using rat femoral fracture model also showed that alendronate increased the size and mineral content of callus, while delaying remodeling of the callus woven bone into lamellar bone at both 6 and 16 weeks after the fracture surgery [15, 16].
The enlarged callus in alendronate-treated animals stabilizes the fracture, despite the increased woven bone content and delayed callus remodeling. Therefore, the larger volume and cross-sectional area (moment of inertia) observed in alendronate treatment appear to be an adaptation that compensates for the alendronate-induced delay of woven bone remodeling into lamellar bone, which is structurally and mechanically superior to woven bone [15]. Essentially, the same results were also obtained in a previous study using another bisphosphonate incadronate [12], indicating that increased size, greater mineral content, and strong inhibition of callus remodeling are the characteristic class effects of bisphosphonates as a class of antiresorptive. In contrast, other antiresorptive agents such as estrogen, raloxifene and vitamin D3 analogue mildly suppress callus remodeling and do not greatly alter the structural and mechanical fracture healing process when compared with control groups [15, 16].
Data on the efficacy of injectable calcitonin on fracture are limited. Only one open randomized controlled study showed that elcatonin reduced vertebral fracture risk by 59% with a small increase in BMD compared to placebo after 2 years treatment [19]. Although calcitonin had a mild effect on bone mass, the antiresorptive effect of calcitonin has been validated in many basic and clinical studies [17–21, 27, 28].
In the present study, mineral apposition rate, bone formation rate and mineralizing surface were significantly lower in elcatonin- and alendronate-treated animals than in controls. Especially, the high-dose alendronate-treated group showed the lowest values for all dynamic parameters among the five groups. These results showed that alendronate delayed callus remodeling by strongly suppressing remodeling of woven bone into lamellar bone. In contrast, elcatonin mildly suppressed callus remodeling and did not impair the overall fracture healing process. Generally the anti-resorptive action of calcitonin is weaker than those of bisphosphonates as also demonstrated in the current study. Possible explanation for this may be the difference in the duration of pharmacological action of these agents. Calcitonin inhibits osteoclastic bone resorption through its receptor abundantly expressed on the plasma membrane of osteoclast [29–31]. Therefore, this agent dose not accumulate in the bone and the duration of calcitonin action should be limited. In contrast, bisphosphonates usually accumulate in the bone due to the high affinity for hydroxyapatite, and they have much longer durations of pharmacological action. The high binding affinities of bisphosphonates for bone may affect many important biological properties of these compounds including uptake and retention on the skeleton, diffusion of the drug within bone, release of absorbed drug back onto bone surfaces, and recycling of the desorbed drug back onto bone surfaces, and effects on mineral dynamics and cellular function within bone [32].
There is a weakness in the current study. Because antiresorptive agents are administrated for osteoporosis patients in a clinical situation, osteoporotic animal models, such as ovariectomized, would provide more clinically related information than normal animal model regarding the effect of antiresorptive agents on fracture healing in the osteoporotic patient. However, we used old enough monkey (18–22 years) with naturally reduced ovarian function although it was not evaluated exactly.
Based on radiological, histomorphometric and biomechanical evaluation of fractured femur in cynomolgus monkeys treated with elcatonin, we conclude that elcatonin mildly suppresses callus remodeling and has no effect on the natural fracture healing process and intrinsic material properties. In contrast, high-dose alendronate induces the formation of large, strong callus but delays the structural and material restoration of the callus. Regarding clinical relevance, these animal fracture data suggest that physicians need not consider discontinuation of elcatonin treatment when a patient undergoing elcatonin therapy for osteoporosis sustains osteoporotic fractures. Moreover, the fact that calcitonin has a potent analgesic effect may be an additional benefit for the continuous use of this agent after fracture.
References
Rodan GA, Fleisch HA (1996) Bisphosphonates: mechanisms of action. J Clin Invest 97:2692–2696
Russell RG, Rogers MJ (1999) Bisphosphonates: from the laboratory to the clinic and back again. Bone 25:97–106
Delmas PD (2002) Different effects of antiresorptive therapies on vertebral and nonvertebral fractures in postmenopausal osteoporosis. Bone 30:14–17
Delmas PD (2002) Treatment of postmenopausal osteoporosis. Lancet 359:2018–2026
Fleisch H (2001) Can bisphosphonates be given to patients with fractures? J Bone Miner Res 16:437–440
Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289:1508–1514
Fujita T, Fujii Y, Miyauchi A, Takagi Y (1999) Comparison of antiresoptive activities of ipriflavone, an isoflavone derivative, and elcatonin, an eel carbocalcitonin. J Bone Miner Metab 17:289–295
Hori M, Takahashi H, Konno T, Inoue J, Haba T, Sakurada T, Noda T, Fujimoto K (1984) Effect of elcatonin on experimental osteoporosis induced by ovariectomy and low calcium diet in beagles. Nippon Yakurigaku Zasshi 84:91–98
Yoshikawa S, Shiba M, Hoshino T, Igarashi M, Orimo H, Sakuma A, Tsuyama N (1983) Effect of eel calcitonin derivative(elcatonin) in osteoporosis. Nippon Seikeigeka Gakkai Zasshi 57:1717–1728
Sato M, Rippy MK, Bryant HU (1996) Raloxifene, tamoxifen, nafoxidine, or estrogen effects on reproductive and nonreproductive tissue in ovariecyomized rat. FASEB J 10:905–912
Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ (1997) Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest 100:1475–1480
Li J, Mori S, Kaji Y, Mashiba T, Kawanishi J, Norimatsu H (1999) Effect of bisphosphonate (incadronate) on fracture healing of long bones in rats. J Bone Miner Res 14:969–979
Li J, Mori S, Kaji Y, Kawanishi J, Akiyama T, Norimatsu H (2000) Concentration of bisphosphonate (incadronate) in callus area and its effects on fracture healing in rats. J Bone Miner Res 15:2042–2051
Li C, Mori S, Li J, Kaji Y, Akiyama T, Kawanishi J, Norimatsu H (2001) Long-term effect of incadronate disodium (YM-175) on fracture healing of femoral shaft in growing rats. J Bone Miner Res 16:429–436
Cao Y, Mori S, Mashiba T, Westmore MS, Ma L, Sato M, Akiyama T, Shi L, Komatsubara S, Miyamoto K, Norimatsu H (2002) Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res 17:2237–2246
Cao Y, Mori S, Mashiba T, Kaji Y, Manabe T, Iwata K, Miyamoto Kensaku, Komatsubara S, Yamamoto T (2007) 1α-25-Dihydroxy-2β(3-hydroxypropoxy)vitamin D3 (ED-71) suppressed callus remodeling but did not interfere with fracture healing in rat femora. Bone 40:132–139
Chambers TJ, Moore A (1983) The sensitivity of isolated osteoclasts to morphological transformation by calcitonin. J Clin Endocrinol Metab 57:819–824
Knopp JA, Diner BM, Blitz M (2005) Calcitonin for treating acute pain of osteoporotic vertebral compression fractures: a systematic review of randomized, controlled trials. Osteoporos Int 16:1281–1290
Ishida Y, Kawai S (2004) Comparative efficacy of hormone replacement therapy, etidronate calcitonin, alfacalcidol, and vitamin K in postmenopausal women with osteoporosis: Yamagata Osteoporosis Prevention Study. Am J Med 117:549–555
Fujita T, Fujii Y, Goto B (1997) A three-year comparative trial in osteoporosis treatment: effect of combined alfacalcidol and elcatonin. J Bone Miner Metab 15:223–226
Orimo H, Morii H, Inoue T (1996) Effect of elcatonin on involutional osteoporosis. J Bone Miner Metab 14:73–78
Aro HT, Wippermann BW, Hodgson SF, Chao EY (1990) Internal remodeling of periosteal new bone during fracture healing. J Orthop Res 8:238–246
Schenk RK, Hunziker EB (1994) Histologic and ultrastructural features of fracture healing. In: Brington CT, Friedlaender GE, Lane JM (eds) Bone regeneration and repair, 1st edn. American Academy of Orthopedic Surgeons, Rosemont, IL, USA, pp 117–146
Einhorn TA (1998) The cell and molecular biology of fracture healing. Clin Orthop 355S:S7–S21
Frost HM (1998) The biology of fracture healing. Clin Orthop 248:283–309
McKibbin B (1978) The biology of fracture healing in long bones. J Bone Joint Surg Br 60-B:150–162
Ikegame M, Ejiri S, Ozawa H (1994) Histochemical and autoradiographic studies on elcatonin internalization and intracellular movement in osteoclasts. J Bone Miner Res 9:25–37
Meschia M, Brincat M, Barbacini P (1993) A clinical trial on effects of a combination of elcatonin (carbo-calcitonin) and conjugated estrogens on vertebral bone mass in early postmenopausal women. Calcif Tissue Int 53:17–20
Nicholson GC, Moseley JM, Sexton PM, Mendelsohn FAO, Martin TJ (1986) Abundant calcitonin receptors in isolated rat osteoclasts. J Clin Invest 78:355–360
Suda T, Takahashi N, Martin TJ (1992) Modulation of Osteoclast differentiation. Endocr Rev 13:66–80
Lin HY, Harris TL, Flannery MS, Azuffo A, Kaji EH, Gorn A, Kolakowski LF Jr, Lodish HF, Goldring SR (1991) Expression cloning of an adenylate cyclase-coupled calcitonin receptor. Science 254:1022–1024
Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RGG, Ebetino FH (2006) Novel insights into actions of bisphonates on bone: Differences in interactions with hydroxyapatite. Bone 38:617–627
Acknowledgments
The authors thank Mika Kawada and Yoshiko Fukuda for histological preparation and Asahi Kasei Co. for kindly supplying the elcatonin.
Conflict of interest statement
All authors have no conflict of interest associated with this study and publication.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Manabe, T., Mori, S., Mashiba, T. et al. Eel calcitonin (elcatonin) suppressed callus remodeling but did not interfere with fracture healing in the femoral fracture model of cynomolgus monkeys. J Bone Miner Metab 27, 295–302 (2009). https://doi.org/10.1007/s00774-009-0046-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-009-0046-x