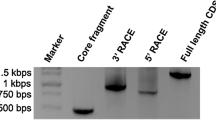
Abstract
Caffeic acid-O-methyltransferase (COMT), an important enzyme governing the process of lignification in plants, functions at the level of caffeic acid methylation along with 3-O-methylation of monolignol precursors. The present investigation was carried out to decipher the role of COMT in tall fescue lignification and to clone and characterize the COMT gene. The study on COMT activity variation at different growth stages of tall fescue exhibited a significant increase in activity over all the growth stages of tall fescue. A significant relative increase of 47.8% was observed from the first vegetative to reproductive stage. COMT activity exhibited a strong positive correlation with lignin content suggesting it to be an important enzyme of tall fescue lignification. Amplification and sequencing of tall fescue COMT gene resulted in an amplicon of size 1662 (Accession No.-MW442832) and an ORF of 346 amino acids. The deduced protein was hydrophobic, thermally stable and acidic with molecular formula C1679H2623N445O482S20, molecular mass 37.4 kDa and theoretical pI of 6.12. The protein possesses a conserved dimerization domain with a highly conserved SAM binding site. The COMT protein was found to be a homo-dimer with 1 catalytic SAH/SAM ligand per monomer interacting with 14 amino acid residues within 4 Å region.





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding author (SS) on request.
References:
AOAC International (2000) Official methods of analysis of the association of official analytical chemists (17th ed). AOAC International
Bhuiya M-W, Liu C-J (2009) A cost-effective colorimetric assay for phenolic O- methyltransferases and characterization of caffeate 3-O-methyltransferases from Populus trichocarpa. Ana Chem 384:151–158. https://doi.org/10.1016/j.ab.2008.09.031
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546. https://doi.org/10.1146/annurev.arplant.54.031902.134938
Broxterman SE, Schols HA (2018) Interactions between pectin and cellulose in primary plant cell walls. Carbo Pol 192:263–272. https://doi.org/10.1016/j.carbpol.2018.03.070
Chen L, Auh C, Chen F, Cheng X, Aljoe H, Dixon RA, Wang Z (2002) Lignin deposition and associated changes in anatomy, enzyme activity, gene expression and ruminal degradability in stems of Tall Fescue at different developmental stage. J Agric F Chem 50:5558–5565. https://doi.org/10.1021/jf020516x
Chen L, Auh CK, Dowling P, Bell J, Chen F, Hopkins A, Dixon RA, Wang ZY (2003) Improved forage digestibility of tall fescue (Festuca arundinacea) by transgenic down-regulation of cinnamyl alcohol dehydrogenase. Biotech J 1(6):437–449. https://doi.org/10.1046/j.1467-7652.2003.00040.x
Chen L, Auh CK, Dowling P, Bell J, Lehmann D, Wang ZY (2004) Transgenic down-regulation of caffeic acid O-methyltransferase (COMT) led to improved digestibility in tall fescue (Festuca arundinacea). Funct Biol 31(3):235–245
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: John MW (ed) The proteomics protocols handbook. Humana Press, Totowa, pp 571–607
Gupta SK, Rai AK, Kanwar SS, Sharma TR (2012) Comparative analysis of zinc finger proteins involved in plant disease resistance. PLoS ONE 7:e42578. https://doi.org/10.1371/journal.pone.0042578
Hand ML, Cogan NO, Forster JW (2012) Molecular characterization and interpretation of genetic diversity within globally distributed germplasm collections of tall fescue (Festuca arundinacea Schreb.) and meadow fescue (F. pratensis Huds.). Theor App Gen 124(6):1127–1137. https://doi.org/10.1007/s00122-011-1774-6
Hu D, Liu XB, She HZ, Gao Z, Ruan RW, Wu DQ, Yi ZL (2017) The lignin synthesis related genes and lodging resistance of Fagopyrum esculentum. Biol Planta 61(1):138–146. https://doi.org/10.1007/s10535-016-0685-4
Humphreys JM, Chapple C (2002) Rewriting the lignin roadmap. Curr Opin Plant Biol 5:224–229. https://doi.org/10.1016/S1369-5266(02)00257-1
Kirk TK, Obst JR (1988) Lignin determination. Methods Enzymol 161:96–99
Konstantin A, Lorenza B, Jurgen K, Torsten S (2006) The SWISS MODEL workspace: a web-based environment for protein structure homology modeling. Bioinform 22:195–201. https://doi.org/10.1093/bioinformatics/bti770
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. Mol Bio 157:105–132. https://doi.org/10.1016/0022-2836(82)90515-0
Lakshmi K, Kalaivani A (2015) Expression of Caffeic acid-O-methyltransferase gene involved in lignin biosynthesis of sugarcane. J Sugarcane Res 5(2):78–82
MacAdam JW, Grabber JH (2002) Relationship of growth cessation with the formation of diferulate cross-links and p-coumaroylated lignins in tall fescue leaf blades. Planta 215(5):785–793
Makkar HPS (2003) Measurement of total phenolics and tannins using Folin-Ciocalteu method. In: Quantification of tannins in tree and shrub foliage. Springer, Berlin
Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY, Geer RC, He J, Gwadz M, Hurwitz DI, Lanczycki CJ, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Bryant SH (2015) CDD: NCBI’s conserved domain database. Nuc Ac Res 43:D222–D226. https://doi.org/10.1093/nar/gku1221
Moore KJ, Moser LE, Vogel KP, Waller SS, Johnson BE, Pedersen JF (1991) Describing and quantifying growth stages of perennial forage grasses. Agron J 83:1073–1077. https://doi.org/10.2134/agronj1991.00021962008300060027x
Parvathi K, Chen F, Guo DJ, Blount JW, Dixon RA (2001) Substrate preferences of O-methyltransferases in alfalfa suggest new pathways for 3-O-methylation of monolignols. Plant J 25:193–202. https://doi.org/10.1046/j.1365-313x.2001.00956.x
Raes J, Rohde A, Christensen JH, Van de Peer Y, Boerjan W (2003) Genome-wide characterization of the lignification toolbox in Arabidopsis. Plant Physiol 133:1051–1071. https://doi.org/10.1104/pp.103.026484
Raja I, Rajendran K, Kumariah M, Rajasekaran S (2016) Isolation and characterization of mannose-binding lectin gene from leaves of Allium ascalonicum (Shallot) and its putative role in insect resistance South Indian. J Biol Sci 2:245–255
Robert X, Gouet P (2014) Deciphering key features in protein structures with the new ENDscript server. Nuc Ac Res 42:W320–W324. https://doi.org/10.1093/nar/gku316
Singh S, Sharma N (2020) Lignin associated anatomical changes at different growth stages of tall fescue (Festuca arundinacea Schreb.). Him J Agric Res 46(1):79–83
Singh S, Katoch R, Sharma N (2021) Enzymatic and biochemical alterations in relation to lignin deposition at different growth stages of tall fescue. Crop Sci 61:2848–2859. https://doi.org/10.1002/csc2.20493
Singh S, Sharma N, Malannavar AB, Badiyal A, Sharma PN (2022) Cloning and in silico characterization of cinnamyl alcohol dehydrogenase gene involved in lignifications of Tall fescue (Festuca arundinacea Schreb.). Mol Gen Genom 297:437–447. https://doi.org/10.1007/s00438-022-01858-6
Stephen FA, Thomas LM, Alejandro AS, Jinghui Z, Zheng Z, Webb M, David JL (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nuc Ac Res 25:3389–3402. https://doi.org/10.1093/nar/25.17.3389
Studer G, Tauriello G, Bienert S, Biasini M, Johner N, Schwede T (2021) ProMod3—A versatile homology modelling toolbox. PLOS Comput Biol 17(1):e1008667. https://doi.org/10.1371/journal.pcbi.1008667
Tang R, Zhang X-Q, Li Y-H, Xie X-M (2014) Cloning and in silico analysis of a cinnamyl alcohol dehydrogenase gene in Pennisetum purpureum. J Gen 93(1):145–158. https://doi.org/10.1007/s12041-014-0355-2
Tu Y, Rochfort S, Liu Z, Ran Y, Griffith M, Badenhorst P, Louie GV, Bowman ME, Smith KF, Noel JP, Mouradov A, Spangenberg G (2010) Functional analysis of caffeic acid-O-methyltransferase and cinnamoyl-CoA-reductase genes from perennial ryegrass (Lolium perenne). Plant Cell 22:3357–3373. https://doi.org/10.1105/tpc.109.072827
Updegraff DM (1969) Semimicro determination of cellulose in biological materials. Ana Biochem 32(3):420–424. https://doi.org/10.1016/S0003-2697(69)80009-6
Acknowledgements
Acknowledgement is due to Hon’ble Vice-Chancellor, Central Agricultural University, Imphal for granting study leave to Dr. Siddhartha Singh for PhD pursuance. Authors are also grateful to the Head, Department of Plant Pathology, College of Agriculture, CSK HPKV, Palampur for providing necessary facilities.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SS: conceptualization; data curation; formal analysis; investigation; methodology; validation; writing original draft; writing & editing. NS: resources; supervision; writing & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling editor: M. Bromke.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, S., Sharma, N. Biochemical and in silico molecular study of caffeic acid-O-methyltransferase enzyme associated with lignin deposition in tall fescue. Amino Acids 55, 1293–1304 (2023). https://doi.org/10.1007/s00726-022-03225-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-022-03225-6