Abstract
The data reported in the frame of proficiency testing (PT) exercises organized from 2007 to 2016 have been statistically re-evaluated using Algorithm A of the ISO 13528:2015, and the relative standard deviation for reproducibility of multi-residue methods for the determination of pesticides in olive oil was evaluated. Usually, the assigned between-laboratories variability in PTs on pesticide residues is fixed/set to 25 %. This value was compared to the calculated robust relative standard deviation (RRSD). A total of 1527 analytical results were collected in the ten PTs for the determination of pesticides in olive oil. An RRSD of 21 % was obtained, below the maximum value of 25 %. If all participants use the same analytical approach (e.g., multi-residue method and same instrumental technique), a lower value of the reproducibility standard deviation should be expecting. The QuEChERS method, coupled with LC–MS/MS and GC–MS/MS, has become an important methodology for the analysis of pesticide residues. This is due to its simplicity, the use of low quantities of acetonitrile, the possibility to analyze a large number of pesticides with fewer steps and high efficiency. This method may harmonize the future of pesticide residue analyses. Recently, it was successfully applied to the analysis of olive oil by 70 % of the laboratories participating to our last PT exercise. An expanded uncertainty of 50 % was systematically applied in Europe since 2006 for the analyses of pesticides; the use of the QuEChERS methodology may reduce to 40 %. This work could contribute to promote the comparability of measurements of pesticide residues in foodstuffs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Measurement uncertainty is a quantitative indicator describing the dispersion around the measured quantity value that should encompass the “true” value of the measurand [1]. A number of guidelines present various approaches to estimate measurement uncertainty [2,3,4,5,6]. Precision and trueness are often considered as major sources of uncertainty [7].
In the field of pesticide residues in foodstuffs, a measurement standard uncertainty of 25 % is considered as the Fit—for—Purpose Relative Standard Deviation. Consequently an expanded uncertainty of 50 % was systematically applied in Europe since 2006. This procedure is well established and routinely employed, assuming that laboratories have demonstrated—in the frame of validation studies—that their own expanded uncertainty is smaller than 50 % [8].
Our laboratory organized proficiency tests for the determination of pesticides in olive oils since 2007 and collected a large number of external quality control data. The results and information received from participants provided indications about satisfactory/unsatisfactory performance and potential problems related with the analytical accuracy. The complexity of this olive oil matrix could serve as a worst case scenario for the evaluation of analytical reproducibility for pesticide residue analysis.
In the document SANTE/11813/2017—on analytical quality control and method validation procedures for pesticides residues analysis in food and feed—olives are included in the commodity groups of “high oil content and intermediate water content”. Therefore, olives could serve as a model for the validation studies of pesticide analysis in complex vegetable products and high fat content products.
Olives and olive oils contain high levels of lipid substances that may disturb the analysis of pesticides analysis, as they are soluble in many organic solvents used for extraction. Lipids should be removed from the extracts by a cleanup step before the chromatographic analysis. In the last 10 years, a remarkable evolution of the analytical methods has been observed for this kind of matrix. At first, methodologies were based on gas chromatography with selective detectors (flame ionization—FID, Electron Capture Detector—ECD, nitrogen phosphorus detector—NPD) after an extensively cleanup such as liquid–liquid partitioning, gel permeation chromatography or solid phase extraction [9, 10].
The need for differentiated analysis systems for the various compound classes and type of material constituted a strong limitation. This was overcome in 2003 by Anastassiades et al. [11] introducing a new multi-residue and multi-matrix approach—known as “QuEChERS” (Quick, Easy, Cheap, Effective, Rugged, and Safe)—that drastically changed the understanding of pesticide residue analysis. The QuEChERS method, coupled with LC–MS/MS and GC–MS/MS, became an important methodology for the analysis of pesticide residues in particular for the new generation of polar pesticide. This is due to its simplicity, the use of low quantities of acetonitrile, the possibility to analyze a large number of pesticides with fewer steps, and its high efficiency. This method may harmonize the future analyses of pesticide residues.
Pesticides cover a wide variety of bioactive compounds, with different physico-chemical properties. They are present in the environment and foodstuffs at trace levels. The Pesticide Manual lists over 1000 pesticides [12], of which more than 500 have a legal limit (MRL—Maximum Residue Limit) set by the European Commission [13]. QuEChERS is the robust and sensitive method allowing measuring many pesticides, metabolites and degradation products in one single run.
QuEChERS was successfully applied to analyze olives and olive oils with some minor modifications [14]: a mixture of different sorbents including C18 and GCB was used for the cleanup step, instead of the classical PSA sorbent used for fruits and vegetables with lower fat content. Up to 70 % of the laboratories participating in our last PTs exercise applied QuEChERS as their routine method with few minor modifications.
The data reported—by different laboratories applying different analytical methods—in the frame of proficiency testing (PT) exercises organized from 2007 to 2016 have been statistically re-evaluated using robust statistics [14] to evaluate (1) the long-term trend of relative reproducibility standard deviation of multi-residue methods for the determination of pesticides in olive oil; (2) trueness (based on the reported recovery results); and (3) the uncertainty of the assigned values.
Methods
Data sources
A total of 1527 analytical results were collected from the ten organized PTs. They were re-evaluated applying the robust statistical protocol described in ISO 13528:2015. Usually, a portion of the test material was spiked with a maximum number of pesticides (not more than 8). The individual compounds were selected for each exercise from a possible target list of 23–26 different compounds. The possible list of compounds includes mainly those considered in the official control plans, with spiked concentration levels around the maximum residue levels set in the European Regulations.
Each participant was required to provide detailed information about the analytical procedures used. Laboratories were requested to analyze the test sample in duplicate. Homogeneity and stability tests were passed in all PTs.
Statistical analyses
The assigned values (xpt) and the respective standard uncertainties (u(xpt)) were obtained applying robust statistics, namely the Algorithm A described in the ISO 13528: 2015 [15]. The standard uncertainty was calculated using the robust standard deviation (s*) and the total number of results (n):
Results and discussion
These 1527 analytical results obtained by different European laboratories on complex matrices could serve as an example of the evaluation of the reproducibility standard deviation of the determination of pesticide residues using multi-residues analytical procedures. The analysis of low levels of analytes in such a complex matrices with a variety of interfering (and co-eluting) substances was very challenging [16,17,18].
The purpose of the current evaluation was to investigate the possible reduction of the target (i.e., maximum admissible) measurement uncertainty based on the mean of relative standard deviations of results by the participating laboratories in the proficiency tests for multi-residue methods. Only if all laboratories use the same procedure, systematic effects attributed to the measurement procedure are under control. In fact, systematic effects become null, so the uncertainty could be directly estimated from the reproducibility between laboratories (sR) according to ISO 21748 [19].
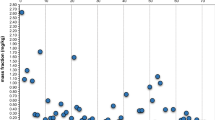
Figure 1 compares the preset between-laboratories standard deviation in PTs on pesticide residues of 25 % to the calculated individual robust relative standard deviation for reproducibility, ranging from 10 % to 45 %, and characterized by a median value of 21 %.
In the past, an extensive sample preparation and clean up step were required for the determination of pesticide residues by chromatography (GC/LC) coupled to different selective detectors. The use of various detectors (e.g., ECD, FPD, NPD and DAD) needed different and specific sample treatment methods, while providing only a limited specificity. Their use did not provide unambiguous identification, even in combination with different polarity columns.
Coupling gas or liquid chromatography to mass spectrometry allowed fast, robust and simple extraction procedures detecting several analytes in a single extraction with good recoveries, even for olives and olive oils. A smaller value of the reproducibility standard deviation is expected if all participants use the same instrumental technique and validated sample preparation procedure, such as the QuEChERS methodology. In the recent years, this method has seen a widespread diffusion, due to its simplicity, and the large number of samples that can be processed in an hour. This easy and quick analytical approach includes limited steps, thus reducing the uncertainty contributions to precision and trueness.
Table 1 indicates the wide variety of analytical methods used by the laboratories having participated to our PTs in the last ten years. Some laboratories routinely used selective detectors but reported unsatisfactory results [20]. Most of the laboratories used mass spectrometry coupled with gas chromatography or liquid chromatography to determine certain polar pesticides in complex matrix [21]. In recent years, the participating laboratories have reached and defined very low quantification limits (in most cases ≤ 0.01 mg/kg). This aspect is essential if combined with the high specificity of tandem mass spectrometry as the reduction of the target uncertainty should be justified with the ability to distinguish smaller deviations to the MRL.
In 2014, the majority of participant laboratories (about 70 %) used the QuEChERS with good performance (Fig. 2). In this specific round, the robust relative standard deviation was well below the value of 25 %, ranging from 14 % for Fenoxycarb and 22 % for Terbutylazine (Fig. 2). The uncertainty of the assigned value was estimated considering a relative standard deviation (σpt) of 21 %.
Case study PT 2014 (Chlorpyrifos Methyl): correlation between methods, z-score results and code laboratories. Classification method: (red circles) QuEChERS or based on QuEChERS, (white circles) In house method, Method EURL-FV, Based on Manual of Pesticide Analysis Vol. 1 DFG, Lentza Rizos, J Chromatogr A 921, 2011, 297–304 (color figure online)
ISO 13528:2015 recommends a ratio u(xpt)/σpt below 0.3 [15]. The standard uncertainty was calculated using the robust standard deviation (s*) and the total number of results (n); where s* was obtained by the algorithm A described in ISO 13528.
Figure 3 presents the trend of this ratio from 2007 to 2016. In the last rounds, the ratio was often fit for the intended use in accordance with the requirements from the ISO 13528:2015, corresponding to the period when laboratories applied routinely the QuEChERS method using GC–MS/MS and LC–MS/MS. In the first rounds, most participants used mainly gas chromatography as instrumental technique.
Two anomalous cases were inconsistent with other results: Fenthion Sulfoxide and Omethoate: well-known to be marked as difficult and tailing in particular in gas chromatography analysis [22].
Recovery results provide information on the trueness parameter. Figure 4 presents the mean recovery submitted by participants in the last 10 years versus concentration. 75 % of the recoveries fall in the range 80 % to 120 %. Recoveries should be determined over as wide a range of analyte concentration because the recovery of the compounds may be concentration dependent. At low concentrations, a larger recovery range is observed (from 70 % to 120 %); this could be attributed to potential chemisorption on the matrix or irreversible adsorbed onto surfaces of the analytical vessels. In these cases and typically with multi-residue methods, recoveries outside these ranges may be acceptable.
It is common practice that pesticide analysis results are not corrected for recovery, when the recovery rates range between 80 and 120 %, according to the method validation and quality control procedures for pesticide residues analysis in food and feed. When the average recovery is significantly different from 1, in the analysis of pesticide residues it is highly recommended to use the approach of standard addition or isotopically—labeled internal standard.
The uncertainty due to bias was estimated from the mean value of recoveries. Satisfactory values for recovery (with an average of 90 %) were submitted by participants in the last three PTs, mainly due to the implementation of the QuEChERS method.
Since the European regulator of the monitoring of pesticide residues in foodstuffs does not allow the correction of results for observed mean recoveries between 80 and 120 %, the results produced by different laboratories will agree significantly more if the same procedure is used by laboratories. In many fields, the widespread use of the same reference procedure ensures the comparability of measurement results. Bettencourt da Silva and Camões [23] discussed also the metrological merits of the use of a reference procedure such as QuEChERS method. This evaluation shows experimental evidences that defining a specific measurement procedure as a reference for measurements of pesticide residues in foodstuffs improves the agreement between results.
Taking into account the expanded uncertainties predicted by Horwitz and Thompson [24, 25] one could expect expanded uncertainties reported by laboratories of the order of 44 % for concentrations below 0.1 mg/kg. This value is in good agreement with the one we calculated value (standard deviation of 21 %), based on the recent results reported in the frame of our recent PTs, where laboratories applied the same analytical methodology: the QuEChERS method.
Conclusion
The QuEChERS procedure has become a popular methodology for the analysis of pesticide residues because of its simplicity, the use of low quantities of acetonitrile, the possibility to analyze a large number of pesticides with good recovery rates. Moreover, this procedure is an adaptable extraction platform that has been successfully implemented for a wide range of foodstuffs in many routine laboratories.
The generalized use of the QuEChERS for the sample preparation in the analysis of pesticide residues in foodstuffs can guarantee the comparability of the measurements produced by different laboratories.
The idea that the QuEChERS may harmonize the analysis of pesticide residue is now widespread. This procedure was successfully applied to the olive oil matrix. The systematic use of the QuEChERS in the analysis of pesticide residue may reduce the target relative standard deviation allowing laboratories to distinguish smaller deviation to the maximum residue level.
References
JCGM 200 (2012) International vocabulary of metrology – Basic and general concepts and associated terms (VIM). 3rd edn. JCGM member organizations (BIPM, IEC, IFCC, ILAC, ISO, IUPAC, IUPAP and OIML)
NORDEST Report TR 537 (2012) Handbook for calculation of measurement uncertainty in environmental, 3.1st edn. Nordic Innovation, Oslo, Norway
EURACHEM/CITAC Guide CG4 (2012) In: Ellison SLR, Williams A (eds) Quantifying uncertainty in analytical measurement, 3rd edn
EUROLAB Technical Report 1/2007: measurement uncertainty revised: alternative approaches to uncertainty evaluation, European Federation of National Associations of Measurement, Testing and Analytical Laboratories
European Commission. Directorate General for Health and Food Safety. SANTE/11813/2017 Guidance document on analytical quality control and method validation procedures for pesticides residues analysis in food and feed
Codex Alimentarius Commission Guideline CAC/GL 59-2006, Guidelines on estimation of uncertainty of results
Alder L, Korth W, Patey AL, van der Schee HA, Scoeneweiss S (2001) Estimation of measurement uncertainty in pesticide residue analysis. J AOAC Int 84(5):1569–1578
Stefanelli P, Generali T, Barbini DA, Girolimetti S, Dommarco R (2013) Uncertainty estimation in the analysis of pesticide residues in olive oil using data from proficiency tests. J Environ Sci Health 48:523–529
Garcia Reyes JF, Ferrer C, Gomez-Ramoz MJ, Molina-Diaz A, Fernandez-Alba R (2007) Determination of pesticide residues in olive oil and olives. Trends Anal Chem 26(3):239–251
Zhang L, Liu S, Cui X, Pan C, Zhang A, Chen F (2012) A review of sample preparation methods for the pesticide residue analysis in foods. Cent Eur J Chem 10(3):900–925
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in products. J AOAC Int 86:412–431
Tsipi D, Botitsi H, Economou A (2015) Mass spectrometry for the analysis of pesticide residues and their metabolites. Wiley, New Jersey
European Council. Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. Off J Eur Union L70/1
Chamkasem N, Harmon T (2015) Analysis of pesticides in olive oil using a modified QuEChERS method with LC-MS/MS and GC-MS/MS. J Regul Sci 3(1):16–35
International Organization for Standardization ISO 13528 (2015) Statistical methods for use in proficiency testing by interlaboratory comparison. International Standard. 2nd edn, 2015 08 01. Geneva, Switzerland
Di Muccio A, Pelosi P, Barbini DA, Generali T, Girolimetti S, Stefanelli P, Leonelli A (1999) Determination of pyrethroid pesticide residues in fatty materials by solid-matrix dispersion partition, followed by mini-column size-exclusion chromatography. J Chromatogr A 833:19–34
Patel K, Fussell RJ, Hetmanski D, Goodall BJ, Keely BJ (2005) Evaluation of gas chromatography-tandem quadrupole mass spectrometry for the determination of organochlorine pesticides in fats and oils. J Chromatogr A 1068:289–294
Lentza-Rizos C, Avramides EJ (1995) Pesticide residues in olive oil. Rev Environ Cont Toxicol 141:111–134
International Organization for Standardization ISO 21748 (2017) Guidance for the use of repeatability, reproducibility and trueness estimates in measurement uncertainty evaluation. 2nd edn, 2017 – 04. Geneva, Switzerland
Generali T, Stefanelli P, Girolimetti S, Barbini DA (2015) Proficiency tests on olive oil organized by the Italian National Reference Laboratory for pesticides: long-term performance of laboratories. Accred Qual Assur 20(4):247–253
Esturk O, Yakar Y, Ayhan Z (2014) Pesticide residue analysis in parsley, lettuce and spinach by LC-MS/MS. J Food Sci Technol 51(3):458–466
Data Pool of the EU Reference Laboratories for Residues of Pesticides. http://www.eurl-pesticides-datapool.eu. Accessed Feb 2018
Bettencourt da Silva RJN, Camões MF (2010) Comparability of measurement results for pesticide residues in foodstuffs: an open issue? Accred Qual Assur 15:691–704
Horwitz W (1997) A heuristic derivation of the horwitz curve. Anal Chem 69(4):789–790
Thompson M (2000) Recent trends in inter-laboratory precision at ppb and sub-ppb concentrations in relation to fitness for purpose criteria in proficiency testing. Analyst 125:385–386
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stefanelli, P., Generali, T., Girolimetti, S. et al. Evaluation of the reproducibility standard deviation in the pesticide multi-residue methods on olive oil from past proficiency tests. Accred Qual Assur 24, 19–24 (2019). https://doi.org/10.1007/s00769-018-1330-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00769-018-1330-z