Abstract
In this work, a commercially available, chirally pure pharmaceutical containing carboxylic group, namely, levofloxacin was utilized as chiral selector for the enantioresolution of sotalol. The TLC plates were prepared by mixing levofloxacin in silica gel slurry. The racemic mixture of sotalol was applied on TLC plates using MeCN–MeOH–H2O (5.2:2.0:0.8, V/V; pH = 5.0) as the mobile phase at 22 (± 2) °C. The separation conditions were optimized in terms of concentration of chiral selector, pH, and temperature to get successful separation. The resolution value was observed to be 2.6. The enantiomers so separated were isolated from TLC plates and characterized using various techniques. The method found to be linear with calibration equation [y = 0.79x + 0.054] and a regression coefficient (r2) value of 0.996. The limits of detection and quantification for the enantiomers were 2.3 μg/spot and 6.7 μg/spot, respectively. The recovery of the enantiomers isolated from TLC plates was in the range of 76–85%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
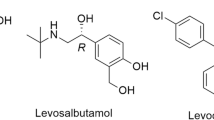
Sotalol (Fig. 1) (4-[1-hydroxy-2-(isopropylamino)ethyl]-methanesulfonanilide) (SOT) is a β-blocker, used in the treatment of hypertension, angina pectoris, and cardiac arrhythmia, and is available as racemate dosage formulations [1, 2]. However, unlike other β-blockers, its β-blocking activity is mainly featured to its (R)-enantiomer 30–60 times higher than its (S)-counterparts. As a result, SOT enantiomers produce different effects on the heart [3]. For instance, intoxication, control of therapeutic compliance, doping control, and pharmacokinetic interactions, etc. need selective, efficient and reproducible methods. Chiral switch from the racemic mixture to pure enantiomers provides improved therapeutics and pharmacokinetics through lower doses and diminished adverse consequences. The enantioresolution of SOT was carried out by high-performance liquid chromatography [4] and capillary electrophoresis [5]. However, these methods involve costly column and complicated optimization conditions. Bhushan and co-workers pioneered an approach based on thin-layer chromatography (TLC) (using home-made plates) for the successful resolution of enantiomers of a variety of analytes [6, 7]. Among a variety of approaches of liquid chromatographic techniques, TLC is recognized for its assured advantages, for example, simple, economical, easy optimization and for successful resolution together with the ability to take pictures of the chromatograms as noticeably visible proofs of resolution [8]. TLC is the most readily available approach for the enantioresolution of racemates and enantiomeric control of purity.
Levofloxacin (LFX; (S)-(‒)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid) (Fig. 1), a wide spectrum antibiotic belongs to the class of fluoroquinolone and is marketed as pure enantiomer at low cost. LFX contains a stereogenic center, polar carboxylic group and good chromophoric moiety (high π e− density); these characteristics make LFX a suitable candidate as a chiral selector (CS). Due to its high molar absorptivity and structural characteristics, it has been utilized as chiral derivatizing reagent for the resolution of racemic analytes [9].
Despite several advantages of TLC, there is no literature available on the enantioresolution of SOT. In view of this, we designed and developed a sensitive method for the enantioresolution of (RS)-SOT using LFX as CS in TLC. Further, the method was optimized for successful separation conditions by changing pH, temperature, and concentration of CS was investigated. Further, the developed method was validated for accuracy, precision, and limit of detection. The novelty of the present method lies in (1) the use of LFX as CS for the first time in TLC, and (2) separation of enantiomers of SOT using TLC.
2 Experimental
2.1 Chemicals and apparatus
Chirally pure pharmaceuticals, LFX (≥ 98.0%, anhydrous basis, \(\left[ \alpha \right]_{D}^{25}\) = − 104 ± 4° in chloroform) and (S)-SOT (\(\left[ \alpha \right]_{D}^{25}\) = + 36.2° [c, 1 M in MeOH]) were procured from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. The pharmaceutical tablet preparation, Sotalar (Cipla, Hyderabad, India) containing 40 mg of active pharmaceutical ingredient (API), i.e., SOT, was purchased from the local market. All the solvents used were of analytical reagent (AR) grade and obtained from Merck (Mumbai, India). Silica gel G (with 13% CaSO4 as binder) was also obtained from Merck, India. UV–Vis spectra were recorded on Hitachi U2001 spectrometer (Tokyo, Japan). The specific rotation of the isolated enantiomers was determined by using a polarimeter (model P3001RS; KRÜSS GmbH, Hamburg, Germany), and pH was measured with a CyberScan 510 pH meter (Thermo Fisher Scientific India Pvt. Ltd. Mumbai, India).
2.2 Extraction of API from tablets
The extraction, isolation, and purification of pharmaceuticals from tablets were carried out as per our earlier report [10]. Briefly, 25 Sotalar tablets (containing 40 mg of rac-SOT) were finely powdered in a mortar. The powder was taken in a conical flask containing 100 mL of water and the mixture was subjected to sonication for 10 min. The resulting solution was centrifuged at 3,000 rpm for 10 min; the residue was further extracted several times with water. The resulting clear solution was concentrated in a vacuum and recrystallized in MeOH. Melting points (m. pt.) of the purified compound were recorded and found to be consistent with racemic analyte, i.e., SOT (m. pt. 212 °C). The isolated compound was considered as racemic drug.
2.3 Stock solutions
Stock solutions of SOT (25 mg/mL) isolated from commercial formulations were prepared in distilled water and (S)-LFX (100 mM) was prepared in MeOH. Further, for optimization of the method, the working standard solutions of (RS)-SOT were prepared by diluting the stock solution with MeOH to obtain 5, 10, 15, 20, 25, and 50 μg/mL. In addition, the stock solution of the CS was diluted with MeOH to get the working stock solutions of concentrations 10, 20, 30, 40, 50, and 100 μg/mL.
3 Thin-layer chromatography
3.1 Preparation of TLC plates
The TLC plates were prepared by spreading the slurry of silica gel G (25 g in 50 mL of 10 mM (S)-LFX) over glass plates (dimension, 10 cm × 5 cm × 0.5 mm) using Stahl-type applicator. To investigate the effect of pH, TLC plates of different pH values, viz, 4, 5, 6, 7, 8, 9, 10 were prepared. For the preparation of TLC plates of pH 4–6, slurry of silica gel was prepared in dilute HCl and pH was suitably adjusted by varying the amount of dilute HCl in aqueous solution. Similarly, TLC plates of pH 8–10 were prepared by using aqueous triethylamine (TEA) in silica gel slurry. TLC plate of pH 7 was prepared by using slurry prepared in double distilled water. TLC plates were activated by placing plates in oven at a temperature of 60 °C overnight prior to use.
3.2 Development of chromatograms
The TLC plates were developed in ascending order from bottom to top using the mobile phase at room temperature (22 ± 2 °C) for 20 min. The TLC plates were then used for the separation of racemic analytes. 10 μL of the stock solution of analyte (10 mM) were applied on the TLC plates. The chromatograms were developed in a rectangular glass chamber containing mobile phase for about 10 min. The TLC plates were then dried at room temperature and placed in chamber containing iodine vapor. The plates show two spots representing the enantiomers of analytes.
3.3 Optimization of separation conditions
A number of mobile phase systems (binary, ternary and quaternary) containing varying compositions of different solvents such as MeCN, MeOH, DCM, CHCl3, and H2O along with organic modifiers like trifluoroacetic acid, and TEA were tried. The optimization of the separation conditions was carried out by changing the concentration of the CS, pH (both mobile phase and stationary phase), and temperature.
3.4 Extraction of separated enantiomers
The spots corresponding to the enantiomers of SOT were marked, and iodine was permitted to evaporate off. The silica gel corresponding to spots was scratched from about 50 plates and the silica gel so accumulated for every spot was extracted with water. Since LFX is feebly soluble in H2O, whereas SOT is freely soluble, the aqueous extract contains only SOT. The total extracts containing pure enantiomers were filtered and dried in a vacuum. To characterize the isolated enantiomers, melting point, λmax, and specific rotation were recorded for enantiomers of analyte drugs.
4 Results and discussion
4.1 Resolution of enantiomers through direct TLC
As discussed in the experimental section, chiral TLC was prepared and used for the separation of enantiomers of (RS)-SOT. Out of various mobile phase systems tried, the successful mobile phase combination was MeCN–MeOH–H2O (5.2:2.0:0.8, V/V, pH = 5.0) for the separation of enantiomers of SOT. The separation conditions were optimized in terms of the solvent system, change in temperature, and change in pH; the results obtained in terms of hRF (RF × 100) values, and Rs values are shown in Table 1S.
4.2 Effect of concentration of chiral selector
The TLC plates containing different amounts of the CS (10, 20, 30, 40, 50, and 100 μg/mL) were prepared for this purpose. It was observed that as the amount of CS increases from 10 to 50 μg/mL, the Rs value is not much affected; on further increase in the amount of CS, the shape of the chromatograms becomes eight-shaped or results in tailing. Therefore, the optimum amount of CS was considered as 10 μg/mL.
4.3 Effect of pH
The effect of the pH on the enantioresolution of SOT was investigated by varying the pH of both mobile phase and stationery phase. The TLC plates of different pH were prepared by adjusting the pH of slurry. The pH of the mobile phase was varied between 4 and 10. The results obtained indicate that good resolution was observed on the TLC plates with basic pH, i.e., with pH 8, 9 and 10; whereas either poor or no resolution was observed with TLC plates of neutral pH or acidic pH (i.e., pH 4, 5 and 6). TLC plates with pH 9 provided the best resolution. The pH of the mobile phase was maintained acidic, i.e., pH = 5.0.
4.4 Effect of temperature
The effect of temperature on the resolution of racemates of β-blockers was investigated by varying the temperature and its effect was identified in terms of tailing, shape of spots, and resolution. The experimental conditions were maintained, and the chamber was pre-equilibrated with mobile phases for about 20 min at different temperatures (18, 20, 22, 24 and 26 °C, where 22 °C was considered to be the room temperature). A good resolution was observed at a temperature near 22 (± 2) °C.
Through the above discussion, it can be proposed that varying the separation conditions (i.e., the concentration of CS, temperature, and pH), the resolution parameters are also affected. Only at certain conditions, good resolution can be observed. Any alteration in these factors resulted in poor or breakdown of resolution. Results of optimization of working temperature in terms of hRF (RF × 100), Rs value for the separation of enantiomers of SOT are presented in Table 2S.
4.5 Isolation of separated enantiomers
As described in the experimental section, the separated enantiomers were isolated and purified. The characterization data were found to be in good agreement with the pure enantiomeric forms of SOT [11]. The recovery of enantiomers from TLC plates was 76–85% of each enantiomer out of approximately 25 mg of amount applied on about 50 plates. The data obtained are as follows: First eluting enantiomer, m. pt. 203 °C; \(\left[ \alpha \right]_{D}^{25}\) = + 36.6° (c, 1 M in MeOH); UV, λmax = 234 nm; Second eluting enantiomer, m. pt. 204 °C; \(\left[ \alpha \right]_{D}^{25}\) = − 38.1° (c, 1 M in MeOH); UV, λmax = 231 nm. As per the specific rotation measurement, specific rotation of the first eluting enantiomer corresponds to (R)-enantiomer, where that of the second eluting enantiomer corresponds to (S)-enantiomer.
4.6 Mechanism of TLC separation
The separation of enantiomers in liquid chromatographic techniques has been considered to occur through a “three-point rule” [12] relating to a minimum of three simultaneous interactions with at least one being stereochemically dependent. The possible interaction may be an “electrostatic attraction, charge-transfer, dipole–dipole interactions, π–π interactions, H-bonding, and steric interaction”, etc. The best resolution was obtained with TLC plates with pH 9.0 and the mobile phase of pH 5.0. Under these conditions, the carboxylic group present in LFX becomes –COO−, whereas the secondary amino group present in the analyte (SOT) becomes −NH+ (Fig. 1S). Thus, it can be believed that (1) ionic interactions between –COO− of the CS and the cationic part of the analyte molecule, (2) H-bonding, and (3) steric interactions are playing a significant role, resulting in in situ formation of diastereomers. As the diastereomers show different physical properties, the in situ diastereomers of transitory nature get resolved in the given chromatographic environment.
To evaluate the elution order of the enantiomers, a sample of pure enantiomer, (S)-SOT was applied in parallel to the racemate, on the TLC plates. The photograph showing the chromatograms representing the racemic mixture and that of pure enantiomer, (S)-SOT is presented in Fig. 2. It was observed that the spot of the chromatogram of a pure enantiomer, (S)-SOT corresponds to the second eluting enantiomer from the racemic mixture. It can be concluded that the (S)-enantiomer interacted less firmly with the chiral stationary phase, and got eluted later.
4.7 Method validation
The racemic mixture of (RS)-SOT (0.01 M) was applied five times on chiral TLC plates to determine the repeatability of the method. The mean precision (as relative standard deviation, RSD) was in the range of 1.7–4.6% for the enantiomers of (RS)-SOT. Accuracy was determined by measuring the recovery of pure enantiomers from the racemic mixture. The recovery of the enantiomers was in the range of 76–85%. The results obtained revealed a good linear calibration fit between the band volume (concentration in micrograms) and the raw volume in the range of 5–30 µg/spot. The calibration curve was established by plotting band volume (y) versus raw volume (x) with the regression equation [y = 0.79x + 0.054] and a regression coefficient (r2) value of 0.996. The limit of detection (LOD) was calculated for nine bands that could be identified from the TLC plates. Whereas the limit of quantification (LOQ) was evaluated after applying (RS)-SOT (1.0 µg/spot) nine times and calculation of the RSD value of the response (raw volume). The LOD value was 2.3 µg/spot, and the LOQ was 6.7 µg/spot.
In addition, the enantiomeric purity was checked by using solutions containing 1% of the inactive form (S)-SOT in the presence of (R)-SOT (where (R)- and (S)-enantiomers were separated and isolated from the racemic mixture). Therefore, the present method can be utilized to determine the enantiomeric purity up to a 1% level of the active enantiomers required for quality control in pharmaceutical industries. Thus, the detection was successful up to 1% of analytes. Therefore, the method can be considered as a sensitive method for the detection of the enantiomers of SOT.
5 Conclusion
In the present work, a direct TLC method was developed and validated for the separation of (RS)-SOT. As LFX contains a stereogenic center, a polar carboxylic group and chromophoric moiety (probable π–π interaction), these characteristics make LFX a suitable candidate as CS. The present method provides advantages such as simplicity, easy optimization of separation condition and lower values of LOD and LOQ. Thus, it can be effectively applied for establishing and control of enantiomeric purity of β-blockers consistently in laboratories associated with regulatory agencies and pharmaceutical industries. The methods described in the literature (cited herein) require costly columns, tiresome procedures, and complicated optimization steps. Therefore, the present method can be utilized to determine the enantiomeric purity up to 1% level of the active enantiomers required for quality control in pharmaceutical industries.
References
Singh BN, Deedwania P, Nademanee K, Ward A, Sorkin EM (1987) Sotalol. Drugs 34:311–349
Antonaccio MJ, Gomoll A (1990) Pharmacology, pharmacodynamics and pharmacokinetics of sotalol. J Am Coll Cardiol 65:12–21
Vashistha VK, Kumar A (2020) Stereochemical facets of clinical β-blockers: an overview. Chirality 32:722–735
Schlauch M, Fulde K, Frahm AW (2002) Enantioselective determination of (R)-and (S)-sotalol in human plasma by on-line coupling of a restricted-access material precolumn to a cellobiohydrolase I-based chiral stationary phase. J Chromatogr B 775:197–207
Hancu G, Sămărghiţan C, Rusu A, Mircia E (2014) Sotalol chiral separation by capillary electrophoresis. J Chil Chem Soc 59:2559–2562
Bhushan R, Thiongo GT (1998) Direct enantioseparation of some β-adrenergic blocking agents using impregnated thin-layer chromatography. J Chromatogr B 708(1–2):330–334
Bhushan R, Parshad V (1996) Thin-layer chromatographic separation of enantiomeric dansylamino acids using a macrocyclic antibiotic as a chiral selector. J Chromatogr A 736(1–2):235–238
Vashistha VK, Bhushan R (2020) Thin-layer chromatographic enantioseparation of atenolol and propranolol using (S)-naproxen as chiral selector: direct and indirect approaches. J Planar Chromatogr 33:101–107. https://doi.org/10.1007/s00764-020-00017-0
Vashistha VK, Martens J, Bhushan R (2017) Sensitive RP-HPLC enantioseparation of (RS)-ketamine via chiral derivatization based on (S)-levofloxacin. Chromatographia 80:1501–1508
Vashistha VK, Bhushan R (2015) Chirality recognition for assessing the enantiomeric purity of betaxolol. Tetrahedron Asymmetry 26:304–311
Foster RT, Carr RA (1992) Sotalol. In: Brittain HG (ed) Analytical profiles of drug substances and excipients. Academic Press, Cambridge, p 501
Dalgliesh CE (1952) The optical resolution of aromatic amino-acids on paper chromatograms. J Chem Soc 137:3940–3942
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vashistha, V.K., Kumar, A. Development of a thin-layer chromatographic method for the enantioresolution of sotalol using levofloxacin as chiral selector. JPC-J Planar Chromat 33, 663–667 (2020). https://doi.org/10.1007/s00764-020-00068-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00764-020-00068-3