Abstract
Mechanistic target of rapamycin complex1 (mTORC1) activation and protein synthesis varied with methionine sources; however, the related mechanisms are largely unknown. Porcine mammary epithelial cells (PMEC) and mammary tissue slices (MTS) were used to test whether methionine precursors differ in providing the available methionine and thus differ in mTORC1 signaling-associated protein synthesis. PMEC with methionine deprivation for 8 h and MTS from lactating sows were cultured for 24 and 2 h, respectively, with treatment media without methionine (negative control, NC) or supplemented with 0.6 mM (for PMEC) and 0.1 mM (for MTS) of l-methionine (l-MET), d-methionine (d-MET), dl-2-hydroxy-4-(methylthio) butyric acid (HMTBA), or keto-methyl(thio)butanoic acid (KMB). The measurements included: phosphorylation of mTORC1 signaling, fractional protein synthesis rate (FSR), amino acids (AA) profile, and enzyme activities. Compared with the NC treatment, activated mTORC1 signaling as manifested by higher (P < 0.05) protein abundance of phosphorylated-S6 Kinase 1 (P-S6K1) and phosphorylated-4E-binding Protein 1 (P-4E-BP1) in PMEC and MTS, and increased protein synthesis as indicated by higher (P < 0.05) FSR in MTS occurred in l-MET and HMTBA treatments rather than in d-MET treatment. Compared with the NC treatment, methionine concentration and ratio of methionine to lysine in MTS increased (P < 0.05) in l-MET and HMTBA treatments but not in d-MET treatment, and activities of enzymes responsible for conversion of d-MET and HMTBA to keto-methionine in mammary tissues were about 10 and 50%, respectively, of that in liver. Taken together, mTORC1 signaling-associated protein synthesis in porcine mammary glands was regulated by the local available methionine depending on methionine sources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Milk synthesis referred to a lot of anabolic biological processes, such as the proliferation of mammary epithelial cells, protein synthesis, and lipid synthesis. mTOR (mechanistic target of Rapamycin) has emerged as a major signaling node to control over these anabolic processes (Howell et al. 2013; Huang et al. 2013). It has been shown that methionine could promote protein synthesis, lipid synthesis, and proliferation of mammary cells through mTOR signaling pathway (Appuhamy et al. 2012; Arriola Apelo et al. 2014; Huang et al. 2013; Zhang et al. 2014). However, these researches were all based on the model of bovine epithelial cells which may differ from species (Shennan and Peaker 2000). Furthermore, the regulation of methionine sources on mTOR signaling pathway was inconsistent and poorly elucidated so far. A previous study conducted with QM7 myoblast cells (Avian muscle cell line) showed that S6 Kinase 1 (S6K1) phosphorylation and protein synthesis were promoted by l-methionine (l-MET), not by methionine precursors including dl-2-hydroxy-4-(methylthio) butyric acid (HMTBA) and d-methionine (d-MET) (Métayer-Coustard et al. 2010). In contrast, studies in porcine kidney fibroblast cells showed that d-MET and HMTBA both promoted cell proliferation and protein synthesis (Schreiner and Jones 1987).
In addition to cell models, studies in animal models also showed some differences in utilization of methionine precursors. Our recent studies in sows have shown that increasing consumption of methionine as HMTBA rather than as dl-MET improved milk fat, lactose, cysteine, and taurine concentrations (Zhang et al. 2015). Further studies in dairy cows indicated that d-MET could not be utilized by mammary glands (Lapierre et al. 2012), while HMTBA was utilized in a constant rate by mammary glands (Lapierre et al. 2011). It is well documented that conversion to l-MET, the only methionine directly utilized by organisms, is required for the utilization of d-MET and HMTBA, in which oxidizing d-MET and HMTBA to keto-methionine is a rate-limiting step (Dibner and Ivey 1992). Moreover, different organs or tissues in sheep (Mccollum et al. 2000), pigs (Fang et al. 2010a) and poultry (Knight and Dibner 1984) have varying capacities for conversion of methionine precursors. However, little is known about whether the differences in conversion capacities have resulted in differences in mTOR activation and protein synthesis between different cells (muscle myoblast cell vs. kidney fibroblast cell) or between methionine sources (d-MET vs. HMTBA).
In the present study, we hypothesized that d-MET and HMTBA might differ in providing the available methionine and thus differ in mTOR signaling-associated protein synthesis. To test the hypothesis, porcine mammary epithelial cells (PMEC) and lactating mammary tissue slices (MTS) were used as models, and the measurements included the phosphorylation of mTOR and its downstream proteins S6K1 and eukaryotic initiation factor 4E-Binding Protein 1 (4E-BP1), protein synthesis rates, and the relative activities of enzymes essential for conversion of methionine precursors. The PMEC experiment was to determine mTOR activation status in response to methionine sources and the MTS experiment was to confirm the association of mammary protein synthesis with methionine source-induced changes in mTORC1 signaling.
Methods
The study protocol was approved by the Animal Care and Use Committee of Animal Nutrition Institute, Sichuan Agricultural University, and the study was carried out in accordance with the NRC Guide for the Care and Use of Laboratory Animals.
Reagents
DMEM/F12, fetal bovine serum, and penicillin–streptomycin–neomycin mixture were purchased from invitrogen (Shanghai, China). DMEM/F12 without methionine and phenylalanine was custom-made by Merck (Beijing, China). l-MET and d-MET were purchased from Ryon (Shanghai, China) and HMTBA was offered by Adisseo (Antony, France). Epidermal growth factor (EGF) and recombinant human prolactin were products of Peprotech (Suzhou, China). Insulin, hydrocortisone, MTBSTFA, CH3CN, Hepes, sulfosalicylic acid, trichloroacetic acid, sucrose, phenylalanine, 1-13C-phenylalanine, and 2-keto-4-(methylthio)butyric acid (KMB) were products of Sigma (Shanghai, China). Lysis buffer for Western blot and immunoprecipitation (IP), bovine serum albumin, and phenylmethanesulfonyl fluoride (PMSF) were products of Beyotime (Shanghai, China). Polyvinylidene fluoride (PVDF) membrane, non-fat dry milk, Tris, and chemiluminescent HRP substrate were purchased from Bio-rad (Hercules, USA). Antibodies of phospho-mTOR (Ser2448), mTOR, phospho-S6K1 (Thr389), S6K1, phospho-4E-BP1 (Thr37/46), 4E-BP1, and anti-rabbit IgG were products of cell signaling technology (Danvers, USA), and corresponding catalog numbers were 3018, 2972, 9205, 9202, 2855, 9452, and 7074. The ion-exchange chromatography was a product of Agela (Chengdu, China). Anti-pan cytokeratin antibody, histostain-SP kit, and DAB kit were products of Bioss (Beijing, China). Stadie-Riggs hand-held microtome was product of Thomas Scientific (Swedesboro, USA).
Cells and tissues
Two separate experiments were conducted using primary PMEC and MTS from lactating sows. The primary culture of PMEC was conducted exactly as previously described (Dahanayaka et al. 2015) and characterized by expression of cytokeratin. Briefly, cytokeratin was identified by a histostain-SP kit with anti-pan cytokeratin antibody according to manufacturer’s instructions and then visualized by a DAB kit. β-casein mRNA (GenBank accession number X54974) was also detected to confirm a normal function of PMEC by primers as follows: forward 5′-TCATCCTTGCCTGCTTCGTG-3′ and reverse 5′-ATTCTGGCGTTCATTCTCTGTT-3′. The tenth generation of PMEC was seeded on six-well plates at ~ 30% confluency, cultured at 37 °C in 5% CO2, and grown to ~ 90% confluency in complete DMEM/F12 supplemented with 10% (v:v) fetal bovine serum, 1% (v:v) Penicillin–Streptomycin–Neomycin mixture and 10 ng/mL EGF before treatment exposure. Six primiparous lactating sows (Landrace × Yorkshire), deemed free of mastitis, were anaesthetised at the fourth hour after receiving a same amount of meal at day 28 postpartum. Immediately after anaesthesia, suckled mammary glands were split down the mid-line and mammary tissues were excised from the center portion of the anterior two pairs of mammary glands and immediately kept in D-Hanks buffer. Then, sows were slaughtered to collect stomach, intestine, liver, kidney, muscle, subcutaneous fat, and mammary tissues used for enzyme assay. Obvious pieces of connective tissue and fat attached to mammary tissues were removed. MTS (~ 200 mg) were prepared within 30 min of slaughter using a Stadie-Riggs hand-held microtome (to make sure the thickness of 0.5 mm) and placed in 25-mL Erlenmeyer flasks containing one of five treatment media (5 mL).
PMEC experiment
PMEC cells were placed in six-well plates and grown to ~ 90% confluence in complete medium. Cells were incubated for 8 h in serum-free, DMEM/F12 media without methionine to set a methionine-deprived status. Then cells were washed twice with PBS, followed by incubation with one of six treatment media. The treatment medium without methionine was referred to the negative control (NC) and the rest five treatment media were prepared by adding 0.6 mM of l-MET, d-MET, HMTBA, 70:30 mix of l-MET:d-MET, or 70:30 mix of l-MET:HMTBA to the NC medium. Total methionine concentration in PMEC was according to a previous research (Zhang et al. 2014). The total sulfur AA (TSAA) in each medium was 0.7 mM and thus in either of the mixes methionine precursor (d-MET or HMTBA) was up to 25% of TSAA, which was based on our previous study in vivo (Zhang et al. 2015). All media were adjusted to a pH of 7.4, were serum-free, and contained 15 mM Hepes, 5 μg/mL insulin, 10 ng/mL EGF, 1 μg/mL hydrocortisone, 100 ng/mL prolactin, 35.48 mg/L phenylalanine, and 0.02 mM phenol red. After incubation for 24 h, cells were harvested with lysis buffer for Western blot and IP for protein extraction. Cell lysates from six wells were combined. The experiment was repeated three times on three different days.
MTS experiment
The treatment medium without methionine was referred to the NC and the rest four treatments media were prepared by adding 0.1 mM of l-MET, d-MET, HMTBA, or KMB. Total methionine concentration in MTS was set up according to a previous research (Arriola Apelo et al. 2014) and it was close to methionine concentration in vivo. Phenylalanine in the treatment media was all replaced with 1-13C-phenylalanine (1-13C-Phe) for the measurement of fractional synthesis rate of protein (FSR). MTS from each sow were cultured in duplicate by each of the five treatments media in 25-mL Erlenmeyer flasks, purged with 95:5 mix of O2:CO2 for 20 s, sealed with rubber stoppers, and placed in a shaking (60 oscillation/min) water bath at 37 °C (time 0 h). At 120 min, the flasks were placed on ice and 5 mL of the ice-cold stop buffer was added to each flask. MTS were retrieved, washed twice in the ice-cold stop buffer, dried with filter paper, and stored in − 80 °C for further analysis. The media maintained pH throughout the incubation as indicated by the phenol red. We identified the viability of MTS by measuring the ATP levels present in MTS (Supplemental Figure 1). The 2-h culture time was based on previous reports that various metabolic and synthetic processes remained linear for at least 2 h in incubated mammary tissues (Arriola Apelo et al. 2014; Baumrucker and Stemberger 1989; Dimenna and Emery 1980).
Protein abundance for P-mTOR, mTOR (a), P-S6K1, S6K1 (b), and P-4E-BP1, 4E-BP1 (c) in porcine mammary epithelial cells (PMEC). PMEC were cultured for 24 h by the medium without methionine (negative control, NC) or supplemented with 0.6 mM of l-methionine (l-MET), d-methionine (d-MET), dl-2-hydroxy-4-(methylthio) butyric acid (HMTBA), 70:30 mix of l-MET:d-MET (l + d), or 70:30 mix of l-MET:HMTBA (l + H). Values are means ± SEMs (n = 3). Means without a common letter differ, P < 0.05
Western blot analysis of mTOR-pathway proteins
For total protein extraction, PMEC and frozen MTS samples were homogenized with cell lysis buffer for Western blot and IP which was supplemented with PMSF. The proteins were separated on 10% polyacrylamide gels and blotted onto PVDF membrane. The membrane was blocked in TBS-T buffer (50 mM Tris–HCl, 150 mM NaCl, 0.1% Tween, pH 7.6) supplemented with 5% non-fat dry milk or 3% bovine serum albumin (for phosphorylated protein), followed by incubation of the specific primary antibody of phospho-mTOR (Ser2448), mTOR, phospho-S6K1 (Thr389), S6K1, phospho-4E-BP1 (Thr37/46), 4E-BP1, and alpha-tubulin. Blots were then incubated with HRP-linked secondary antibody anti-rabbit IgG. The signal was detected with chemiluminescent HRP substrate. Densitometric analyses of Western blot bands were performed using the Image Lab™ software system (Bio-Rad).
Fractional synthesis rate of protein
A 20-μL aliquot of MTS homogenate containing 5-mg protein/mL was combined with 20 μL of 6% (m:v) sulfosalicylic acid to precipitate protein and centrifuged at 15,000×g for 20 min at 4 °C. Precipitates were hydrolyzed with 6 mol/L HCl at 110 °C for 20 h. The hydrolysate and the supernatant collected from precipitation were desalted by ion-exchange chromatography and converted to N-(tert-butyldimethyl) amino acid derivatives with CH3CN and MTBSTFA as previously described (Calder and Smith 1988). Protein-bound 1-13C-Phe enrichment (EPB) (%) and intracellular free 1-13C-Phe enrichment (EIC) (%) were quantified by GC–MS (GC–MS: GC6890/MS5973, Agilent) using selected ion monitoring to determine the ration of the 239 and 234 m/z ions. Calibration curves were gravimetrically generated to determine EPB and EIC. Fractional synthesis rate of protein (FSR) was calculated as
where t is the total incubation time of MTS in media (2 h) containing 1-13C-Phe and the units of FSR are % h−1.
Amino acid analysis
For free AA analysis, 1-g frozen tissue was homogenized in 5-mL pure water. The protein precipitation procedure was carried out as described previously (10). Briefly, 1 mL of the sample and 2.5 mL of 7.5% (w/v) trichloroacetic acid solution were mixed thoroughly and centrifuged at 12,000×g and 4 °C for 15 min. Then, the supernatant was collected and analyzed for AA by ion-exchange chromatography using an L8800 high-speed AA analyzer (Hitachi) as described by our previous study (Li et al. 2014).
Enzyme activity assay
Tissue samples including the stomach, duodenum, jejunum, ileum, liver, kidney, muscle, subcutaneous fat, and mammary gland were dissected free from connective tissue and/or intraperitoneal fat, rinsed, and homogenized into four volumes of homogenizing buffer and diluted 1:1 with potassium phosphate buffer as previously described (Dibner and Ivey 1992). The homogenizing buffer consisted of 0.25-M sucrose, 0.01-M PMSF, and 0.02-M potassium phosphate. The potassium phosphate buffer consisted of 0.02-M potassium phosphate, pH 7.5. The specific activity of the enzymes involved in the conversion of HMTBA and d-MET to KMB in each of the tissues was determined as described (Fang et al. 2010a) with substrates of HMTBA and d-MET. One unit of enzyme activity was defined as that amount yielding 1 nmol of KMB per gram of tissue under the standard reaction conditions.
Statistical analysis
Data were analyzed using the GLM procedures of SAS statistical package (V8.1, SAS Institute Inc., Cary, NC, USA). Duncan’s multiple range test was used to evaluate differences among treatments when the treatment effect was significant. A simple linear regression model (PROC REG, SAS V8.1) was used to test the statistical correlation of FSR with each of the measurements including phosphorylation ratios of mTOR and its target proteins, free amino acids levels and ratios of these amino acids to lysine. It was considered statistically significant at P values ≤ 0.05.
Results
Protein abundance of mTOR signaling in PMEC
Compared with the NC treatment, the addition of methionine source mixes l + d and l + H improved (P < 0.05) the abundance of phosphorylated-mTOR (P-mTOR) and mTOR (Fig. 1a). However, the abundance of P-mTOR and mTOR proteins was not affected (P > 0.05) by the addition of single methionine sources including l-MET, d-MET, and HMTBA. Compared with the NC treatment, the protein abundance of phosphorylated-S6K1 (P-S6K1) (Fig. 1b) and phosphorylated-4E-BP1 (P-4E-BP1) (Fig. 1c) both were increased (P < 0.05) by the addition of single methionine sources including l-MET and HMTBA and by the addition of methionine source mixes l + d and l + H. The protein abundance of 4E-BP1 was also increased by the addition of l-MET (Fig. 1c). In contrast, the addition of d-MET showed no effect (P > 0. 05) on the protein abundance of S6K1, P-S6K1, 4E-BP1, and P-4E-BP1.
Protein abundance of mTOR signaling and FSR in MTS
Compared with the NC treatment, the protein abundances of P-mTOR (Fig. 2a), P-S6K1 (Fig. 2b), and P-4E-BP1 (Fig. 2c) were all increased (P < 0.05) by the addition of either l-MET or HMTBA, while decreased P-4E-BP1 (P < 0.05) was observed following the addition of d-MET. The protein abundances of P-mTOR, P-S6K1, and P-4E-BP1 in the KMB treatment did not differ from those in the NC treatment (P > 0.05), but was higher than those in the d-MET treatment. Compared with the NC treatment, the addition of l-MET and HMTBA both resulted in increased FSR (P < 0.05), and no difference (P > 0.05) was observed among the NC, d-MET, and KMB treatments (Fig. 3).
Protein abundance for P-mTOR, mTOR (a), P-S6K1, S6K1 (b), P-4E-BP1, 4E-BP1 (c) in porcine mammary tissue slices (MTS). MTS were cultured for 2 h by the medium without methionine (negative control, NC) or supplemented with 0.1 mM of l-methionine (l-MET), d-methionine (d-MET), dl-2-hydroxy-4-(methylthio) butyric acid (HMTBA), or 2-keto-4-(methylthio) butyric acid (KMB). Values are means ± SEMs (n = 4). Means without a common letter differ, P < 0.05
Fractional synthesis rate of protein (FSR) in porcine mammary tissue slices (MTS). MTS were cultured for 2 h by the medium without methionine (negative control, NC) or supplemented with 0.1 mM of l-methionine (l-MET), d-methionine (d-MET), dl-2-hydroxy-4-(methylthio) butyric acid (HMTBA), or 2-keto-4-(methylthio) butyric acid (KMB). Values are means ± SEMs (n = 4). Means without a common letter differ, P < 0.05
Free amino acid profile in MTS
Concentrations of free essential amino acids (lysine, methionine, threonine, leucine, isoleucine, valine, phenylalanine, and histidine) and most of non-essential amino acids (arginine, proline, serine, glycine, alanine, and tyrosine) present in MTS were decreased (P < 0.05) following a 2-h culture by the treatment media with and without the addition of methionine sources (Table 1). Interestingly, compared with the MTS before treatment (at 0 h), the ratios of methionine, leucine, proline, and serine to lysine were decreased (P < 0.05), while the ratios of threonine and ornithine to lysine were increased (P < 0.05) following a 2-h culture by the NC treatment (Table 2). Compared with the NC treatment, the addition of l-MET, HMTBA, and KMB all increased (P < 0.05) the ratio of methionine to lysine in MTS, while no difference (P > 0.05) was observed between the NC and d-MET treatments. The addition of l-MET also resulted in a higher ratio of arginine to lysine (P < 0.05) compared with the HMTBA and KMB treatments. However, the ratios of most of amino acids (isoleucine, valine, phenylalanine, histidine, aspartate, glutamate, glycine, alanine, cysteine, and tyrosine) to lysine were not affected (P > 0.05) following a 2-h culture by the treatment media (Table 2).
Enzyme activity
Kidney had the highest activity of d-MET oxidase, followed by liver, duodenum, and jejunum, with the lowest d-MET oxidase activity observed in mammary gland, which is about 10% of that in the liver (Fig. 4). In contrast, liver had the highest activities of enzymes responsible for conversion of HMTBA to KMB, followed by muscle, subcutaneous fat, and kidney (Fig. 5). Activities of enzymes responsible for conversion of HMTBA to KMB in stomach, duodenum, jejunum, ileum, and mammary gland were about half of that in liver.
Correlation of FSR with amino acids levels and phosphorylation state of signaling proteins
FSR was positively correlated (P < 0.05) with P-mTOR/tubulin, P-S6K1/tubulin, and P-4E-BP1/tubulin (Table 3). Moreover, FSR was positively correlated (P < 0.05) with methionine and methionine/lysine. The P-mTOR/tubulin was positively correlated (P < 0.05) with arginine, while P-S6K1/tubulin and P-4E-BP1/tubulin were positively correlated (P = 0.060 and P < 0.05, respectively) with methionine (Table 3).
Discussion
Our previous study in vivo indicated a strong association between milk composition and extra addition of methionine sources in lactating sows’ diets (Li et al. 2014). Specifically, sows fed HMTBA showed higher milk protein, fat, and lactose concentrations than those fed dl-MET (Zhang et al. 2015), which indicates the significant difference in milk synthesis between sows fed different methionine sources. Further analysis revealed that though milk-free methionine levels were not affected by methionine sources, milk cysteine and taurine concentrations were higher in the HMTBA fed than in the dl-MET fed sows, which suggested that the difference in milk synthesis might be associated with the metabolism of methionine sources in mammary glands. Thus, in the present study, PMEC and MTS were further used as models to test whether methionine precursors differ in providing the available methionine and thus differ in mTOR signaling-associated protein synthesis.
Given that activation of mTOR signaling was affected by concentrations of methionine (Nan et al. 2014), PMEC in a methionine-deprived status was first used to test how mTOR and its downstream signaling proteins respond to different methionine sources. The comparison of protein abundances between the NC and l-MET treatments indicated that continuous methionine-deprived status in PMEC caused a lower S6K1 and 4E-BP1 phosphorylation, but the mTOR phosphorylation was not changed. Consistent with our results, studies in bovine mammary epithelial cells also observed unchanged mTOR phosphorylation following methionine deprivation (Appuhamy et al. 2012). It has been indicated that mTOR interacts with several proteins to form two distinct complexes named mTOR complexes 1 (mTORC1) and 2 (mTORC2). The activation of mTORC1 has been correlated with increased nutrient uptake and metabolism, which leads to cell proliferation and growth (Csibi et al. 2013). In contrast, mTORC2 plays a central role in negatively controlling whole-body growth size (Cybulski et al. 2009). Moreover, it is well documented that mTORC1 and mTORC2 have different sensitivities to rapamycin as well as upstream inputs and downstream outputs, which might explain why mTOR phosphorylation was unchanged, though mTORC1 activation was attenuated as manifested by lower S6K1 and 4E-BP1 phosphorylation in the NC treatment. These observations suggested that mTOR phosphorylation was not always an indication of mTORC1 activation. This notion was further supported by the evidence that among methionine sources evaluated, l + d and l + H treatments showed the highest increase in mTOR phosphorylation, but no difference was observed in mTORC1 activation as indicated by S6K1 and 4E-BP1 phosphorylation status among l-MET, l + d, and l + H treatments.
An important finding in the present study was that among different methionine sources, HMTBA and l-MET showed a similar effect while d-MET showed no effect on mTORC1 activation. Most recently, a study in mouse C2C12 cells indicated that mTORC1 activation via T1RI/T1R3-PLCβ-Ca2+-ERK1/2 pathway required the specificity of amino acids, among which l-MET showed the highest activity (Zhou et al. 2016) and another study indicated that amino acids activated mTORC1 in an obligate two-step mechanism including priming and activation, with l-MET and branched-chain amino acids acting as the activating amino acids (Dyachok et al. 2016). Further evidence indicated that activation of S6K1, the downstream protein of mTORC1 signaling, occurred when leucine was available as the l-isoform rather than the d-isoform (Nagamori et al. 2016), which further indicates the importance of the spatial structures of amino acids. It would appear that methionine precursors including d-MET and HMTBA might not activate mTORC1 signaling unless converted to l-MET.
To our knowledge, protein synthesis is positively correlated with mTORC1 activation (Dickinson et al. 2011) and mTORC1 activation can be associated with relative phosphorylation of S6K1 and 4E-BP1 (Nicklin et al. 2009) or relative abundance of P-S6K1 and P-4E-BP1 (Gundermann et al. 2014). To confirm the importance of methionine availability in mTORC1 activation and protein synthesis, we further evaluated the response of MTS to treatment media with different methionine precursors. In this MTS model, we found that FSR was linearly correlated with the abundance of P-mTOR, P-S6K1, and P-4E-BP1 rather than relative phosphorylation of these proteins except 4E-BP1. This might be associated with culture time, given that total protein would be more consistent in a relatively shorter time from 5 min to 1 h (Dennis et al. 2013; Gordon et al. 2014; Nicklin et al. 2009), while total protein would always change when culture time increased to a longer time such as 24 h (Huang et al. 2013; Lu et al. 2013; Zhang et al. 2014). Furthermore, mTORC1 activation, as manifested by increased abundance of P-S6K1 and P-4E-BP1, was linearly correlated with the concentration of methionine. Notably, the addition of l-MET and its precursor HMTBA both increased methionine concentration in the MTS and resulted in mTORC1 activation. In contrast, d-MET showed no effect on methionine concentration and mTORC1 activation compared with the NC treatment. Taken together, these results indicated that mTORC1 signaling-associated protein synthesis was regulated by the local availability of methionine in porcine mammary glands. Noting that the absolute value of most of free amino acids in MTS appeared lower than the initial value observed in MTS0, we proposed that the 2 h of incubation did not allow treatments to recover the MTS0 value. Mammary gland undergoes great anabolism at lactation period and amino acids are largely consumed to synthesize protein or involve other metabolism. It has been indicated that the blood flow in lactating sows was about 7000 L/days through the mammary gland, which means about 5 L blood flow per minute (Guan et al. 2004). Thus, it is plausible that the amino acids in medium may be not sufficient to maintain an original concentration of amino acids in mammary gland tissues following a 2-h incubation.
Given that l-MET is the only methionine directly utilized by organisms, activities of enzymes essential for conversion of methionine precursors might play an important role in the decision of the local availability of methionine and thus mTORC1 activation. The rate-limiting enzymes for conversion of d-MET, d- and l-isoforms of HMTBA to l-MET are d-amino acid oxidase (d-AAOX), d-2-hydroxy acid dehydrogenase (d-HADH), and L-2-hydroxy acid oxidase (l-HAOX), respectively (Knight and Dibner 1984). The appropriate pH required in enzyme assay is 7.5 and 8.24 for d-HADH and l-HAOX, respectively. However, limited by the availability of methionine sources, HMTBA with d- and l-isoforms, respectively, accounting for 50% of the total, instead of single d- or l-HMBTA, was used as the substrate for enzyme assay, with pH 7.5 set in the reaction system, which might have resulted in underestimation of capability of HMTBA conversion by mammary tissues. This notion was supported by the evidence that there were lower yields of KMB, converted from HMTBA, by most of tissues of sows compared with those determined in the same tissues of piglets in our previous study (Fang et al. 2010a). In contrast, the reaction conditions for the assay of D-AAOX activity was the same as that used in our previous study (Fang et al. 2010a). Accordingly, the yields of KMB, converted from d-MET, determined in most of tissues of sows were close to those determined in the same tissues of piglets, indicating actual estimation on capability of d-MET conversion by target tissues. Thus, we proposed that mammary tissues had higher capability of converting HMTBA than converting d-MET, though similar yields (around 20 nmol/g tissue/min) of KMB, converted from these two methionine sources, were observed in the present enzyme assay conditions. In addition, activities of enzymes responsible for conversion of d-MET and HMTBA to KMB in mammary tissues were about 10 and 50%, respectively, of that in liver, which implies that mammary tissues may play more important roles in HMTBA conversion than in d-MET conversion, while these two methionine sources are metabolized in vivo. This notion might be supported by the observation that milk cysteine and taurine levels were higher in HMTBA fed than in dl-MET fed sows, while these two methionine sources were supplied at equi-molar basis (Zhang et al. 2015), which suggested that more amount of methionine or its precursors was metabolized in mammary tissues, while sows were fed HMTBA, instead of dl-MET. Another important finding in the present study was that the concentration of arginine and its ratio to lysine in MTS decreased following a 2-h culture by media with either HMTBA or KMB compared with l-MET treatment. There is evidence that arginine underwent great catabolism in mammary gland (O’Quinn et al. 2002) and thus served as a donor of amino group for other amino acids (Mepham 1982). It would appear that arginine might serve as the main amino group donor in the process of HMTBA and KMB conversion to l-MET in porcine mammary tissues.
Despite that methionine was positively correlated with P-S6K1 and P-4E-BP1, the relatively higher methionine in HMTBA than in l-MET treatment did not cause a difference in P-S6K1 and P-4E-BP1 abundance. This might be associated with relatively higher arginine level in l-MET than in HMTBA treatment. In support of this view, arginine was positively correlated with P-mTOR which induces phosphorylation of target protein S6K1 and 4E-BP1. Interestingly, though equi-molar amounts of methionine sources were supplied, HMTBA showed higher increment than l-MET in increasing methionine levels and ratio of methionine to lysine present in MTS. This might be associated with the competition of amino acid transporters. Studies in rat retinal capillary endothelial cells (Tomi et al. 2005) and human tumor cell lines (Yanagida et al. 2001) showed that high concentration of d-MET and l-MET could result in a lower transport efficiency of l-leucine and vice versa. Studies in sows (Zhang et al. 2015) and laying hens (Wan 2016) indicated that systemic plasma lysine levels were decreased by increased consumption of methionine as Dl-MET but not as HMTBA. Lysine and methionine have been reported to share the B0,+ and b0,+ transport systems (Broer 2008), and mutual inhibition occurs among lysine, methionine, leucine, and arginine when transported across mammary gland tissues from lactating sows (Hurley et al. 2000). In the present study, culture media contained 0.7-mM arginine, 0.5-mM lysine, 0.45-mM leucine, and 0.1-mM methionine. It would appear that the relatively higher levels of arginine, lysine, and leucine might affect methionine uptake. In contrast, HMTBA, a chemically short chain fatty acid, was transported through diffusion or monocarboxylate transporters (Martin-Venegas et al. 2007) and, therefore, could be efficiently transported into cells without compromising or being compromised by other amino acids (Fang et al. 2010b). Similarly, KMB could also be absorbed by diffusion, and higher concentration of methionine present in MTS was observed following KMB supplementation compared with the NC and d-MET treatments. Accordingly, significantly increased or a tendency (P < 0.10) to increase mTORC1 signaling activation was observed in the KMB treatment compared with the d-MET and NC treatment. However, just a tendency (P < 0.10) to increase FSR was observed in the KMB treatment compared with the NC treatment. This might be associated with that KMB could activate CTBP1 (Achouri et al. 2007) which is a transcriptional co-repressor involved in a wide range of cellular events (Stankiewicz et al. 2014) and the methanethiol released as a result of the instability of KMB may cause enzyme inhibition (Yang 1969). Furthermore, the instability of KMB might explain that concentration of methionine present in the MTS was lower in the KMB treatment than in the HMTBA treatment. A similar study in porcine kidney fibroblast cells (Schreiner and Jones 1987) indicated that high concentration (5 mM) of KMB could inhibit the growth of cells, while similar doses of d-MET and HMTBA did not, indicating the negative control of higher concentration of KMB on cell proliferation, an anabolic biological process conversely regulated by mTORC1 signaling.
Conclusions
This study provided the first evidence that mTORC1 signaling-associated protein synthesis in porcine mammary glands was regulated by the local availability of methionine, which is dependent on supplemental methionine sources.
References
Achouri Y, Noel G, Van Schaftingen E (2007) 2-Keto-4-methylthiobutyrate, an intermediate in the methionine salvage pathway, is a good substrate for CtBP1. Biochem Biophys Res Commun 352:903–906. https://doi.org/10.1016/j.bbrc.2006.11.111
Appuhamy JRN, Knoebel NA, Nayananjalie WD, Escobar J, Hanigan MD (2012) Isoleucine and leucine independently regulate mTOR signaling and protein synthesis in MAC-T cells and bovine mammary tissue slices. J Nutr 142:484–491
Arriola Apelo SI et al (2014) Casein synthesis is independently and additively related to individual essential amino acid supply. J Dairy Sci 97:2998–3005
Baumrucker CR, Stemberger BH (1989) Insulin and insulin-like growth factor-I stimulate DNA synthesis in bovine mammary tissue in vitro. J Anim Sci 67:3503
Broer S (2008) Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88:249–286. https://doi.org/10.1152/physrev.00018.2006
Calder AG, Smith A (1988) Stable isotope ratio analysis of leucine and ketoisocaproic acid in blood plasma by gas chromatography/mass spectrometry. Use of tertiary butyldimethylsilyl derivatives. Rapid Commun Mass Spectrom 2:14–16
Csibi A et al (2013) The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell 153:840–854
Cybulski N, Polak P, Auwerx J, Rüegg MA, Hall MN (2009) mTOR complex 2 in adipose tissue negatively controls whole-body growth. Proc Natl Acad Sci USA 106:9902–9907
Dahanayaka S et al (2015) Technical note: Isolation and characterization of porcine mammary epithelial cells. J Anim Sci 93:5186–5193
Dennis MD, Kimball SR, Jefferson LS (2013) Mechanistic target of rapamycin complex 1 (mTORC1)-mediated phosphorylation is governed by competition between substrates for interaction with raptor. J Biol Chem 288:10–19
Dibner JJ, Ivey FJ (1992) Capacity in the liver of the broiler chick for conversion of supplemental methionine activity to l-methionine. Poult Sci 71:700–708
Dickinson JM et al (2011) Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr 141:856–862
Dimenna GP, Emery RS (1980) Factors affecting fatty acid oxidation in bovine mammary tissue. Lipids 15:497–503
Dyachok J, Earnest S, Iturraran EN, Cobb MH, Ross EM (2016) Amino acids regulate mTORC1 by an obligate two-step mechanism. J Biol Chem 291:22414–22426. https://doi.org/10.1074/jbc.M116.732511
Fang Z, Luo H, Wei H, Huang F, Qi Z, Jiang S, Peng J (2010a) Methionine metabolism in piglets fed dl-methionine or its hydroxy analogue was affected by distribution of enzymes oxidizing these sources to keto-methionine. J Agric Food Chem 58:2008–2014
Fang Z, Huang F, Luo J, Wei H, Ma L, Jiang S, Peng J (2010b) Effects of dl-2-hydroxy-4-methylthiobutyrate on the first-pass intestinal metabolism of dietary methionine and its extra-intestinal availability. Br J Nutr 103:643–651. doi:10.1017/S0007114509992169
Gordon BS, Kazi AA, Coleman CS, Dennis MD, Chau V, Jefferson LS, Kimball SR (2014) RhoA modulates signaling through the mechanistic target of rapamycin complex 1 (mTORC1) in mammalian cells. Cell Signal 26:461–467
Guan X, Pettigrew JE, Ku PK, Ames NK, Bequette BJ, Trottier NL (2004) Dietary protein concentration affects plasma arteriovenous difference of amino acids across the porcine mammary gland. J Anim Sci 82:2953–2963
Gundermann DM, Walker DK, Reidy PT, Borack MS, Dickinson JM, Volpi E, Rasmussen BB (2014) Activation of mTORC1 signaling and protein synthesis in human muscle following blood flow restriction exercise is inhibited by rapamycin. Am J Physiol Endocrinol Metab 306:E1198–E1204
Howell JJ, Ricoult SJ, Ben-Sahra I, Manning BD (2013) A growing role for mTOR in promoting anabolic metabolism. Biochem Soc Trans 41:906–912
Huang Y et al (2013) SOCS3-mediated blockade reveals major contribution of JAK2/STAT5 signaling pathway to lactation and proliferation of dairy cow mammary epithelial cells in vitro. Molecules 18:12987–13002
Hurley WL, Wang H, Bryson JM, Shennan DB (2000) Lysine uptake by mammary gland tissue from lactating sows. J Anim Sci 78:391–395
Knight JJDA, Dibner JJ (1984) Conversion of 2-hydroxy-4-(methylthio) butanoic acid to l-methionine in the chick: a stereospecific pathway. J Nutr 114:1723–1726
Lapierre H, Vázquez-Añón M, Parker D, Dubreuil P, Holtrop G, Lobley GE (2011) Metabolism of 2-hydroxy-4-(methylthio) butanoate (HMTBA) in lactating dairy cows. J Dairy Sci 94:1526–1535
Lapierre H, Holtrop G, Calder AG, Renaud J, Lobley GE (2012) Is d-methionine bioavailable to the dairy cow? J Dairy Sci 95:353–362
Li H et al (2014) Changes in plasma amino acid profiles, growth performance and intestinal antioxidant capacity of piglets following increased consumption of methionine as its hydroxy analogue. Br J Nutr 112:855–867
Lu L, Li Q, Huang J, Gao X (2013) Proteomic and functional analyses reveal MAPK1 regulates milk protein synthesis. Molecules 18:263–275. https://doi.org/10.3390/molecules18010263
Martin-Venegas R, Rodriguez-Lagunas MJ, Geraert P, Ferrer R (2007) Monocarboxylate transporter 1 mediates dl-2-hydroxy-(4-methylthio)butanoic acid transport across the apical membrane of Caco-2 cell monolayers. J Nutr 137:49–54
Mccollum MQ, Vázquezañón M, Dibner JJ, Webb KE (2000) Absorption of 2-hydroxy-4-(methylthio)butanoic acid by isolated sheep ruminal and omasal epithelia. J Anim Sci 78:1078–1083
Mepham TB (1982) Amino acid utilization by lactating mammary gland. J Dairy Sci 65:287–298
Métayer-Coustard S et al (2010) Methionine deprivation regulates the S6K1 pathway and protein synthesis in avian QM7 myoblasts without activating the GCN2/eIF2 alpha cascade. J Nutr 140:1539–1545
Nagamori S et al (2016) Structure–activity relations of leucine derivatives reveal critical moieties for cellular uptake and activation of mTORC1-mediated signaling. Amino Acids 48:1045–1058
Nan X et al (2014) Ratio of lysine to methionine alters expression of genes involved in milk protein transcription and translation and mTOR phosphorylation in bovine mammary cells. Physiol Genom 46:268–275
Nicklin P et al (2009) Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136:521–534
O’Quinn PR, Knabe DA, Wu G (2002) Arginine catabolism in lactating porcine mammary tissue. J Anim Sci 80:467–474
Schreiner CL, Jones EE (1987) Metabolism of methionine and methionine hydroxy analogue by porcine kidney fibroblasts. J Nutr 117:1541–1549
Shennan DB, Peaker M (2000) Transport of milk constituents by the mammary gland. Physiol Rev 80:925–951
Stankiewicz TR, Gray JJ, Winter AN, Linseman DA (2014) C-terminal binding proteins: central players in development and disease. Biomol Concepts 5:489–511
Tomi M, Mori M, Tachikawa M, Katayama K, Terasaki T, Hosoya K (2005) l-type amino acid transporter 1-mediated l-leucine transport at the inner blood-retinal barrier. Invest Ophthalmol Vis Sci 46:2522–2530. https://doi.org/10.1167/iovs.04-1175
Wan JM (2016) Effects of methionine source and level on hepatic methionine and lipid metabolism of laying hens. PhD dissertation, Sichuan Agricultural University
Yanagida O et al (2001) Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta (BBA) Biomembr 1514:291–302
Yang SF (1969) Further studies on ethylene formation from α-Keto-γmethylthiobutyric acid or β-methylthiopropionaldehyde by peroxidase in the presence of sulfite and oxygen. J Biol Chem 244:4360–4365
Zhang X et al (2014) GSK3β regulates milk synthesis in and proliferation of dairy cow mammary epithelial cells via the mTOR/S6K1 signaling pathway. Molecules 19:9435–9452
Zhang X et al (2015) Differences in plasma metabolomics between sows fed dl-methionine and its hydroxy analogue reveal a strong association of milk composition and neonatal growth with maternal methionine nutrition. Br J Nutr 113:585–595
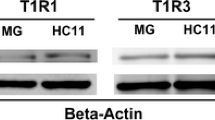
Zhou Y, Ren J, Song T, Peng J, Wei H (2016) Methionine regulates mTORC1 via the T1R1/T1R3-PLCβ-Ca2+-ERK1/2 signal transduction process in C2C12 cells. Int J Mol Sci 17(10):1684
Acknowledgements
The project was funded by the Rhodimet Research Grant from Adisseo France S.A.S., Briand, Antony Cedex, France, and the National Natural Science Foundation of China (31472109).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y Zhang, P Wang, S lin, H Yin, Y Song, X Zhang, L Che, Y lin, S Xu, B Feng, D Wu, and Z Fang, no conflicts of interest; Y Mercier is the member of Adisseo, the producer of methionine.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Handling Editor: G. J. Peters.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Wang, P., Lin, S. et al. mTORC1 signaling-associated protein synthesis in porcine mammary glands was regulated by the local available methionine depending on methionine sources. Amino Acids 50, 105–115 (2018). https://doi.org/10.1007/s00726-017-2496-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-017-2496-0