Abstract
Protein accretion in some fish species is affected by dietary lipids, starch and their interactions, but this aspect of nutrition is largely unknown in largemouth bass (LMB). Therefore, we designed six experimental diets with three starch levels (5%, 10%, and 15%; dry matter basis) and two lipid levels (10% and 12.5%; dry matter basis) to evaluate the effects of dietary starch and lipid levels on the protein retention, growth, feed utilization, and liver histology of LMB. There were three tanks (18 fish per tank, ~ 4.85 g per fish) per dietary treatment group and the trial lasted for 8 weeks. Fish were fed to apparent satiation twice daily. Results indicated that increasing the dietary starch level from 5 to 15% reduced (P < 0.05) absolute feed intake (AFI; − 9.0%, − 15% and − 14% on days 14–28, 28–42, and 42–56, respectively) and weight gains (− 4.4% and − 6.5% on days 42 and 56, respectively) of LMB. Increasing the dietary lipid level from 10 to 12.5% reduced (P < 0.05) AFI (− 9.7%, − 11.7% and − 11.9% on days 14–28, 28–42; and 42–56, respectively), weight gains (− 4.2%, − 5.9% and − 6.9% on days 28, 42 and 56, respectively), and survival rate (by a 5.6% unit) of LMB. The retention of dietary protein and some amino acids in the body was affected by dietary starch or lipid levels and their interactions. The viscerosomatic index (VSI), hepatosomatic index (HSI), and intraperitoneal fat ratio (IPFR) increased with increasing the dietary starch level from 5 to 15%. Compared with 10% lipids, 12.5% lipids in diets increased IPFR but had no effect on VSI or HSI. The concentrations of glucose in serum increased with increasing the dietary starch level from 5 to 15% at 4 to 24 h after feeding, with the effect of dietary lipids being time-dependent. Compared with a 5%-starch diet, fish fed a diet with 10%- or 15%-starch exhibited an enlarged and pale liver with excessive glycogen. Based on these findings, we recommend dietary lipid and starch levels to be 10% and < 10%, respectively, for juvenile LMB to maximize the retention of dietary protein in their bodies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish have particularly high requirements for dietary protein, because amino acids are their major metabolic fuels (Jia et al. 2017). To reduce protein requirement and feed costs, it is important to improve amino acid utilization for protein synthesis rather than as an energy source (Mohanta et al. 2008). In swine and poultry nutrition, the non-productive use of ingested protein is generally reduced considerably when proper amounts of starch or lipids are added to diets (Wu 2018). As a major non-protein energy source in fish diets, starch is the least expensive form of dietary energy, and the protein-sparing effect of dietary starch has been reported in some fish species (Jafri 1995; Wang et al. 2005; VásquezTorres and Arias-Castellanos 2013). The inclusion of starch can also improve the physical properties of extrusion and steam pellet feeds (Wilson 1994). However, for carnivorous fish, dietary lipids are regarded as better providers of energy than digestible carbohydrate, because utilization of glucose by their tissues is limited (Cowey and Sargent 1977; Jia et al. 2017). Moreover, appropriate levels of dietary lipids or starch should be carefully evaluated and determined for fish to enhance their growth, health, and product quality. Excessive lipids and starch in diets could lead to growth restriction, hepatopathy, poor immune function, and excess fat accumulation in fish (Han et al. 2014; Li et al. 2015; Wang et al. 2016a, b; Yang et al. 2018; Zhou et al. 2020).
Largemouth bass (LMB) is a carnivorous species, which is cultured in North America (Durborow 2019) and many other countries [including China (Tidwell et al. 2019; Wang et al. 2015)] worldwide as an aquaculture species due to its high market value. Results of our recent studies have indicated that LMB extensively oxidize amino acids as metabolic fuels but have a limited ability to utilize glucose or fatty acids for ATP production (Li and Wu 2019; Li et al. 2020). The reported requirement of this fish for dietary crude protein is 40–50%, depending on fish size and experimental conditions (Huang et al. 2017). However, little is known about the requirements of LMB for dietary lipids and starch or their interactions. Therefore, we conducted this study to evaluate the effects of dietary starch (5%, 10% and 15%) and lipid (10% and 12.5%; dry matter basis) levels on the growth, feed utilization, glucose metabolism, and liver histology of LMB.
Materials and methods
Experimental diets
Six experimental diets were formulated to contain three starch levels (5%, 10%, and 15%; dry matter basis) and two crude lipid levels (10% and 12.5%; dry matter basis) with a fixed level of 45% crude protein. The ingredients and proximate composition of diets are shown in Table 1. The main protein sources used in this study were fishmeal and soybean protein concentrate with defined composition of amino acids, carbohydrate and lipids (Li and Wu 2020). The main lipid sources were fish oil, poultry fat, and soybean oil. All solid ingredients were thoroughly mixed using a mixer and thereafter, fish oil, soybean oil, and water were added to the mixture to form a moist dough. The experimental diets were produced by a screw extruder (Big Bite Meat Grinder, West Chester, OH) and oven-dried at 50 °C until dry matter content was 97.0%. All feeds were kept at – 20 °C.
Experimental animals
Juvenile LMB were obtained from a commercial fish farm (Bait Barn, Bryan, TX, USA), and maintained in a recirculating water system. Prior to starting the experiments, LMB were acclimated to the experimental condition and maintained on a commercial diet (AquaMax® Grower 400, Purina, MO) for 2 weeks. The recirculating experimental system for the culture of fish consisted of 18 tanks, with three tanks for each dietary treatment. Each tank contained 55 l of deionized water (26.0 ± 0.01 °C; mean ± SEM, n = 56). From the outlet of fish tanks, the water flowed through mechanical and biological filters. At the beginning of this trial, 18 fish with a uniform body size [initial mean body weight, about 4.8 g (ranging from 4.7 to 4.9 g) per fish] were randomly distributed into each tank. Air was supplied to the water through air stones connected to air pumps, with its salinity being maintained at 3–5 ppt. The photoperiod of the housing facility was maintained for 14 h per day, with lights being turned off between 10:00 PM and 8:00 AM. Water quality parameters [pH 6.90 ± 0.03, NH4+ (0.48 ± 0.02 mg/l), nitrite (0.25 ± 0.01 mg/l), nitrate (11.1 ± 0.07 ppm), and dissolved O2 (8.0 ± 0.05 ppm); mean ± SEM, n = 56] were monitored daily and remained within acceptable limits. Fish were hand-fed with experimental diets to apparent satiation twice daily at 09:00 and 16:00. Total feed consumption was recorded every day. The amount of the unconsumed diet supplied to each tank was collected to determine the weight of feed based on the number of pellets. We determined that the loss of provided pellet feed into the water was 10%, and therefore, the true feed consumption by fish was calculated as the amount of provided feed × 0.9. Total fish weight in each tank was recorded every 2 weeks to minimize handling and stress. Fish were weighed after a 24-h period of food deprivation. Almost 100% water in tanks was replaced gradually every day by adding fresh water into a central reservoir tank at the rate of ~ 4 l/min for 5 h to make sure the quality of water is optimal. The feeding trial lasted for 56 days.
Sample collection
At the beginning of the trial, 30 fish were euthanized with 140 ppm MS-222 (neutralized by an appropriate amount of NaHCO3) for the analysis of whole-body composition. At the end of the experiment, blood (0.5 ml) was collected from the caudal vein of conscious fish (6 fish/tank) with the use of a hypodermic syringe at various time points after feeding. After blood collection, all fish were euthanized as described previously, with four fish being randomly selected from each tank for the analyses of whole-body composition and with 4 fish being randomly selected from each tank to obtain viscera organs [including the stomach and intestine (without luminal contents), as well as liver, pancreas, and spleen)], peritoneal adipose tissue, and skeletal muscle. Blood samples were immediately centrifuged (2 min at 8000 g) at 4 °C. The supernatant fluid (serum) was obtained and stored at – 80 °C until analyzed.
Biochemical analyses
Crude protein (N × 6.25) was determined by the combustion method, and calcium and phosphorus were determined by optical spectrometry in Servi-Tech laboratories (Amarillo, TX, USA). Moisture was determined by drying at 105 °C in an oven to a constant weight. Lipids were extracted from the samples with chloroform/methanol (2:1 v/v) according to the method of Folch et al. (1957). Glucose in neutralized samples was determined enzymatically using a fluorometric method involving hexokinase and glucose-6-phosphate dehydrogenase (Fu et al. 2005), and lactate was analyzed using lactate dehydrogenase (Wu et al. 1995). The gross energy values of the diets were calculated based on 22.6 kJ/g, 39.3 kJ/g and 17.2 kJ/g for crude protein, crude lipid, and glycogen/starch, respectively. Amino acids in the diets and the bodies of fish were analyzed, as described by Li and Wu (2020).
Syntheses of glycogen and lipids from glucose in the liver and skeletal muscle
Each fresh tissue (20–50 mg) was placed into a tube with 1 ml KHB buffer containing 5 mM glucose and [U-14C]glucose (150 dpm/nmol). Each tube was incubated at 26 °C for 2 h after the incubation medium was gassed with 95% O2/5% CO2 for 20 s. At the end of the 2-h incubation, all tubes were centrifuged for 3 min at 600 g and the tissue was obtained for glycogen analysis, as described by Jobgen et al. (2009) with modifications. Briefly, tissue was washed with ice-cold PBS four times. Thereafter, 0.3 ml of 30% KOH was added to the tissue. The solution was boiled for 5 min in a water bath for solubilization, followed by addition of 0.1 ml of 2% Na2SO4 and 0.9 ml of 100% alcohol. Then, all the tubes were kept at 25 °C for overnight. The pellet (glycogen-sodium sulphate) was obtained by centrifugation at 600 g for 10 min, and the supernatant fluid was saved for the extraction of glycogen associated with protein. The glycogen pellet was washed with 2 ml of 65% alcohol twice. After the alcohol was evaporated in a waterbath at 70 °C, the precipitate was dissolved in 0.1 mL of 1 M H2SO4. The solution was transferred to a counting vial with 5 ml of scintillation cocktail and 14C radioactivity was measured by a liquid scintillation counter (Zhang et al. 2019). The supernatant fluid from the glycogen-sodium sulfate precipitation step above was neutralized with 6 M HCl (about 265 μl), followed by addition of 1 ml of 1.5 M HClO4 and centrifugation (10 min at 600 g rpm). The resultant pellet was washed with 2 ml of 1.5 M HClO4 three times and dissolved in 0.3 ml of 30% KOH. An aliquot (0.2 ml) of this solution was mixed with 5 ml of scintillation cocktail for the measurement of 14C radioactivity in a liquid scintillation counter (Zhang et al. 2019).
14C-lipids was extracted from tissue with the use of isopropylalcohol:heptane:1N H2SO4 (40:10:1; extraction solution), as described by Wu et al. (1991). Briefly, each tissue was homogenized with 5 ml of extraction solution. The homogenate and incubation medium were mixed, and vortex for 1 min. After 5 min, 3 ml Heptane and 3 ml H2O was added to each tube. All the tubules were vortexed for 2 min. After 5 min, the upper layer (heptane layer) was collected, and heptane was dried down to less than 0.5 ml at 65 °C. Finally, 5 ml of scintillation cocktail was added to the tube for the measurement of 14C radioactivity by a liquid scintillation counter.
Histological analysis of the liver
A portion (~ 500 mg) of the liver was fixed with 4% paraformaldehyde (buffered to pH 7.2) for 24 h. The samples were rinsed, dehydrated, and embedded in paraffin for histological analysis at the Veterinary Medicine & Biomedical Sciences Histology Laboratory of Texas A&M University. Each sample was cut into two of 6 μm transverse sections with a rotary microtome. The sections were stained with the periodic acid-Schiff (PAS) solution as described by Fu and Campbell-Thompson (2017). One section was digested by α-amylase, and the other was treated with the vehicle buffer, and both were stained with the PAS solution. The PAS solution containing diastase (an enzyme that digests glycogen) was also used to stain the tissue for differentiating glycogen from other PAS-positive elements in tissue. For each treatment group, the size of hepatocytes was calculated as the area of the cells (10 cells for each liver sample). Hepatocyte images in the histology slides were evaluated using an Axioplan 2 microscope (Carl Zeiss, Thornwood, NY) interfaced with an Axiocam HR digital camera, the cell area was measured by Axiovision 4.3 software (Carl Zeiss).
Calculation and statistical analysis
Growth performance, feed utilization and morphometrical parameters were calculated as follows:
Retention or productive value of dietary nutrient and energy (%) = 100 × nutrient or energy gain/dietary nutrient or energy intake. The term “productive value” is used, because some of the lipids gained in animals (including fish) are derived from de novo synthesis.
All data were tested for homogeneity (the Levene’s test) and normal distribution (the Kolmogorov–Smirnov test). When the tests were statistically significant, the data were log transformed before analysis. A 2 × 3 factorial analysis of variance (ANOVA) was used to analyze the main effects (lipids and starch) and their interactions. The tank for fish rearing was the experimental unit for data analyses. Differences among treatment groups were analyzed by the Student–Newman–Keuls multiple comparison test. P < 0.05 was taken to indicate statistical significance. All statistical analyses were performed using the SPSS package (version 18.0, Chicago, IL).
Results
AFI, growth performance, feed efficiency, and survival of LMB
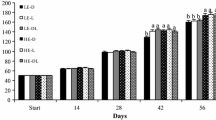
Data on the AFI [as done by other researchers (Borges et al. 2009; Rueda-Jasso et al. 2004)], growth performance, and feed efficiency of LMB are summarized in Tables 2 and 3. Between days 0 and 14 of the trial, increasing the dietary starch level from 5 to 15% or the dietary lipid level from 10 to 12.5% did not affect (P > 0.05) the AFI or weigh gains of LMB. Between days 14 and 28 of the trial, increasing the dietary starch level from 5 to 15% reduced (P < 0.05) the AFI of LMB by 9.0% but had no effect (P > 0.05) on their weight gain; increasing the dietary lipid level from 10 to 12.5% reduced (P < 0.05) the AFI of LMB by 9.7% and their weigh gains by 4.2%. After day 28 of the trial, increasing the dietary starch level from 5 to 15% reduced (P < 0.05) AFI (− 15% and − 14% on days 28–42 and 42–56, respectively) and weight gains (− 4.4% and − 6.5% on days 42 and 56, respectively) of LMB, whereas increasing the dietary lipid level from 10 to 12.5% reduced (P < 0.05) AFI (− 11.7% and − 11.9% on days 28–42 and 42–56, respectively) and weight gains (− 5.9% and − 6.9% on days 28–42 and 42–56, respectively) of the fish. During the entire 8-week experiment, the relative feed intakes (% of body weight per day) of the LMB in the 5%, 10% and 15% starch groups were 2.70 ± 0.13, 2.58 ± 0.12, and 2.56 ± 0.12 (means ± SEM, n = 6 tanks), respectively (P > 0.05), whereas the values for fish in the 10% and 12.5% lipid groups were 2.66 ± 0.09 and 2.56 ± 0.08 (means ± SEM, n = 9 tanks), respectively (P > 0.05). Neither feed conversion ratio (AFI/weight gain) nor protein efficiency ratio (weight gain/protein intake) was affected (P > 0.05) by dietary starch (5% to 15%) or lipid (10% to 12.5%) levels during the entire experimental period. There were no interactions (P > 0.05) between dietary starch and lipid levels in affecting the fed intake or weight gain of LMB. Thus, overall, juvenile LMB consumed less feed and grew more slowly when fed a 45%-protein diet containing either ≥ 10% starch or 12.5% lipids. The absolute weight gain of fish did not appear to be closely related to the percentages of dietary AAs retained in the body (Tables 5 and 6).
The rates of survival of LMB were 93–99%, depending on dietary groups (Table 7). Increasing the dietary starch level from 5 to 15% did not affect (P > 0.05) the survival of LMB. In contrast, increasing the dietary lipid level from 10 to 12.5% reduced (P < 0.05) the survival rate of LMB by a 5.6% unit. There were no interactions (P > 0.05) between dietary starch and lipid levels in affecting the survival of the fish.
Ratios of energy, protein, lipids, phosphorus, calcium and amino acids gained in the body to their dietary intakes of LMB
During the entire 56-day trial, 35–41% of dietary energy, 29–33% of dietary protein, 35–40% of dietary phosphorus, and 46–50% of dietary calcium were retained in the body of LMB, whereas the ratios of lipids gained in the body to their dietary intake (productive value) were 48–71% (Table 4). Increasing the dietary starch level from 5 to 15% or the dietary lipid level from 10 to 12.5% did not affect (P > 0.05) the retention of dietary phosphorus and calcium in the body of LMB. In contrast, increasing the dietary starch level from 5 to 15% had no effect (P > 0.05) on the productive value of dietary energy in the body of LMB, reduced (P < 0.05) the retention of dietary protein by 3.2%, and increased (P < 0.001) the productive value of dietary lipids by 26.7%. Increasing the dietary lipid level from 10 to 12.5% reduced (P < 0.05) the productive value of dietary energy, protein, and lipids by 8.3%, 2.2%, and 17.8%, respectively. There were interactions between dietary starch and lipid levels in affecting the productive of dietary energy (P < 0.001), protein (P < 0.001), lipids (P < 0.05), phosphorus (P < 0.001) and calcium (P = 0.020) in the body of LMB. Specifically, the retention of dietary energy, protein and phosphorus in the body of LMB fed the 10% starch and 10% lipid was the highest (P < 0.05) among all the dietary groups, whereas the productive value of dietary lipids in the body of LMB fed the 15% starch and 10% lipid diet was the highest (P < 0.05) among all the dietary groups. The retention of dietary phosphorus in LMB fed the diet containing 10% starch and 10% lipids was the highest (P < 0.05) among all the dietary groups, whereas the retention of dietary calcium in LMB fed the diet containing 10% starch and 10% lipid diet, the 5% starch and 10% lipid diet, and the 15% starch and 12.5% lipid diet was higher (P < 0.05) than other groups of the fish. Data on the ratios of amino acids gained in the body to their dietary intakes are summarized in Table 5 (for nutritionally dispensable amino acids) and Table 6 (for nutritionally indispensable amino acids). There were interactions between dietary starch and lipid levels in affecting the retention of most dietary amino acids (P < 0.05). Increasing the dietary starch level from 5 to 10% increased the percentages of aspartate, glutamate, cysteine, proline, glycine, and valine gained in the body to their dietary intake, whereas increasing the dietary lipid level from 10 to 12.5% did not affect the retention of dietary amino acids (P > 0.05).
Relative weights of all viscera organs, liver, and intraperitoneal adipose tissue of LMB
Figure 1 shows the pictures of the body, liver and intraperitoneal adipose tissue of LMB fed diets containing different starch and lipid levels. Data on the VSI, HSI, IPFR, and hepatocyte size of juvenile LMB fed different diets are summarized in Table 7. Viscera organs, liver, and IPFR represented 7.3–8.5%, 1.1–1.9%, and 2.2–3.0% of the whole-body weight, respectively. All of the three variables increased (P < 0.05) with increasing the dietary starch level from 5 to 15%. Increasing the dietary lipid level from 10 to 12.5% increased (P < 0.001) IPFR by 16.2% but had no effect (P > 0.05) on VSI or HSI. There were no interactions (P > 0.05) between dietary starch and lipid levels in affecting the VSI, HSI or IPFR of LMB. Increasing the dietary starch level from 5 to 15% augmented (P < 0.001) the hepatocyte size in the liver by 75.9%, but increasing the dietary lipid level from 10 to 12.5% had no effect. There were interactions (P < 0.05) between dietary starch and lipid levels in affecting the hepatocyte size of LMB, in that the hepatocyte size in the liver of LMB fed the 15% starch and 10% lipid diet was the largest (P < 0.05) among all the dietary groups.
Composition of macronutrients in the body of LMB
At the end of the 56-day trial, the content of water, protein, lipids, phosphorus, and calcium in the body of LMB was about 70–72%, 17%, 6.3–7.6%, 0.75%, and 1.2%, respectively, whereas the content of water, protein, and lipids in the skeletal muscle of LMB was about 77%, 20%, and 1.2%, respectively (Table 8). Increasing the dietary starch level from 5 to 15% did not affect (P > 0.05) the content of protein, phosphorus, and calcium in the body of LMB or the content of water and protein in their skeletal muscle, but increased the content of lipids in both the body (+ 20.5%; P = 0.005) and skeletal muscle (+ 14.3%; P = 0.027). Compared with the 5%-starch diet, the LMB fed the 10% starch diet had a 1.8% lower content of water in the body. Increasing the dietary lipid level from 10 to 12.5% had no effect (P > 0.05) the content of protein, phosphorus and calcium in the body or the content of water and lipids in skeletal muscle, increased (P = 0.002) the content of lipids in the body by 16.4%, and reduced (P < 0.01) the content of water in the body by 1.5% and the content of intramuscular protein by 1.5%. There were no interactions (P > 0.05) between dietary starch and lipid levels in affecting the content of water and lipids in the body of LMB or the content of water, protein and lipids in skeletal muscle. However, there were interactions (P < 0.05) between dietary starch and lipid levels in affecting the content of protein, phosphorus and calcium in the body. Specifically, the retention of dietary protein in the body of LMB fed the 10% starch and 10% lipid diet and the 5% starch and 12.5% lipid diet was the highest (P < 0.05) among all the dietary groups, whereas the retention of dietary phosphorus and calcium in the body of LMB fed the 10% starch and 10% lipid diet, the 5% starch and 12.5% lipid diet, and the 15% starch and 12.5% lipid diet was the highest (P < 0.05) among all the dietary groups.
Composition of macronutrients in the liver of LMB
At the end of the 56-day trial, the content of water, protein, lipids, and glycogen in the body of LMB was about 73–77%, 16.9–17.7%, 1.6–2.8%, and 8–12%, respectively (Table 9). Increasing the dietary starch level from 5 to 15% enhanced (P < 0.001) the content of water (+ 3.7%) and glycogen (+ 41%) in the liver of LMB, but decreased (P < 0.001) the content of protein (− 20.8%) and lipids (− 39.1%). Increasing the dietary lipid level from 10 to 12.5% also decreased the content of protein in the liver (− 10.5%; P = 0.002) and lipids (− 9.9%; P = 0.020), but did not affect (P > 0.05) the content of water or glycogen in the liver. There were interactions (P < 0.05) between dietary starch and lipid levels in affecting the content of water in the liver of LMB. Specifically, the content of water in the liver of LMB fed the 5% starch and 12.5% lipid diet was the highest (P < 0.05) among all the dietary groups.
Syntheses of glycogen and lipids from glucose in the liver and skeletal muscle
To assess the metabolic rates of glycogen and lipid syntheses from glucose, livers and skeletal muscle from the six different groups of LMB were incubated with the same extracellular concentration of glucose (5 mM). The rates of glycogen synthesis from glucose in the liver of LMB were about three times those for fatty acid synthesis (Table 10). Compared with the liver, the skeletal muscle of LMB had a very low activity to synthesize glycogen and fatty acids from glucose. The rates of glycogen synthesis in the liver or skeletal muscle did not differ (P > 0.05) between LMB fed 5% and 15% starch diets. Likewise, the rates of fatty acid synthesis from glucose did not differ (P > 0.05) among LMB fed the 5%, 10% and 15% starch diets. Of interest, there was a tissue-specific effect of dietary lipid levels on the rates of fatty acid and glycogen syntheses in the liver and skeletal muscle. Specifically, increasing the dietary lipid level from 10 to 12.5% did not affect (P > 0.05) the rates of fatty acid synthesis from glucose in the liver but increased (P < 0.05) those in skeletal muscle by 19.7%. In contrast, increasing the dietary lipid level from 10 to 12.5% did not affect (P > 0.05) the rates of glycogen synthesis from glucose in skeletal muscle but decreased (P < 0.05) those in the liver by 12.4%. There were no interactions (P > 0.05) between dietary starch and lipid levels in affecting fatty acid and glycogen syntheses in the liver or skeletal muscle of LMB.
Concentrations of glucose and lactate in the serum of LMB
Concentrations of glucose in the serum of LMB were 4.64, 3.92, 3.41, 1.82 and 2.11 mM (pooled SEM = 0.12; n = 18 tanks per time point) at 2, 4, 8, 12, and 24 h after feeding. The concentrations of glucose in serum decreased (P < 0.001) progressively between 2 and 12 h after feeding and remained low at 24 h after feeding, with no difference (P > 0.05) between 12 and 24 h after feeding. Increasing the dietary starch level from 5 to 15% elevated (P < 0.001) the concentrations of glucose in serum at 2 to 24 h after feeding. In contrast, the effect of dietary lipids on the concentrations of glucose in serum was time-dependent. Specifically, increasing the dietary lipid level from 10 to 12.5% decreased (P < 0.05) the concentrations of glucose in serum by 13.7–17.6% at 2 to 8 h after feeding, had no effect (P > 0.05) at 12 h after feeding, and increased (P < 0.05) those by 23.1% at 24 h after feeding. A 4 h after feeding, there were interactions (P = 0.006) between dietary starch and lipid levels in affecting the concentrations of glucose in the serum of LMB. Specifically, the concentrations of glucose in serum was higher when the 45% protein diet contained 15% starch and 10% lipids, compared with the 5% starch and 10% lipid diet, the 10% starch and 10% lipid diet, and the 5–15% starch and 12.5% lipid diets. At other time points, there were no significant interactions (P > 0.05) between dietary starch and lipid levels in affecting the concentrations of glucose in serum.
Concentrations of lactate in the serum of LMB were 2.73, 2.06, 2.14, 2.18 and 2.46 mM (pooled SEM = 0.09; n = 18 tanks per time point) at 2, 4, 8, 12, and 24 h after feeding. The concentrations of lactate in serum decreased (P < 0.001) between 2 and 4 h after feeding, remained at reduced values through 12 h after feeding, and increased (P < 0.05) at 24 h after feeding, compared with 4–12 h after feeding. Increasing the dietary starch level from 5 to 15% enhanced (P < 0.001) the concentrations of lactate in serum at 2 to 24 h after feeding. In contrast, increasing the dietary lipid level from 10 to 12.5% did not affect (P > 0.05) the concentrations of lactate in serum at 2 to 24 h after feeding. At 4 to 12 h after feeding, there were interactions (P < 0.05) between dietary starch and lipid levels in affecting the concentrations of lactate in the serum of LMB. Specifically, the 4-h concentrations of lactate in the serum of LMB fed the 15% starch and 10% lipid diet was the highest (P < 0.05) among all the dietary groups, whereas the 8- and 12-h concentrations of lactate in the serum of LMB fed the 15% starch and 10% lipid diet, 10–15% starch and 12.5% lipid diets were higher (P < 0.05) than those in the 5–10% starch and 10% lipid diets and the 5% starch and 12.5% lipid diet. At 2 and 24 h after feeding, there were not significant interactions (P > 0.05) between dietary starch and lipid levels in affecting the concentrations of lactate in serum.
Liver histology
Histological analyses revealed that, compared with LMB fed the 5% starch diet, LMB fed 10–15% starch diets exhibited an enlarged and pale liver, as well as enlarged hepatocytes with excessive glycogen in the cytosol, as indicated by the PAS stain (Fig. 2). These histologic findings are characteristics of glycogenic hepatopathy (hepatic glycogenosis).
Hepatic histology of juvenile largemouth bass fed diets containing different starch and lipid levels. The PAS stain (without diastase): showing the red staining of the hepatocyte cytoplasm. D refers to the PAS stain with diastase (to break down glycogen): showing a marked decrease in the bright red staining (or the amount of glycogen) of the hepatocyte cytoplasm
Discussion
Results of this study indicated that juvenile LMB adapted well to our culture system on the basis of the rates of their growth and survival. The lean tissue gain in fish is mainly determined by the deposition of protein in the body (Dumas et al. 2007). We noted a few reports that LMB could achieve a rate of up to 40% of dietary nitrogen retention in the body (Li et al. 2018, 2019; Ding et al. 2019). Our lower values (29–33%) for this variable are similar to or better than those for similar size LMB that were fed similar diets, such as 22–30% (Portz et al. 2001), 25–30% (Subhadra et al. 2006), 26–28% (Tidwell et al. 2007), 30% (Gong et al. 2019; Song et al. 2018), 25% (Cai et al. 2020), 17–22% (Guo et al. 2020), ~ 20% (Zhong et al. 2020), and 25–30% (Zhou et al. 2014). The average retention of dietary nitrogen in juvenile LMB is ~ 30% (Tidwell et al. 2019), which is lower than the value of 55–65% in poultry (Rehman et al. 2018) and 45–70% for growing pigs (depending on age; Wu et al. 2014). Fry et al. (2018) indicated that the average rates of the retention of dietary nitrogen as edible protein in aquatic species and chickens were 19% and 37%, respectively, and the value is 41% for pigs (Wu et al. 2014). Dietary amino acids that are not retained in the body are either oxidized or excreted into the environment. A number of environmental factors, such as temperature, oxygen concentration, salinity, density and photoperiod, can influence the growth rate of farmed fish (Chen et al. 2015; Matthias et al. 2018; Ren et al. 2018). In the current study, a relatively high density (18 fish/55 l of water) may be a reason for the relatively lower rate of growth. Moreover, differences in genotypes and the culture systems may also influence the growth rate of fish (Gjedrem 2000; Pierce et al. 2008). Of note, fish generally have 1.5- to two-fold greater requirements for dietary protein (30–55%) than livestock species and poultry (12–20%), and use more amino acids as metabolic fuels (Wu 2018). In support of this view, we recently reported that tissues (the proximal intestine, liver, skeletal muscle, and kidneys) of hybrid-striped bass (Jia et al. 2017) and LMB (Li and Wu 2019; Li et al. 2020) prefer to use glutamate, glutamine and aspartate to provide ATP.
Appropriate dietary starch and lipid levels could improve the protein retention and growth performance of fish, but the nutritional effects likely vary among different species (NRC 2011). Despite the wide use of LMB in aquaculture, little is known about their dietary requirements for starch and lipids for maximum protein accretion. We found that the retention of dietary nitrogen in the LMB, as well as the ratios of some amino acids gained in the body to their dietary intakes, increased with increasing the dietary starch level from 5 to 10% (Tables 5 and 6), and the growth of LMB decreased with increasing the dietary starch level from 5 to 15% or the dietary lipid level from 10 to 12.5% (Table 2). Interestingly, the ratio of glycine gained in the body to its dietary intake was about 43%, which was greater than that for any of the other amino acids (Table 5), suggesting a substantial synthesis of this amino acid in LMB. In addition, the survival rate of LMB fed the 12.5% lipid diet was lower than that of LMB fed the 10% lipid diet (Table 7). The growth performance of some carnivorous fish is impaired when their diets contain more than 15% starch (NRC 2011). For example, Li et al. (2015) reported that the growth of giant croaker (Nibea japonica) decreased with increasing the dietary cornstarch level to more than 12.2%. In addition, juvenile grouper (Epinephelus akaara), a dietary level of 7.64% starch appeared to be sufficient for maximum growth of juvenile grouper (Epinephelus akaara) (Wang et al. 2016a, b). Similarly, we found that even 10% of dietary starch could limit the weight gain (Table 2) of LMB. There are reports that the requirement of LMB for dietary lipids is about 10–15%, dependent on experimental conditions (Huang et al. 2017; Bright et al. 2005). In our another study, we found that LMB grew faster when fed a 10% lipid diet, compared with a 7.5% lipid diet (our unpublished work). Taken together, we recommend that the requirement of juvenile LMB for dietary lipids be 10% (dry matter basis). For comparison, omnivorous fish (e.g., tilapia) tolerate well high dietary starch intake, as increasing the dietary starch level from 10 to 40% improves their growth performance (Amirkolaie et al. 2006), whereas some carnivores (e.g., hybrid grouper) tolerate well 28% cornstarch in diets and do not accumulate excess intraperitoneal fat, compared with a 0% starch diet (Luo et al. 2016). Likewise, many species of fish have dietary requirements for 15–20% starch for optimal growth (Watanabe 1982). The lower limit to the inclusion of starch and lipids in LMB diets is consistent with our recent report that the major tissues of LMB, including the intestine, liver, skeletal muscle, and kidneys, had a low ability to oxidize glucose and fatty acids into CO2 (Li and Wu 2019; Li et al. 2020).
The primary reason for the lower rate of growth in LMB fed the 10–15% starch diets compared with the 5% starch diet or in LMB fed the 10% lipid diet compared with the 12.5% lipid diet may result from metabolic and structural alterations in the liver of the LMB. To our knowledge, such a phenomenon has not been reported for LMB. We found that increasing the dietary starch from 5 to 15% or the dietary lipid level from 10 to 12.5% did not affect the relative feed intake (mg feed/g body weight per day) of the LMB. Interestingly, after the second week until the end of the trial, the AFI of fish (g feed/fish) decreased with increasing dietary starch or lipid levels. Like terrestrial animals (Wu 2018), fish usually regulate their feed consumption to meet energy requirements (Hemre et al. 1995; Sveier et al. 1999). When fed a diet containing excess energy, fish exhibit growth depression due to a reduction in AFI (Ellis and Reigh 1991; El-Sayed and Garling 1988; Han et al. 2014) and protein intakes (Khan and Abidi 2012). Likewise, high dietary starch or lipid levels, along with high dietary energy levels, decrease protein intake by animals, which contributes to a reduced growth rate, as observed for LMB in this study. Moreover, as in terrestrial animals (Wu 2020), reduced AFI by fish may result from pathological changes in tissues of fish (e.g., the intestine and liver) due to excessive starch and lipid intakes (Tan et al. 2007). A decrease in growth rate in association with excessive accumulation of glycogen in the liver (hepatic glycogenosis) was observed in some fish species (e.g., ≥ 10% starch for juvenile grouper and ≥ 7% glucose for juvenile white sturgeon) fed diets containing high starch levels (Wang et al. 2016a, b; Fynn-Aikins et al. 1992). Clearly, LMB cannot tolerate ≥ 10% dietary starch and > 10% dietary lipids (dry matter basis).
The FCR and PER in LMB were not affected by the levels of dietary starch (5–15%) or lipids (10–12.5%). Our results suggest that these dietary levels of starch and lipids do not negatively influence the efficiency of the utilization of dietary nutrients for weight gains of LMB during a 56-day experimental period. However, when the components of weight gains are unknown, values on the FCR and PER may be misleading. For example, we noted that increasing the dietary starch level from 5 to 15% reduced the retention of dietary protein in LMB by 3.2% and increased the retention of dietary lipids in the body by 27% (Table 4), possibly contributing to an unfavorably fatter carcass. Similarly, Lie et al. (1988) suggested that protein retention is a better indicator of feed efficiency for lean tissue growth than the PER in fish species. Likewise, Han et al. (2014) concluded that a body weight change cannot be regarded as an accurate predictor of true protein accretion in fish when their bodies accumulate lipids. It is possible that high dietary starch impairs the digestion of dietary protein and/or the subsequent synthesis of protein from amino acids in extra-intestinal tissues while promoting lipid synthesis from starch-derived glucose in tissues, such as the liver and skeletal muscle (Table 10) as well as white adipose tissue depots (Table 7). Nonetheless, a lower rate of growth in fish (including LMB) will delay the time from their entry into aquaculture facilities to their marketing. This will increase both the costs of farm labors as well as the risks for production losses due to possible infectious diseases, climate changes, and natural disasters, thereby leading to reductions in production efficiency and economic returns. Therefore, excessive levels of starch and lipids in fish diets should always be avoided.
White adipose tissue, which is distributed in abdominal, mesenteric, subcutaneous, and peri-gonadal sites, as well as the liver, skeletal muscle, and other organs, is the main depot for lipid storage primarily as triacylglycerols in fish (Salmerón 2018), as in terrestrial animals (Wu 2018). Juvenile LMB contained 6.33–7.66% lipids (Table 8). Based on the content of lipids in skeletal muscle (1.19–1.36%; Table 8) and assuming that skeletal muscle represents about 50% of the body weight, about 9% of total lipids in LMB are present in the skeletal muscle of the fish (1.19% × 0.5/6.33% = 9.4%; 1.36% × 0.5/7.66% = 8.9%). Considering that lipid content in white adipose tissue is 82% (g/g; Jobgen et al. 2009) and that IPFR was 2.24% to 2.78% of body weight in LMB (Table 7), abdominal white adipose tissue contributed to about 30% (2.24% × 0.82/6.33% = 28.4%; 2.78% × 0.82/7.66% = 29.8%) of total lipids in the fish. Thus, abdominal adipose tissue is a main site of fat deposition in juvenile LMB. Increasing the dietary starch level from 5 to 15% or dietary lipid level from 10 to 12.5% promoted the accumulation of lipids in the abdominal cavity, these results indicate that the nutritional regulation of fat accretion in LMB is depot-specific. At present, it is unknown whether the enlarged fat mass in the body results from an increase in the volume of existing adipocytes (hypertrophy) and/or the number of adipocytes through the proliferation and differentiation of precursor cells (hyperplasia or adipogenesis). Future studies are warranted to address this issue.
In monogastric animals (e.g., swine, poultry, and rats), chronic high intake of dietary starch or lipids results in the development of fatty liver and hepatic dysfunction (Wu 2020). This has been assumed to occur in fish. However, we observed that increasing the dietary starch level from 5 to 15% or the dietary lipid level from 10 to 12.5% did not increase lipid content in the liver of LMB but rather reduced lipid content in this organ (Table 9) likely due to reduced deposition of dietary lipids. Based on the retention of dietary lipids in LMB (Table 4) and their digestibility (90%), 21–46% of absorbed dietary lipids are oxidized to CO2 or excreted from the body. Notably, high dietary starch resulted in hepatic glycogenosis (Figs. 1 and 2). Thus, the hepatopathy in LMB fed high starch diets is not a fatty liver disease. In support of this view, we observed that: (1) the HSI, hepatocyte area, and hepatic glycogen concentrations increased substantially with increasing the dietary starch level from 5 to 15% (Tables 7 and 9; Fig. 1); and (2) excessive amounts of glycogen deposits are present in the cytosol of hepatocytes, as determined by the PAS stain (Fig. 1). The enlargement of the liver in LMB fed the 10–15% starch diets is due, in part, to the deposition of water, because 1 g glycogen is associated with 3 g water (Brooks and Fahey 1984), leading to structural abnormalities in the liver and possibly impairments in some of its metabolic functions (e.g., protein synthesis, bile production, and lipoprotein assembly). Our other study revealed that LMB fed diets with low protein and high starch exhibited hepatic glycogenosis (Li and Wu, unpublished work). Similar results were obtained in the present study, where LMB were fed diets containing 45% protein. Thus, high intake of starch can induce hepatic glycogenosis in LMB regardless of low or adequate protein intake. This species is highly sensitive to starch intake, because it has a limited ability to oxidize glucose in the body (Li and Wu 2019; Li et al. 2020). Of note, Goodwin et al. (2002) reported that juvenile LMB fed a 45% protein diet containing 21, 27 or 35% carbohydrate (nitrogen-free extract that included starch) exhibited hepatic glycogenosis and growth restriction. In contrast, Amoah et al. (2008) did not detect any differences in the PER, hepatosomatic index, or liver glycogen concentration among fish fed three diets containing 13, 19, and 25% starch. This may be explained, based on the findings from the present study, that the control, 13% starch diet might have maximized its adverse effects on these variables.
The mechanisms responsible for the hepatic glycogenosis in LMB fed a diet containing ≥ 10% starch are not fully understood. There is a suggestion that some fish develop this disease due to their limited ability to control blood glucose concentration (Palmer et al. 1972; NRC 2011). In many fish species (Kamalam et al., 2017), as in terrestrial animals (Wu 2020), glucose can be converted into glycogen through glycogenesis in the liver and skeletal muscle. This was also observed in LMB (Table 10). Of particular note, the rates of glycogen synthesis from glucose in the liver of LMB were much greater than those for fatty acid synthesis (Table 10), indicating a high capacity for hepatic glycogenesis in LMB. In this study, we found that high dietary starch resulted in an 84–130% increase in serum glucose concentrations at 2 h after feeding in comparison with time 0 (24 h after feeding) and the elevated glucose levels remained through 8 h after feeding (Table 11). Such a prolonged post-prandial elevation in the circulating levels of glucose is not common in healthy terrestrial animals (Wu 2018) and is a characteristic of metabolism in LMB, possibly due to a low uptake of glucose by extra-hepatic tissues, including skeletal muscle and kidneys (Li and Wu 2019; Li et al. 2020). At the same extracellular glucose concentration (5 mM), the rate of conversion of glucose into glycogen in the liver did not differ among LMB fed the 5%, 10% and 15% starch diets, indicating that the machinery of hepatic glycogenesis (i.e., enzymes and regulation) was not altered by high starch intake. Thus, elevated blood glucose, which is derived from intestinal absorption after feeding, increased the intake of glucose by the liver to drive hepatic glycogen synthesis because of enhanced substrate provision. This leads to hepatic glycogenosis. Such a metabolic disease not only reduces the AFI of LMB and impairs their growth, but also causes their deaths (Goodwin et al. 2002).
In conclusion, the retention of dietary protein in LMB was affected by dietary starch or lipid levels and their interactions. The liver of LMB had a high capacity for synthesizing glycogen from glucose and a much lower capacity for converting glucose into fatty acids. Compared with the liver, the synthesis of fatty acids or glycogen from glucose was limited in the skeletal muscle of LMB. A dietary level of starch ≥ 10% resulted in hepatic glycogenosis due to prolonged elevations of blood glucose for glycogen synthesis in the liver, as well as reductions in the AFI and weight gain of LMB. Compared with a 10%-lipid diet, a dietary level of 12.5%-lipid decreased the AFI, growth and survival of the fish. Increasing the dietary starch level from 5 to 15% or the lipid level from 10 to 12.5% did not lead to a fatty liver in LMB. Based on these results, we recommend dietary lipid and starch levels to be 10% and < 10% (dry matter basis), respectively, for juvenile LMB to maximize the retention of dietary protein in their bodies.
Abbreviations
- AFI:
-
Absolute feed intake (g/fish)
- BW:
-
Body weight
- CP:
-
Crude protein
- FCR:
-
Feed conversion ratio
- HSI:
-
Hepatosomatic index
- IPFR:
-
Intraperitoneal fat ratio
- LMB:
-
Largemouth bass
- NO:
-
Nitric oxide
- PAS:
-
Periodic acid-Schiff
- PER:
-
Protein efficiency ratio
- VSI:
-
Viscerosomatic index
- WG:
-
Weight gain
References
Amirkolaie AK, Verreth JAJ, Schrama JW (2006) Effect of gelatinization degree and inclusion level of dietary starch on the characteristics of digesta and faeces in Nile tilapia (Oreochromis niloticus). Aquaculture 260:194–205
Amoah A, Coyle SD, Webster CD, Durborow RM, Bright LA, Tidwell JH (2008) Effects of graded levels of carbohydrate on growth and survival of largemouth bass, Micropterus salmoides. J World Aquac Soc 39:397–405
Borges P, Oliveira B, Casal S, Dias J, Conceiçao L, Valente LM (2009) Dietary lipid level affects growth performance and nutrient utilisation of Senegalese sole (Solea senegalensis) juveniles. Br J Nutr 102:1007–1014
Bright LA, Coyle SD, Tidwell JH (2005) Effect of dietary lipid level and protein energy ratio on growth and body composition of largemouth bass Micropterus salmoides. J World Aquac Soc 36:129–134
Brooks G, Fahey T (1984) Exercise physiology. Macmillan Publishing Co, New York
Cai ZN, Qian XQ, Xie SQ (2020) Optimal dietary protein concentrations for largemouth bass (Micropterus salmoides) of different sizes (10–500 g). Aquac Inter 28:831–840
Chen YJ, Yuan RM, Liu YJ, Yang HJ, Liang GY, Tian LX (2015) Dietary vitamin C requirement and its effects on tissue antioxidant capacity of juvenile largemouth bass, Micropterus salmoides. Aquaculture 435:431–436
Cowey CB, Sargent JR (1977) Lipid nutrition in fish. Comp Biochem Physiol B 57:269–273
Ding G, Li S, Wang A, Chen N (2019) Effect of chicken haemoglobin powder on growth, feed utilization, immunity and haematological index of largemouth bass (Micropterus salmoides). Aquac Fish. https://doi.org/10.1016/j.aaf.2019.04.003
Dumas A, De Lange CF, France J, Bureau DP (2007) Quantitative description of body composition and rates of nutrient deposition in rainbow trout (Oncorhynchus mykiss). Aquaculture 273:165–181
Durborow R (2019) Largemouth bass. In: USDA national institute of food and agriculture extension resource. https://freshwater-aquaculture.extension.org/largemouth-bass.
Ellis SC, Reigh RC (1991) Effects of dietary lipid and carbohydrate levels on growth and body composition of juvenile red drum, Sciaenops ocellatus. Aquaculture 97:383–394
El-Sayed AFM, Garling DL Jr (1988) Carbohydrate-to-lipid ratios in diets for Tilapia zillii fingerlings. Aquaculture 73:157–163
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Fry JP, Mailloux NA, Love DC, Milli MC, Cao L (2018) Feed conversion efficiency in aquaculture: do we measure it correctly? Environ Res Lett 13:024017
Fu DA, Campbell-Thompson M (2017) Periodic acid-Schiff staining with diastase. Methods Mol Biol 1639:145-149
Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu G (2005) Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr 135:714–721
Fynn-Aikins K, Hung SS, Liu W, Li H (1992) Growth, lipogenesis and liver composition of juvenile white sturgeon fed different levels of D-glucose. Aquaculture 105:61–72
Gjedrem T (2000) Genetic improvement of cold-water fish species. Aquac Res 31:25–33
Gong Y, Yang F, Hu J, Liu C, Liu H, Han D, Jin J, Yang Y, Zhu X, Yi J, Xie S (2019) Effects of dietary yeast hydrolysate on the growth, antioxidant response, immune response and disease resistance of largemouth bass (Micropterus salmoides). Fish Shellfish Immunol 94:548–557
Goodwin AE, Lochmann RT, Tieman DM, Mitchell AJ (2002) Massive hepatic necrosis and nodular regeneration in largemouth bass fed diets high in available carbohydrate. J World Aquac Soc 33:466–477
Guo JL, Kuang WM, Zhong YF, Zhou YL, Chen YJ, Lin SM (2020) Effects of supplemental dietary bile acids on growth, liver function and immunity of juvenile largemouth bass (Micropterus salmoides) fed high-starch diet. Fish Shellfish Immunol 97:602–607
Han T, Li X, Wang J, Hu S, Jiang Y, Zhong X (2014) Effect of dietary lipid level on growth, feed utilization and body composition of juvenile giant croaker Nibea japonica. Aquaculture 434:145–150
Hemre GI, Sandnes K, Lie Ø, Torrissen O, Waagbø AR (1995) Carbohydrate nutrition in Atlantic salmon, Salmo salar L.: growth and feed utilization. Aquac Res 26:149–154
Huang D, Wu Y, Lin Y, Chen J, Karrow N, Ren X, Wang Y (2017) Dietary protein and lipid requirements for juvenile largemouth bass, Micropterus salmoides. J World Aquac Soc 48:782–790
Jafri AK (1995) Protein-sparing effect of dietary carbohydrate in diets for fingerling Labeo rohita. Aquaculture 136:331–339
Jia S, Li X, Zheng S, Wu G (2017) Amino acids are major energy substrates for tissues of hybrid striped bass and zebrafish. Amino Acids 49:2053–2063
Jobgen WJ, Meininger CJ, Jobgen SC, Li P, Lee M-J, Smith SB, Spencer TE, Fried SK, Wu G (2009) Dietary L-arginine supplementation reduces white-fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr 139:230–237
Kamalam BS, Medale F, Panserat S (2017) Utilization of dietary carbohydrates in farmed fishes: new insights on influencing factors, biological limitations and future strategies. Aquaculture 467:3–27
Khan MA, Abidi SF (2012) Effect of varying protein-to-energy ratios on growth, nutrient retention, somatic indices, and digestive enzyme activities of Singhi, Heteropneustes fossilis (Bloch). J World Aquac Soc 43:490–501
Li XY, Wu G (2019) Oxidation of energy substrates in tissues of largemouth bass (Micropterus salmoides). J Anim Sci 97(Suppl 3):68–69
Li P, Wu G (2020) Composition of amino acids and related nitrogenous nutrients in feedstuffs for animal diets. Amino Acids 52:671–691
Li XY, Wang JT, Han T, Hu SX, Jiang YD (2015) Effects of dietary carbohydrate level on growth and body composition of juvenile giant croaker Nibea japonica. Aquac Res 46:2851–2858
Li S, Lian X, Chen N, Wang M, Sang C (2018) Effects of dietary vitamin E level on growth performance, feed utilization, antioxidant capacity and nonspecific immunity of largemouth bass, Micropterus salmoides. Aquac Nutr 24:1679–1688
Li S, Sang C, Wang A, Zhang J, Chen N (2019) Effects of dietary carbohydrate sources on growth performance, glycogen accumulation, insulin signaling pathway and hepatic glucose metabolism in largemouth bass, Micropterus salmoides. Aquaculture 513:734391
Li XL, Zheng SX, Jia SC, Song F, Zhou CP, Wu G (2020) Oxidation of energy substrates in tissues of largemouth bass (Micropterus salmoides). Amino Acids. https://doi.org/10.1007/s00726-020-02871-y
Lie Ø, Lied E, Lambertsen G (1988) Feed optimization in Atlantic cod (Gadus morhua): fat versus protein content in the feed. Aquaculture 69:333–341
Luo Y, Wu X, Li W, Jiang S, Lu S, Wu M (2016) Effects of different corn starch levels on growth, protein input, and feed utilization of juvenile hybrid grouper (male Epinephelus lanceolatus × female E. fuscoguttatus). North Am J Aquac 78:168–173
Matthias BG, Ahrens RN, Allen MS, Tuten T, Siders ZA, Wilson KL (2018) Understanding the effects of density and environmental variability on the process of fish growth. Fish Res 198:209–219
Mohanta KN, Mohanty SN, Jena JK, Sahu NP (2008) Optimal dietary lipid level of silver barb, Puntius gonionotus fingerlings in relation to growth, nutrient retention and digestibility, muscle nucleic acid content and digestive enzyme activity. Aquac Nutr 14:350–359
NRC (National Research Council) (2011) Nutrient requirements of fish and shrimp. National Academy Press, Washington, DC
Palmer RH (1972) Bile acids, liver injury, and liver disease. Arch Intern Med 130:606–617
Pierce LR, Palti Y, Silverstein JT, Barrows FT, Hallerman EM, Parsons JE (2008) Family growth response to fishmeal and plant-based diets shows genotype × diet interaction in rainbow trout (Oncorhynchus mykiss). Aquaculture 278:37–42
Portz L, Cyrino JEP, Martino RC (2001) Growth and body composition of juvenile largemouth bass Micropterus salmoides in response to dietary protein and energy levels. Aquacu Nutr 7:247–254
Rehman ZU, Kamran J, El-Hack MA, Alagawany M, Bhatti SA, Ahmad G, Saleem A, Ullah Z, Yameen RMK, Ding C (2018) Influence of low-protein and low-amino acid diets with different sources of protease on performance, carcasses and nitrogen retention of broiler chickens. Anim Prod Sci 58:1625–1631
Ren X, Wang Y, Chen JM, Wu YB, Huang D, Jiang DL, Li P (2018) Replacement of fishmeal with a blend of poultry byproduct meal and soybean meal in diets for largemouth bass, Micropterus salmoides. J World Aquac Soc 49:155–164
Rueda-Jasso R, Conceiçao LE, Dias J, De Coen W, Gomes E, Rees JF, Soares F, Dinis MT, Sorgeloos P (2004) Effect of dietary non-protein energy levels on condition and oxidative status of Senegalese sole (Solea senegalensis) juveniles. Aquaculture 231:417–433
Salmerón C (2018) Adipogenesis in fish. J Exp Biol 221:jeb161588
Song MQ, Shi CM, Lin SM, Chen YJ, Shen HM, Luo L (2018) Effect of starch sources on growth, hepatic glucose metabolism and antioxidant capacity in juvenile largemouth bass, Micropterus salmoides. Aquaculture 490:355–361
Subhadra B, Lochmann R, Rawles S, Chen R (2006) Effect of dietary lipid source on the growth, tissue composition and hematological parameters of largemouth bass (Micropterus salmoides). Aquaculture 255:210–222
Sveier H, Wathne E, Lied E (1999) Growth, feed and nutrient utilisation and gastrointestinal evacuation time in Atlantic salmon (Salmo salar L.): the effect of dietary fish meal particle size and protein concentration. Aquaculture 180:265–282
Tan Q, Xie S, Zhu X, Lei W, Yang Y (2007) Effect of dietary carbohydrate-to-lipid ratios on growth and feed utilization in Chinese longsnout catfish (Leiocassis longirostris Günther). J Appl Ichthyol 23:605–610
Tidwell JH, Coyle S, Bright LA (2007) Effects of different types of dietary lipids on growth and fatty acid composition of largemouth bass. North Am Aquac 69:257–264
Tidwell JH, Coyle SD, Bright LA (2019) Largemouth bass aquaculture Largemouth bass production in China. 5M published ltd, Sheffield, pp 37–47
Vásquez-Torres W, Arias-Castellanos JA (2013) Effect of dietary carbohydrates and lipids on growth in cachama (Piaractus brachypomus). Aquac Res 44:1768–1776
Wang Y, Liu YJ, Tian LX, Du ZY, Wang JT, Wang S, Xiao WP (2005) Effects of dietary carbohydrate level on growth and body composition of juvenile tilapia, Oreochromis niloticus× O. aureus. Aquac Res 36:1408–1413
Wang QD, Cheng L, Liu JS, Li ZJ, Xie SQ, De Silva SS (2015) Freshwater aquaculture in PR China: trends and prospects. Rev Aquac 7:283–302
Wang J, Li X, Han T, Yang Y, Jiang Y, Yang M, Xu Y, Harpaz S (2016a) Effects of different dietary carbohydrate levels on growth, feed utilization and body composition of juvenile grouper Epinephelus akaara. Aquaculture 459:143–147
Wang J, Jiang Y, Li X, Han T, Yang Y, Hu S, Yang M (2016b) Dietary protein requirement of juvenile red spotted grouper (Epinephelus akaara). Aquaculture 450:289–294
Watanabe T (1982) Lipid nutrition in fish. Comp Biochem Physiol B 73:3–15
Wilson RP (1994) Utilization of dietary carbohydrate by fish. Aquaculture 124:67–80
Wu G (2018) Principles of animal nutrition. CRC Press, Boca Raton
Wu G (2020) Management of metabolic disorders (including metabolic diseases) in ruminant and nonruminant animals. In: Bazer FW, Lamb GC, Wu G (eds) Animal agriculture: challenges, innovations, and sustainability. Elsevier, New York, pp 471–492
Wu G, Field CJ, Marliss EB (1991) Glutamine and glucose metabolism in thymocytes from normal and spontaneously diabetic BB rats. Biochem Cell Biol 69:801–808
Wu G, Knabe DA, Yan W, Flynn NE (1995) Glutamine and glucose metabolism in enterocytes of the neonatal pig. Am J Physiol 268:R334–R342
Wu G, Bazer FW, Cross HR (2014) Land-based production of animal protein: impacts, efficiency, and sustainability. Ann NY Acad Sci 1328:18–28
Yang Y, Han T, Xiao J, Li X, Wang J (2018) Transcriptome analysis reveals carbohydrate-mediated liver immune responses in Epinephelus akaara. Sci Rep 8:639
Zhang JM, He WL, Yi D, Zhao D, Song Z, Hou YQ, Wu G (2019) Regulation of protein synthesis in porcine mammary epithelial cells by L-valine. Amino Acids 51:717–726
Zhong YF, Shi CM, Zhou YL, Chen YJ, Lin SM, Tang RJ (2020) Optimum dietary fiber level could improve growth, plasma biochemical indexes and liver function of largemouth bass, Micropterus salmoides. Aquaculture 518:734661
Zhou YL, Guo JL, Tang RJ, Ma HJ, Chen YJ, Lin SM (2020) High dietary lipid level alters the growth, hepatic metabolism enzyme, and anti-oxidative capacity in juvenile largemouth bass Micropterus salmoides. Fish Physiol Biochem 46:125–134
Acknowledgements
We thank students and research assistants in our laboratory for helpful discussions. Financial support by Guangdong Yeuhai Feeds Group Co., Ltd. is gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
This study involved the chemical analyses of animal-source and plant-source feedstuffs. No approval of animal use protocols was required.
Informed consent
No informed consent is required for this study.
Additional information
Handling Editor: F. Blachier.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, X., Zheng, S., Ma, X. et al. Effects of dietary starch and lipid levels on the protein retention and growth of largemouth bass (Micropterus salmoides). Amino Acids 52, 999–1016 (2020). https://doi.org/10.1007/s00726-020-02869-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-020-02869-6