Abstract
Our goal was to examine associations of infant sleep and feeding patterns with maternal sleep and mood among women at risk for postpartum depression. Participants were 30 women (age ± SD = 28.3 ± 5.1 years) with a history of MDD (but not in a mood episode at enrollment) who completed daily sleep diaries, wore wrist actigraphs to estimate sleep, and had their mood assessed with the Hamilton Depression Rating Scale (HAM-D-17) during four separate weeks of the perinatal period (33 weeks pregnancy and weeks 2, 6, and 16 postpartum). They logged their infants’ sleep and feeding behaviors daily and reported postnatal stress on the Childcare Stress Inventory (CSI) at week 16. Mothers’ actigraphically estimated sleep showed associations with infant sleep and feeding patterns only at postpartum week 2. Shorter duration of the longest infant-sleep bout was associated with shorter maternal sleep duration (p = .02) and lower sleep efficiency (p = .04), and maternal sleep efficiency was negatively associated with the number of infant-sleep bouts (p = .008) and duration of infant feeding (p = .008). Neither infant sleep nor feeding was associated with maternal sleep at 6 or 16 weeks, but more disturbed infant sleep and more frequent feeding at 6 weeks were associated with higher HAM-D scores at 6 and 16 weeks and higher CSI scores. Sleep in the mother-infant dyad is most tightly linked in the early postpartum weeks, but mothers continue to experience disturbed sleep and infant sleep and feeding behaviors continue to be associated with mothers’ depressive symptoms and stress ratings as long as 16 weeks postpartum. These data imply that interventions designed to improve maternal sleep and postpartum mood should include both mothers and infants because improving infant sleep alone is not likely to improve maternal sleep, and poor infant sleep is linked to postpartum depression and stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many reports confirm that women experience significant sleep disruption during pregnancy and the postpartum period (Coble et al. 1994; Doan et al. 2007; Driver and Shapiro 1992; Gay et al. 2004; Goyal et al. 2007; Hedman et al. 2002; Horiuchi and Nishihara 1999; Karacan et al. 1969; Kennedy et al. 2007; Lee et al. 2000a, b; Lee and Lee 2007; Mindell and Jacobson 2000; Montgomery-Downs et al. 2010b; Nishihara and Horiuchi 1998; Parry et al. 2000; Signal et al. 2007; Swain et al. 1997; Wilkie and Shapiro 1992; Wolfson et al. 2003), and several studies have shown associations between disturbed maternal sleep and development of postpartum depressed mood (Dennis and Ross 2005; Goyal et al. 2007, 2009; Lee et al. 2000a; Swain et al. 1997). Although infant sleep and feeding patterns likely contribute to maternal postpartum sleep disturbance, associations of infant sleep and feeding with maternal sleep and mood are understudied, particularly among women at high risk for perinatal depression.
Epidemiologic studies show associations between maternal reports of infant-sleep problems and increased maternal depressive symptoms in the concurrent postpartum period. For example, a study of 738 Australian mothers of 6–12-month-old infants showed that women who reported that their baby had a sleep problem were more than twice as likely to score above the clinical threshold on the Edinburgh Postnatal Depression Scale (EPDS) relative to those who did not (Hiscock and Wake 2001). Similarly, a study of 505 postpartum women who did not exhibit high EPDS scores at postpartum week 1 showed that mothers reporting three or more infant awakenings per night and less than 6 h maternal sleep in a 24-h period had clinically-significant EPDS scores at 4 and 8 weeks postpartum (Dennis and Ross 2005).
Armitage and colleagues (Armitage et al. 2009) compared infant sleep measured with actigraphy at ages 2 and 24 weeks in 11 infants whose mothers had a diagnosis or history of a major depressive disorder (MDD) versus 7 infants born to women with no personal or family history of depression. The infants of mothers with MDD took longer to fall asleep and had shorter nighttime sleep durations, decreased sleep efficiency, and more night awakenings at both ages. Thus, this study showed that associations between maternal history of MDD and infant-sleep behavior are evident at a very early developmental stage (i.e., at week 2 of life) and that sleep differences in infants born to women with MDD persist for at least 6 months. This study did not examine associations between infant sleep and maternal sleep, however, and maternal mood symptoms were not reported.
Infant feeding behaviors have also been examined with respect to maternal sleep and mood. A large epidemiologic study demonstrated that new mothers who were breastfeeding reported that they had longer sleep durations and shorter sleep latencies than formula-feeding women (Kendall-Tackett et al. 2013). In addition, studies that measured sleep objectively with actigraphy in breastfeeding and formula-feeding mothers showed that women who breast fed had equivalent (e.g., (Montgomery-Downs et al. 2010a)) or longer (e.g.,(Doan et al. 2014)) sleep durations than new mothers who used formula. With respect to feeding and mood, a recent survey of 272 mothers of infants up to 24-months old showed that maternal reports of infant sleep and feeding problems were associated independently with higher maternal depression scores on the EPDS (Muscat et al. 2014).
Several studies have measured infant sleep with respect to maternal sleep, depression, or stress in the context of interventions aimed at improving infant sleep and thereby the outcomes for new parents, e.g., (Hiscock et al. 2007; Hiscock and Wake 2002; Mindell et al. 2011; Wolfson et al. 1992). Though methods in these trials varied, several showed that the interventions improved parental reports of infant sleep as well as maternal self-reported mood, stress, or sleep disturbance in those who received the interventions compared to controls. This area of research is not without controversy, however, as one recent systematic review of 43 such studies indicated that behavioral interventions aimed at improving infant sleep during the first 6 months of life do not improve long-term outcomes for mothers or infants and may increase risk of unintended outcomes, such as early discontinuation of breastfeeding and increased anxiety among new mothers (Douglas and Hill 2013).
Complementary to studies on improving infant sleep, a small number of intervention trials have focused on improving maternal sleep during the postpartum period (Lee and Gay 2011; Stremler et al. 2006, 2013; Swanson et al. 2013). These interventions used actigraphy or sleep diaries to measure maternal sleep but did not measure infant sleep. Furthermore, the interventions had mixed results in improving maternal sleep, but taken together, the data indicate that women at higher risk of postpartum sleep problems are more likely to benefit from sleep intervention.
In summary, despite a burgeoning interest in research on perinatal sleep and maternal mood, gaps in this literature remain, including describing associations between maternal sleep and infant sleep across the early weeks of development and associations of these sleep patterns with maternal mood and stress. A deeper understanding of sleep in the mother-infant dyad, particularly in women at high risk for postpartum sleep and mood difficulties, likely is necessary to develop more effective interventions to improve both maternal and infant sleep. Thus, the aims of this study were (1) to examine associations between maternal sleep and infant sleep and feeding patterns at 2, 6, and 16 weeks postpartum in women with a history of MDD and (2) to assess associations between infant sleep and feeding and maternal depressed mood and maternal stress during the postpartum period. We hypothesized that shorter, more interrupted infant sleep and longer and more frequent feedings would be associated with more maternal sleep disruption, higher depression scores on the Hamilton Depression Rating Scale, and higher reported stress on the Childcare Stress Inventory.
Methods
Participants
We recruited expectant mothers ages 18–40 at ∼33 weeks gestation calculated by last menstrual period who fulfilled DSM-IV criteria for history of MDD and were not in a current mood episode. Interested women telephoned the laboratory in response to flyers, brochures, newspaper advertisements, and a direct mailing. History of MDD and absence of a mood episode at enrollment were confirmed by a Structured Clinical Interview for DSM-IV Disorders (SCID I/P, (First et al. 2002)). We excluded potential participants with a primary Axis I diagnosis other than MDD; learning disability, mental retardation, or developmental delay; diagnosis of a sleep disorder (including primary insomnia); high-risk pregnancy; current employment as night shift worker; disability that interfered with testing; current alcohol/drug dependence; or expectation that infants would not be living in the home or would have a nighttime caregiver other than the mother. Women with comorbid anxiety disorders were not excluded. We did not select participants on the basis of parity, feeding plans, tobacco use, or use of antidepressants, anxiolytics, antipsychotics, or mood stabilizers. The Rhode Island Hospital and Women and Infants Hospital institutional review boards approved the study. Participants gave signed informed consent and were paid for participating.
Maternal sleep measurements
Sleep measures were taken for one week each at 3rd trimester (∼33 weeks gestation) and at postpartum weeks 2, 6, and 16. Participants completed a daily sleep/wake diary developed for perinatal women (Wolfson et al. 2003) and called the laboratory’s time-stamped voicemail each day when they woke up. Diary items include bedtime, rising time, amount of sleep, naps, and times when they removed the actigraph, as well a chart to record their infant’s sleep (see below).
Women wore a wrist actigraph (Octagonal Basic or Micro Motionlogger Watch, AMI, Ardsley, NY) continuously on the nondominant wrist during each study week. Actigraphy data were recorded in 1-min bins using the zero-crossing mode with a filter setting of 18 and were analyzed using Action-W software (AMI) algorithm, which has been validated with polysomnography (Sadeh et al. 1994). We estimated the following sleep measures from actigraphy: sleep onset time (first of three continuous epochs of sleep occurring after the bedtime reported on the sleep diary), sleep offset time (last epoch of 5 continuous epochs of sleep occurring before the wake time reported on the sleep diary), time in bed (TIB, hours between sleep onset and sleep offset), total sleep time (TST, hours of estimated sleep occurring between sleep onset and sleep offset), and sleep efficiency (TST ÷ TIB * 100). We scored sleep periods occurring between sleep offset and sleep onset as naps. To distinguish between daytime sleep episodes and periods when the participant had removed the actigraph or was engaged in quiet wakefulness, naps identified by actigraphy were confirmed by sleep diary and/or by questioning participants about periods of inactivity when we downloaded the actigraph.
Infant-sleep measures
We derived the following sleep measures from the “Baby’s Sleep/Wake Chart” included on the mother’s daily sleep/wake diary and calculated weekly averages for each measure (see Fig. 1 for an example of a completed sleep chart):
-
Number of infant-sleep bouts per 24 h where one bout was defined as the infant going to sleep and waking up.
-
Infant-sleep duration: duration of sleep (in hours, reported in 15-min increments) in all sleep bouts per 24 h.
-
Infant bedtime: the last time the infant fell asleep with no awakenings before the mother went to bed. If the infant was awake at the time of the mother’s actigraphically estimated sleep onset, infant bedtime was defined as the beginning of the first infant-sleep episode lasting longer than 30 min after the mother’s sleep onset.
-
Mother-infant bedtime difference: difference between mother and infant bedtimes in minutes; larger values indicate a longer interval of maternal wakefulness after infant bedtime.
-
Longest infant night sleep bout (h): duration of the longest interval that the infant slept continuously during the mother’s actigraphically measured sleep time.
-
Number of infant feeds per 24 h where each feed was defined as a discreet interval of continuous feeding.
-
Feed duration: total duration of infant feeding (reported in 15-min increments) per 24 h.
Participants received the sleep diary booklet that contained the “Baby’s Sleep/Wake Chart” on the first day of each study week, but since the drop-off times varied from person-to-person and from week-to-week, variables for maternal and infant sleep were taken from bedtime on day 1 through wake time on day 7 provided that diaries were complete and the actigraph was worn. Data from individual diary days less than 50 % complete were excluded from analyses. Reliability of diary scoring was determined by comparing two scorers (INI and KMS) in a randomly selected sample of all records. Eleven percent of the records was double-scored and showed high inter-scorer reliability; for example, Kappa was 0.83 for infant-sleep duration.
Maternal mood and stress measures
We assessed symptoms of depressed mood with the 17-item Hamilton Rating Scale for Depression (HAM-D, (Hamilton 1960)) performed by a trained, board-certified psychiatrist (KMS) at the end of each monitoring week. Maternal stress was measured at 16 weeks postpartum with the Childcare Stress Inventory (CSI) (Cutrona 1983), which asks how many of 21 stressful experiences occurred for them since the baby was born. Previous work has shown an average of five stress items endorsed by postpartum women (Cutrona 1983). Among our participants, Cronbach’s alpha was 0.86 for the CSI.
Statistical analyses
We used SPSS Version 19 (IBM, Chicago, IL) and SAS version 9.4 (The SAS Institute, Cary, NC) for data analysis. Data are summarized as means with standard deviations or 95 % confidence intervals. Changes across time in infant sleep and feeding measures and maternal sleep patterns were examined with analysis of variance, and post-hoc comparisons were made using Tukey HSD. We modeled associations between infant and maternal sleep using generalized mixed linear models (SAS proc glimmix) to account for the expectation that individual participants’ data would be correlated over time and to provide flexibility with regards to the distributions of the dependent variables. Each model contained terms for the fixed effects of time point, the independent variable of interest, and their interaction (reported as an F-statistic). Subsequently, the slopes of the associations between the independent variable with the dependent variable were tested separately for each postpartum week (reported as a t-statistic) with alpha maintained at 0.05 across these multiple tests using the Holm method.
Results
Participants
Thirty women (mean age ± SD = 28.3 ± 5.1 years) were eligible for inclusion in these analyses. Twenty-eight women participated at all four time points; two women did not complete the 16-week assessment. Ten participants (33 %) were first-time mothers, and the median number of children among those who were already mothers was 1 (range 1–3 children). Median number of lifetime depressive episodes was 2 (range = 1–10 episodes). Other measures describing the sample are shown in Table 1.
Actigraphically estimated maternal sleep
Maternal sleep measures estimated with wrist actigraphy at the 3rd trimester and 2, 6, and 16 weeks postpartum are shown in Table 2. Average nighttime sleep time was just over 6 h at 2 weeks postpartum and by 16 weeks postpartum had increased to an average of about 6.5 h, which is not a statistically significant change. Average maternal sleep efficiency was lowest at postpartum week 2 at 72.4 %, but increased significantly to 82.7 % by 16 weeks postpartum, back to 3rd trimester levels. Analysis of variance showed main effects of perinatal week for time in bed, minutes of wakefulness, and sleep efficiency. Post-hoc analyses showed significantly longer time in bed at 2 weeks compared with 16 weeks postpartum, significantly greater nocturnal wake at 2 and 6 weeks postpartum compared with 33 weeks gestation and 16 weeks postpartum, and significantly lower sleep efficiency at 2 and 6 weeks postpartum compared with 33 weeks gestation and 16 weeks postpartum. Sleep onset and sleep offset times did not differ significantly across the perinatal period in our sample.
Median number of naps was 2 at the 3rd trimester and postpartum weeks 2 and 6 and 1 nap at week 16. All participants reported napping at least one time during the study period. The number of women who reported zero naps at the 3rd trimester was five, four at postpartum week 2, five at postpartum week 6, and nine at postpartum week 16. Number of naps, nap length, and nap duration did not differ across the perinatal period.
Maternally reported infant sleep
We attempted to include as many infant-sleep diaries as possible for each participant at each time point. We were able to include 92 % of diary days from postpartum week 2, 92 % from week 6, and 81 % at week 16. The main reason for individual day non-inclusions was that >50 % of the diary was incomplete. Median completed diary days was 7.
Infant-sleep data are summarized in Table 3. At 2 weeks postpartum, mothers reported average infant-sleep times of 15.3 h per day, with sleep divided into ∼8 bouts per day; mean feeding duration was 3.9 h per day. Infant behavior showed expected developmental changes from postpartum week 2 to postpartum week 16, including fewer bouts of sleep per 24 h, decreased sleep duration, fewer feedings, shorter total feeding duration, and longer sleep bouts at night. Infants’ “bedtimes” shifted earlier from week 2 to week 16, while the interval between infant bedtime and mothers’ actigraphically estimated sleep onset lengthened from postpartum week 2 to postpartum week 16.
Associations between infant sleep and feeding and maternal nighttime sleep
The GLIMMIX procedure showed significant overall fixed effects of infant’s longest sleep bout on maternal sleep efficiency (F 1,46 = 9.37, p = .004), number of infant-sleep bouts on maternal sleep efficiency (F 1,48 = 10.40, p = .002), and infant feeding duration on maternal sleep time (F 1,48 = 4.50, p = .04), as well as trends for fixed effects of infant’s longest sleep on maternal sleep time (F 1,46 = 3.06, p = .09) and for infant feeding duration on maternal sleep efficiency (F 1,48 = 2.86, p = .09).
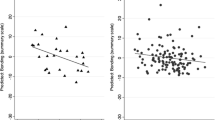
Further analyses of the slopes of individual weeks revealed significant associations between infant sleep and feeding patterns and maternal nighttime sleep at 2 weeks postpartum. Specifically, at postpartum week 2, shorter duration of longest infant-sleep bout was associated with shorter maternal sleep duration (t 46 = 2.89, adjusted p = .02, Fig. 2a) and lower maternal sleep efficiency (t 46 = 2.55, adjusted p = .04, Fig. 2b). In addition, at 2 weeks postpartum, maternal sleep efficiency was negatively associated with number of infant-sleep bouts (t 48 = −3.16, adjusted p = .008, Fig. 2c) and with duration of feeding (t 48 = −2.59, adjusted p = .008, Fig. 2d).
Associations of infant sleep and feeding with maternal sleep at 2, 6, and 16 weeks postpartum. Solid lines represent the slopes of the associations between longest infant nighttime sleep bout and maternal TST (a), longest infant nighttime sleep bout and maternal sleep efficiency (b), number of daily infant sleep bouts and maternal sleep efficiency (c), and average daily duration of infant feeding and maternal sleep efficiency (d). Shaded bars indicate the 95 % confidence intervals around the slope estimates
We did not observe similar associations at postpartum weeks 6 and 16. Infant total sleep time, infant bedtime, difference between infant and maternal bedtimes, and number of feeds were not associated significantly with maternal sleep measures at any time point.
Maternal depression and stress and associations with infant sleep
Average HAM-D scores were 6.9 ± 3.6 at 33 weeks gestation, 7.9 ± 5.3 at 2 weeks postpartum, 8.1 ± 4.1 at 6 weeks postpartum, and 9.2 ± 7.4 at 16 weeks postpartum. We observed HAM-D scores greater than 13 (indicating moderate depression) in two participants at 33 weeks gestation, four at week 2, three at week 6, and six at week 16.
There was an overall fixed effect of average number of reported infant-sleep bouts on maternal HAM-D score (F 1,48 = 5.43, p = .02), with a higher number of bouts associated with more depressive symptoms; associations at 2 weeks (t 48 = 2.05, adjusted p = .09) and 6 weeks (t 48 = 2.46, adjusted p = .052) postpartum accounted for this effect. This finding was consistent, but significance was reduced to a trend, when maternal sleep items were removed from the calculated HAM-D score (F 1,48 = 3.68, p = .06).
Average CSI score at week 16 was 6.2 ± 4.7, range 0–19. Lower total infant-sleep duration at postpartum weeks 2 (F 1,27 = 7.81, p = .009) and 6 (F 1,25 = 5.73, p = .02) were associated with a higher number of stressors reported by mothers at week 16, but this finding was reduced to a trend at week 16 (F 1,23 = 3.31, p = .08). Number of infant-sleep bouts at week 16, however, was positively associated with mothers’ reported stressors at week 16 (F 1,23 = 12.57, p = .002).
Feeding behaviors also showed an association with maternal reported stress on the CSI. Report of a higher number of stressors at week 16 was associated with a higher number of daily feedings at postpartum weeks 2 (F 1,26 = 8.67, p = .007) and 6 (F 1,26 = 8.19, p = .008), with a similar trend observed at week 16 (F 1,23 = 3.23, p = .09).
Discussion
These analyses show associations of infant sleep and feeding behaviors with maternal nighttime sleep efficiency and sleep duration in the second week postpartum. We observed lower sleep efficiencies and shorter sleep durations among mothers who reported that their infants had shorter bouts of sleep at night during postpartum week 2. We also found an association between longer reported infant daily feeding durations at two weeks and less maternal sleep. These data indicate that maternal sleep is linked to her reports of infant sleep and feeding behaviors in the early postpartum period. After 2 weeks postpartum, however, associations between infant sleep and feeding patterns and maternal sleep were not seen. Thus, although average maternal sleep durations measured with actigraphy (week 2 mean TST = 6.2 h, week 6 mean TST = 6.3 h) and maternally reported infant-sleep durations (week 2 mean TST = 15.3 h, week 6 mean TST = 14.5 h) did not change significantly from 2 to 6 weeks postpartum, maternal and infant sleep behaviors were not coupled as tightly after 2 weeks postpartum. This implies that factors other than infant sleep and feeding patterns impact maternal sleep in later weeks.
We are not aware of comparable studies that have assessed associations between maternal and infant sleep at various time points. Indeed, the few studies that have assessed both maternal and infant sleep have not focused on associations between sleep behaviors in the mother-infant dyad (e.g., (Quillin and Glenn 2004)). Behavioral patterns within the mother-infant dyad undergo dynamic changes during the early postpartum weeks (Acebo and Thoman 1995; Anders et al. 1992), and we may have been unable to show associations in maternal and infant-sleep patterns after 2 weeks because of variability in infant development, as well as differences and changes in maternal responsiveness during that time period. Nevertheless, the data presented here imply that infant behaviors are not the main factor driving sleep disturbance in new mothers after 2 weeks postpartum. This interpretation is supported by the work of Doering (Doering 2013) who examined sleep in 143 socioeconomically disadvantaged women at 2, 4, and 8 weeks postpartum. Although waking at night to care for their infant was the most commonly reported source of sleep disturbance among these new mothers, their sleep was also disturbed by factors known to contribute to shortened or disturbed sleep in the general adult population. For instance, at 4 weeks postpartum, 84 % of participants reported caffeine use, 26 % reported smoking cigarettes, >20 % reported sleeping with a child other than the infant in her bed, and 52 % reported sleeping with the television on for all or part of the night.
Our data on maternal and infant-sleep times are similar to those reported in the literature at similar time points. For instance, Quillin and Glenn (Quillin and Glenn 2004) reported an average maternally reported infant-sleep time of 13.9 h and maternal nocturnal sleep time of 5.8 h at 4 weeks postpartum in their study comparing sleep between breast- and bottle-feeding mothers. Lee and colleagues (Lee et al. 2000b) reported a mean polysomographically recorded sleep duration of 379 min in mothers at 3–4 weeks postpartum, which is nearly identical to our sleep durations estimated with actigraphy. In our sample, new mothers obtained an average of just over 6 h of nighttime sleep during postpartum weeks 2 and 6, with ranges across women of 4.7–7.8 h at 2 weeks postpartum and 4.7–8.1 h at 6 weeks postpartum. By 16 weeks, average sleep duration had increased to only 6.5 h with a range of 3.8–8.5 h. Although napping was common in our sample, on average, naps added less than 20 min of sleep per day to the mother’s total sleep time. This represents a pattern of insufficient sleep and also indicates that maternal sleep disturbance did not improve much from 2 weeks postpartum to 16 weeks postpartum. Such chronically short sleep durations would be expected to produce impairments in cognition, mood, and physiologic regulation (Basner and Dinges 2012; Insana et al. 2013; Vgontzas et al. 2004). Nevertheless, there are no guidelines for perinatal women and the clinicians who care for them regarding how severe and for how long shortened and disturbed sleep should be tolerated before treatment is considered during pregnancy and the postpartum period. Further work is needed in this area.
Though infant sleep and feeding patterns did not show significant associations with maternal sleep after 2 weeks postpartum in our sample, we did find associations between infants’ sleep and feeding and new mothers’ depression and stress during later postpartum weeks. Mothers who reported more infant-sleep bouts at 6 weeks were rated as more depressed, and those who reported less infant sleep and more feeding bouts at 2 and 6 weeks postpartum reported more postpartum stress. This is consistent with previous reports in the literature showing associations between reports of infant-sleep problems and maternal depressed mood (Dennis and Ross 2005; Hiscock and Wake 2001). Thus, although maternal sleep in our sample only tracked infant sleep in the very early postpartum weeks, infant sleep and feeding behaviors continue to associate with mothers’ mood and stress ratings at later postpartum time points. Taken together, these findings indicate that interventions designed to improve maternal sleep and mood must focus on both mother and infant because improving infant sleep alone is not likely to improve maternal sleep and poor infant sleep can contribute to maternal depression and stress even if it is not affecting sleep directly.
Studies examining the maternal infant dyad often raise questions about whether the phenomena of interest represent “nature” or “nurture.” In other words, are infants born to mothers with a history of depression more likely to have sleep disruption for biological reasons, or do women with a history of depression treat their infants differently, thus resulting in more sleep problems in their infants? Armitage and colleagues’ data shows that infants with “exposure” to a mother with a history of depression had more sleep disturbance than controls as early as 2 weeks postpartum (Armitage et al. 2009) regardless of the mother’s symptoms at the time of the assessment, suggesting that factors other than maternal behavior—i.e., in utero environment or genetics—likely play a role in sleep behavior in infants. This notion is supported by animal studies that show differential responsiveness of circadian and sleep behavior in mice pups depending on their mothers’ prenatal light-dark exposures (Ciarleglio et al. 2011). We speculate that expectant mothers’ prenatal sleep behaviors may influence infants’ behavioral regulation and that biological and behavioral factors during pregnancy and the postpartum period contribute to the development of sleep and feeding patterns.
Our data have implications for interventions designed to improve maternal and infant sleep and prevent perinatal depression. If maternal sleep patterns during pregnancy do indeed forecast infant behavioral outcomes, early interventions, i.e., during pregnancy, may result in improved sleep in mother and child after birth. Treatment strategies that address sleep problems during pregnancy may also improve obstetric outcomes, since disturbed sleep during pregnancy has been shown to predict untoward birth outcomes, such as increased pain perception (Beebe and Lee 2007; Lee and Gay 2004). Introducing a sleep intervention during pregnancy also has the practical advantage of allowing expectant mothers time to focus on their sleep before the arrival of the infant. If future studies show that improving maternal sleep during pregnancy has lasting positive effects postpartum for mother and baby, this may improve compliance with such treatments, particularly if clinicians promote healthy sleep in expectant mothers instead of ascribing perinatal sleep difficulties to pregnancy or a new baby regardless of duration or severity of symptoms.
One limitation of this study is that we examined a modestly sized, heterogeneous sample of women with a previous history of depression. Thus, since our study focused on women who are vulnerable to postpartum depression, our findings may not generalize to non-depressed mothers and their infants. That being said, relatively few of our participants developed a postpartum major depressive episode during the study, and we speculate that we may have observed greater sleep disturbance among new mothers and their infants if more had experienced postpartum depression. In addition, our measure of infant sleep was through maternal self-report, and although reported infant sleep behaviors followed expected developmental trends, mothers’ reports of infant sleep might be different than objective measures, i.e., actigraphy or direct observation, in infants. Another limitation is that although most women in our sample reported some involvement with the father of the baby, we did not assess the quality or quantity of support provided by fathers or others, and these factors likely impact mothers’ sleep, mood, and stress levels. Similarly, we did not measure infants’ sleeping arrangements (i.e., co-sleeping), the impact of the infants’ siblings on maternal sleep, or other characteristics of the infant that might impact the mother-infant relationship, e.g., excessive crying, colic, or feeding difficulties. More “difficult” infants might be expected to increase mood disturbances in new mothers. Finally, we acknowledge that associations between infant behaviors and maternal sleep disturbance, stress, and depression are likely bidirectional, and our study did not assess causality. Nevertheless, we note that it has been argued that direction of influence within the mother-infant is not a meaningful or useful construct in the early postpartum period because the dyad is so intertwined at this time (Acebo and Thoman 1995).
In summary, in our sample of perinatal women and their infants, insufficient maternal sleep was evident at 2, 6, and 16 weeks postpartum but was related significantly to infant-sleep patterns only at postpartum week 2. Nevertheless, more disturbed infant sleep continued to be associated with higher maternal stress and depressed mood at later time points. Interventions aimed at preventing postpartum depression and improving postpartum sleep should focus on both members of the mother-infant dyad.
References
Acebo C, Thoman EB (1995) Role of infant crying in the early mother-infant dialogue. Physiol Behav 57:541–547
Anders TF, Halpern LF, Hua J (1992) Sleeping through the night: a developmental perspective. Pediatrics 90:554–560
Armitage R, Flynn H, Hoffmann R, Vazquez D, Lopez J, Marcus S (2009) Early developmental changes in sleep in infants: the impact of maternal depression. Sleep 32:693–696
Basner M, Dinges DF (2012) An adaptive-duration version of the PVT accurately tracks changes in psychomotor vigilance induced by sleep restriction. Sleep 35:193–202. doi:10.5665/sleep.1620
Beebe KR, Lee KA (2007) Sleep disturbance in late pregnancy and early labor. J Perinat Neonatal Nurs 21:103–108. doi:10.1097/01.JPN.0000270626.66369.26
Ciarleglio CM, Axley JC, Strauss BR, Gamble KL, McMahon DG (2011) Perinatal photoperiod imprints the circadian clock. Nat Neurosci 14:25–27. doi:10.1038/nn.2699
Coble PA, Reynolds CF 3rd, Kupfer DJ, Houck PR, Day NL, Giles DE (1994) Childbearing in women with and without a history of affective disorder. II. Electroencephalographic sleep. Compr Psychiatry 35:215–224
Cutrona CE (1983) Causal attributions and perinatal depression. J Abnorm Psychol 92:161–172
Dennis CL, Ross L (2005) Relationships among infant sleep patterns, maternal fatigue, and development of depressive symptomatology. Birth 32:187–193. doi:10.1111/j.0730-7659.2005.00368.x
Doan T, Gardiner A, Gay CL, Lee KA (2007) Breast-feeding increases sleep duration of new parents. J Perinat Neonatal Nurs 21:200–206. doi:10.1097/01.JPN.0000285809.36398.1b
Doan T, Gay CL, Kennedy HP, Newman J, Lee KA (2014) Nighttime breastfeeding behavior is associated with more nocturnal sleep among first-time mothers at one month postpartum. J Clin Sleep Med 10:313–319. doi:10.5664/jcsm.3538
Doering JJ (2013) The physical and social environment of sleep in socioeconomically disadvantaged postpartum women. J Obstet Gynecol Neonatal Nurs 42:E33–E43. doi:10.1111/j.1552-6909.2012.01421.x
Douglas PS, Hill PS (2013) Behavioral sleep interventions in the first six months of life do not improve outcomes for mothers or infants: a systematic review. J Dev Behav Pediatr JDBP 34:497–507. doi:10.1097/DBP.0b013e31829cafa6
Driver HS, Shapiro CM (1992) A longitudinal study of sleep stages in young women during pregnancy and postpartum. Sleep 15:449–453
First M, Spitzer R, Gibbon M, Williams J (2002) Structured clinical interview for DSM-IV-TR axis 1 disorders, research version, patient edition (SCID-I/P). Biometrics Research, New York State Psychiatric Institute, New York
Gay CL, Lee KA, Lee SY (2004) Sleep patterns and fatigue in new mothers and fathers. Biol Res Nurs 5:311–318. doi:10.1177/1099800403262142
Goyal D, Gay CL, Lee KA (2007) Patterns of sleep disruption and depressive symptoms in new mothers. J Perinat Neonat Nurs 21:123–129
Goyal D, Gay C, Lee K (2009) Fragmented maternal sleep is more strongly correlated with depressive symptoms than infant temperament at three months postpartum. Arch Womens Ment Health 12:229–237. doi:10.1007/s00737-009-0070-9
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62
Hedman C, Pohjasvaara T, Tolonen U, Suhonen-Malm AS, Myllyla VV (2002) Effects of pregnancy on mothers’ sleep. Sleep Med 3:37–42
Hiscock H, Wake M (2001) Infant sleep problems and postnatal depression: a community-based study. Pediatrics 107:1317–1322
Hiscock H, Wake M (2002) Randomised controlled trial of behavioural infant sleep intervention to improve infant sleep and maternal mood. BMJ 324:1062–1065
Hiscock H, Bayer J, Gold L, Hampton A, Ukoumunne OC, Wake M (2007) Improving infant sleep and maternal mental health: a cluster randomised trial. Arch Dis Child 92:952–958. doi:10.1136/adc.2006.099812
Horiuchi S, Nishihara K (1999) Analyses of mothers’ sleep logs in postpartum periods. Psychiatry Clin Neurosci 53:137–139
Insana SP, Williams KB, Montgomery-Downs HE (2013) Sleep disturbance and neurobehavioral performance among postpartum women. Sleep 36:73–81. doi:10.5665/sleep.2304
Karacan I, Williams RL, Hursch CJ, McCaulley M, Heine MW (1969) Some implications of the sleep patterns of pregnancy for postpartum emotional disturbances. Br J Psychiatry 115:929–935
Kendall-Tackett K, Cong Z, Hale TW (2013) Depression, sleep quality, and maternal well-being in postpartum women with a history of sexual assault: a comparison of breastfeeding, mixed-feeding, and formula-feeding mothers. Breastfeed Med 8:16–22. doi:10.1089/bfm.2012.0024
Kennedy HP, Gardiner A, Gay C, Lee KA (2007) Negotiating sleep: a qualitative study of new mothers. J Perinat Neonatal Nurs 21:114–122. doi:10.1097/01.JPN.0000270628.51122.1d
Lee KA, Gay CL (2004) Sleep in late pregnancy predicts length of labor and type of delivery. Am J Obstet Gynecol 191:2041–2046. doi:10.1016/j.ajog.2004.05.086
Lee KA, Gay CL (2011) Can modifications to the bedroom environment improve the sleep of new parents? Two randomized controlled trials. Res Nurs Health 34:7–19. doi:10.1002/nur.20413
Lee SY, Lee KA (2007) Early postpartum sleep and fatigue for mothers after cesarean delivery compared with vaginal delivery: an exploratory study. J Perinat Neonatal Nurs 21:109–113. doi:10.1097/01.JPN.0000270627.73993.b0
Lee KA, McEnany G, Zaffke ME (2000a) REM sleep and mood state in childbearing women: sleepy or weepy? Sleep 23:877–885
Lee KA, Zaffke ME, McEnany G (2000b) Parity and sleep patterns during and after pregnancy. Obstet Gynecol 95:14–18
Mindell JA, Jacobson BJ (2000) Sleep disturbances during pregnancy. J Obstet Gynecol Neonatal Nurs 29:590–597
Mindell JA, Du Mond CE, Sadeh A, Telofski LS, Kulkarni N, Gunn E (2011) Long-term efficacy of an internet-based intervention for infant and toddler sleep disturbances: one year follow-up. J Clin Sleep Med 7:507–511. doi:10.5664/JCSM.1320
Montgomery-Downs HE, Clawges HM, Santy EE (2010a) Infant feeding methods and maternal sleep and daytime functioning. Pediatrics 126:e1562–e1568. doi:10.1542/peds.2010-1269
Montgomery-Downs HE, Insana SP, Clegg-Kraynok MM, Mancini LM (2010b) Normative longitudinal maternal sleep: the first 4 postpartum months. Am J Obstet Gynecol 203(465):e461–e467. doi:10.1016/j.ajog.2010.06.057
Muscat T, Obst P, Cockshaw W, Thorpe K (2014) Beliefs about infant regulation, early infant behaviors and maternal postnatal depressive symptoms. Birth 41:206–213. doi:10.1111/birt.12107
Nishihara K, Horiuchi S (1998) Changes in sleep patterns of young women from late pregnancy to postpartum: relationships to their infants’ movements. Percept Mot Skills 87:1043–1056
Parry BL, Curran ML, Stuenkel CA, Yokimozo M, Tam L, Powell KA, Gillin JC (2000) Can critically timed sleep deprivation be useful in pregnancy and postpartum depressions? J Affect Disord 60:201–212
Quillin SI, Glenn LL (2004) Interaction between feeding method and co-sleeping on maternal-newborn sleep. J Obstet Gynecol Neonatal Nurs 33:580–588
Sadeh A, Sharkey KM, Carskadon MA (1994) Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep 17:201–207
Signal TL, Gander PH, Sangalli MR, Travier N, Firestone RT, Tuohy JF (2007) Sleep duration and quality in healthy nulliparous and multiparous women across pregnancy and post-partum. Aust N Z J Obstet Gynaecol 47:16–22. doi:10.1111/j.1479-828X.2006.00672.x
Stremler R, Hodnett E, Lee K, MacMillan S, Mill C, Ongcangco L, Willan A (2006) A behavioral-educational intervention to promote maternal and infant sleep: a pilot randomized, controlled trial. Sleep 29:1609–1615
Stremler R, Hodnett E, Kenton L, Lee K, Weiss S, Weston J, Willan A (2013) Effect of behavioural-educational intervention on sleep for primiparous women and their infants in early postpartum: multisite randomised controlled trial. BMJ 346:f1164. doi:10.1136/bmj.f1164
Swain AM, O’Hara MW, Starr KR, Gorman LL (1997) A prospective study of sleep, mood, and cognitive function in postpartum and nonpostpartum women. Obstet Gynecol 90:381–386
Swanson LM, Flynn H, Adams-Mundy JD, Armitage R, Arnedt JT (2013) An open pilot of cognitive-behavioral therapy for insomnia in women with postpartum depression. Behav Sleep Med 11:297–307. doi:10.1080/15402002.2012.683902
Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP (2004) Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 89:2119–2126. doi:10.1210/jc.2003-031562
Wilkie G, Shapiro CM (1992) Sleep deprivation and the postnatal blues. J Psychosom Res 36:309–316
Wolfson A, Lacks P, Futterman A (1992) Effects of parent training on infant sleeping patterns, parents’ stress, and perceived parental competence. J Consult Clin Psychol 60:41–48
Wolfson AR, Crowley SJ, Anwer U, Bassett JL (2003) Changes in sleep patterns and depressive symptoms in first-time mothers: last trimester to 1-year postpartum. Behav Sleep Med 1:54–67
Acknowledgements
This study was funded by K23-MH086689 from the National Institutes of Health and a J. Christian Gillin Award from the Sleep Research Society Foundation to KMS and a grant from the Brown University/Women and Infants Hospital National Center of Excellence in Women’s Health Seed Grant to KMS and TP. The authors thank Julie Quattrucci, Aubree Hoepper, and Emily Mepham for assistance with data collection. We acknowledge Christine Acebo, PhD and Mary A. Carskadon, PhD for helpful comments on the manuscript. Finally, we are grateful to the study participants and their families for participating in our study.
Ethical standards
This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Participants gave informed consent prior to their inclusion in the study and were paid for their participation.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharkey, K.M., Iko, I.N., Machan, J.T. et al. Infant sleep and feeding patterns are associated with maternal sleep, stress, and depressed mood in women with a history of major depressive disorder (MDD). Arch Womens Ment Health 19, 209–218 (2016). https://doi.org/10.1007/s00737-015-0557-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00737-015-0557-5