Abstract
Taurine has been reported to influence bone metabolism, but the role of taurine on osteogenic differentiation of human mesenchymal stem cells (hMSCs) remains unclear. In the present study, we investigated the effect of taurine on osteogenic differentiation of hMSCs. The results showed that taurine increased the alkaline phosphatase (ALP) activity and mineralized nodules in hMSCs induced by osteogenic induced medium. Meanwhile, RT-PCR analysis showed that taurine up-regulated the mRNA expression of ALP, osteopontin, Runt-related transcription factor 2 (Runx2) and Osterix in a dose-dependent manner. Furthermore, taurine induced activation of extracellular signal regulated kinase (ERK) and pretreatment with the ERK inhibitor U0126 abolished the taurine-induced osteogenesis of hMSCs. Taken together, our study reveals that taurine promotes the osteogenesis of hMSCs by activating the ERK pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is the most common metabolic bone disease which is characterized by a low bone mass and the deterioration of bone microarchitecture, leading to bone fragility and susceptibility to fracture (Kanis et al. 2009; Rachner et al. 2011). Bone mass homeostasis depends on the balance between bone formation and bone resorption (Deal 2009; Kanis et al. 2009). Osteoblasts and osteoclasts are two types of cells that form and resorb bone, respectively. The main mechanism of osteoporosis is an imbalance between the activity of osteoblasts that form bone and osteoclasts that break it down (Ke et al. 2012; Rachner et al. 2011).
Mesenchymal stem cells (MSCs) are multipotent stromal cells that can differentiate into a variety of cell types such as osteoblasts, adipocytes, chondrocytes, myoblasts and neurons (Pittenger et al. 1999). Studies showed that osteoblasts arose from MSCs and the osteogenic differentiation of MSCs was decreased in osteoporotic patients (Benisch et al. 2012; Dalle Carbonare et al. 2009). Osteoporosis might be due to defects in MSCs that lead to reduced proliferation and osteoblast differentiation (Prall et al. 2013). Recent studies have demonstrated that MSCs could be used to treat bone fracture and osteoporotic bone defect (Wang et al. 2013; Cao et al. 2012). Furthermore, the study by Guan et al. (2012) found that directing MSCs to bone could augment bone formation and increase bone mass. Therefore, enhancing osteogenesis of MSCs is thought to be a useful therapeutic strategy for bone diseases such as osteoporosis (Pino et al. 2012).
Taurine (2-aminoethanesulfonic acid) is an organic acid widely distributed, and is detected in high concentration in mammalian tissues (Terauchi et al. 1998; Wang et al. 2011). Taurine has a variety of biological actions such as antioxidation, osmoregulation, modulation of ion movement, and conjugation of bile acids (Rahman et al. 2011; Schaffer et al. 2010). Recently, studies showed that taurine transporter was expressed in human osteoblast-like MG63 cells, murine osteoblasts MC3T3-E1 cells, and human primary osteoblast (Yuan et al. 2006). Taurine also increased ALP activity and collagen synthesis in osteoblast-like UMR-106 cells (Park et al. 2001). Furthermore, taurine also stimulated ALP activity and osteocalcin secretion (Yuan et al. 2006) and inhibited osteoclastogenesis through the taurine transporter (Yuan et al. 2010). However, the role of taurine on osteogenic differentiation of hMSCs remains unclear. In this study, we investigated the effect of taurine on osteogenic differentiation of hMSCs.
Materials and methods
Reagents and cell culture
Taurine, dexamethasone, beta-glycerophosphate, l-ascorbic acid-2-phosphate, dimethyl sulfoxide (DMSO) and methyl thiazolyl tetrazolium (MTT) were purchased from Sigma Chemical Company (St. Louis, MO, USA). Taurine was dissolved in modified Eagle’s medium of alpha (a-MEM) (Gibco) and sterilized via a 0.2-μm filter. Human fetal bone marrow-derived MSCs were from the Stem Cell Bank in the Prince of Wales Hospital. The hMSCs were kept in a-MEM (Gibco) supplemented 10 % fetal bovine serum (FBS) (Gibco) and 1 % penicillin/streptomycin (Gibco).
Osteogenic differentiation and taurine treatment
Osteogenic differentiation of MSCs was induced by osteogenic induction medium (OIM) containing 100 nmol/L dexamethasone, 10 mmol/L beta-glycerophosphate, and 0.05 mmol/L l-ascorbic acid-2-phosphate. After cells reached confluence, the culture medium was changed into OIM and the OIM was changed every 3 days. The concentration and exposure time of taurine were changed according to different experiments.
Cell viability
Cells were seeded in a 96 flat-bottomed well plate at density 5 × 103 per well. After 24 h, cells were incubated by media containing different concentrations taurine at 37 °C for 1 and 3 days. Then, cells were treated with the 0.5 mg/ml MTT solution for 4 h at 37 °C. After removal of the MTT solution, cells were treated with 150 μL DMSO and the plate was shaken for 10 min. The absorbance was measured at 570 nm with a microplate reader.
Alkaline phosphatase (ALP) activity
After MSCs were treated with OIM and taurine for 7 days, the cells in 12-well plates were washed with PBS and lysed by lysis buffer consisting 20 mM Tris–HCl (pH 7.5), 150 mM NaCl, and 1 % Triton X-100. The ALP activity was determined using the ALP assay kit (BioSystems, Spain). The protein concentration of cell lysates was measured using the Bradford assay at 595 nm on a microplate spectrophotometer (Bio-Rad, USA). ALP activity was normalized according to the total protein concentration of cell lysate.
ALP staining
After MSCs were treated with OIM and taurine for 7 days, the cells were washed with PBS twice and fixed with 70 % ethanol for 10 min. The cells were equilibrated with ALP buffer (0.15 M NaCl, 0.15 M Tris–HCl, 1 mM MgCl2, PH 9.0) for 15 min. Then the cells were incubated with ALP substrate solution (5 μL BCIP and 10 μL NBT in l mL ALP buffer) at 37 °C in dark for 60 min and the reaction was stopped by distilled water.
Mineralization assay
After 14 days of osteogenic induction, the MSCs were fixed with 70 % ethanol for 10 min. Then the cells were stained with 0.5 % alizarin red S (pH 4.1) for 10 min at room temperature and washed three times with deionized water. Orange red staining indicated the position and intensity of calcium deposits. Alizarin red was extracted by the addition of 10 % cetylpyridinium chloride (CPC, Sigma) and quantified by measuring the OD of the extract at 550 nm.
Quantitative real-time PCR
Total cellular RNA was extracted with RNeasy Mini Kit (Qiagen, USA) and first-strand cDNA was synthesized using M-MLV reverse transcriptase (Promega, USA) according to the manufacturer’s instructions. Quantitative real-time PCR was performed according to procedures in a previous study (Yao et al. 2012). In brief, amplification conditions were as follows: initial denaturation at 95 °C for 5 min, and then 40 cycles of 95 °C for 15 s and 60 °C for 60 s. Primer sequences were as follows: ALP—forward, 5′ctcccagtctcatctcct3′; reverse, 5′aagacctcaactcccctgaa3′; osteopontin (OPN)—forward, 5′gtaccctgatgctacagacg3′; reverse, 5′ttcataactgtccttcccac3′; Runx2—forward, 5′acttcctgtgctcggtgct3′; reverse, 5′gacggttatggtcaaggtgaa3′; Osterix (Osx)—forward, 5′ccaggcaacactcctactcc3′; reverse, 5′gccttgccatacaccttgc3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH)—forward, 5′ggcatggactgtggtcatgag3′; reverse, 5′tgcaccaccaactgttagc3′. The relative gene expression was presented with the 2−ΔΔCT method, and each sample was normalized to the expression level of GAPDH.
Western blot
Cells were harvested and equal amounts of proteins were separated on 10 % sodium dodecyl sulfate (SDS)-polyacrylamide gels. Then proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore) for 75 min at 100 V and the membrane was blocked in TBST (10 mM Tris, pH 7.6, 150 mM NaC1, 0.05 % Tween 20) containing 5 % skim milk for 1 h at room temperature. The membranes were washed five times with PBS containing 0.1 % Tween 20 (PBST) and incubated, respectively, with anti-ERK1/2 (BD) or anti-p-ERK1/2 (BD) antibodies at 4 °C overnight. After the membranes were washed five times with PBST, proteins were detected with the enhanced chemiluminescence blotting reagents (Amersham Biosciences) according to the manufacturer’s instruction. The band intensity was quantified using Quantity One software.
Statistical analysis
Data were presented as the mean ± standard deviation and statistical analysis was performed using SPSS software (version 13.0, SPSS). One-way analysis of variance (ANOVA), followed by the least significant difference (LSD) test, was adopted for multiple-group comparison.
Results
Effect of taurine on viability of hMSCs
The effect of taurine on cell viability was investigated at concentrations ranging 1–10 mM after 1, 3, 7 and 14 days of culture. The result showed that viability of hMSCs was not affected after exposure to taurine at concentrations of 1, 5 and 10 mM for 1, 3, 7 and 14 days (Fig. 1).
Taurine increased ALP activity of hMSCs
ALP is an early marker of osteogenic differentiation, so we examined the effects of taurine on the ALP activity of hMSCs. Data showed that taurine increased the ALP activity of hMSCs in a dose-dependent manner and maximal effect was observed at a concentration of 10 mM. As expected, similar result was observed by ALP staining (Fig. 2).
Taurine increased ALP activity of hMSCs. a ALP was stained with BCIP/NBT kit after hMSCs were incubated with taurine at different concentrations for 7 days. b The ALP activity of hMSCs was measured after they were incubated with different concentrations of taurine for 7 days. a p < 0.01 compared with Con
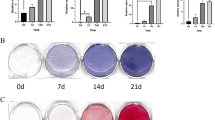
Taurine promoted mineralization of hMSCs
In addition, we examined the effects of taurine on the mineralization of hMSCs. Alizarin Red S (ARS) staining showed that taurine increased mineralized nodule formation in OIM after 14 days of culture (Fig. 3a). Quantification showed that taurine markedly increased calcium deposition in a dose-dependent manner compared with the control and maximal effect was observed at a concentration of 10 mM (Fig. 3b).
Taurine promoted mineralization of hMSCs. a MSCs were treated with taurine in OIM for 14 days, then the mineralized nodules were stained by Alizarin Red S. b Quantification of four independent mineralization experiments. The mineralization was quantified by extraction of Alizarin Red S dye with 10 % CPC. a p < 0.01 compared with Con
Taurine up-regulated mRNA expression levels of ALP, osteopontin (OPN), Runx2 and Osterix (Osx) in hMSCs
Furthermore, we detected the gene expression of ALP, OPN, Runx2 and Osx in hMSCs. Real-time PCR results showed that taurine significantly increased the mRNA expression levels of ALP, OPN, Runx2 and Osx in a dose-dependent manner (Fig. 4).
Taurine activated ERK pathway in hMSCs
ERK signaling pathway plays an important role in the osteogenic differentiation of MSCs (Lai et al. 2001; Ge et al. 2007), so we detected the level of p-ERK1/2. Western blot analysis revealed that taurine significantly increased the levels of p-ERK1/2 in a time-dependent manner. The levels of p-ERK1/2 dramatically increased after 15 min of exposure compared with control, and phosphorylated ERK remained activated for 90 min after taurine treatment (Fig. 5).
Effect of taurine on activation of ERK 1/2 in hMSCs. MSCs were treated with taurine for 15–90 min, and proteins were separated by 10 % SDS-PAGE and detected with the indicated antibodies. a Western blot analysis of p-ERK 1/2 levels in hMSCs. b Quantification of three independent Western blot experiments with the mean level of p-ERK normalized to total ERK. b p < 0.01 compared with Con
U0126 inhibited taurine-induced osteogenic differentiation in hMSCs
Furthermore, we detected the effect of U0126, inhibitors of ERK1/2, on taurine-induced osteogenic differentiation. As expected, 10 μM U0126 inhibited the increase of ALP, the mRNA expression of OSX, Runx2, OPN, and mineral deposition induced by taurine (Fig. 6).
Inhibition of taurine-induced osteogenic differentiation by U0126. MSCs were pretreated with U0126 for 1 h, followed by addition of 10 μM taurine. The medium was changed every 3 days. a The ALP activity of hMSCs was measured on 7 days. b The mineralized nodules were stained by Alizarin Red S and the mineralization was quantified by extraction of Alizarin Red S dye with 10 % CPC on 14 days. c The gene expressions of OSX, Runx2 and OPN were detected by real-time PCR on 7 days. a p < 0.01 compared with Con, b p < 0.01 compared with Taurine. A representative result from three independent experiments is shown
Discussion
Taurine, 2-aminoethanesulfonic acid, is an organic acid widely distributed and has been identified in high concentration in bone (Park et al. 2001). Recently, data showed that taurine played an important role in bone metabolism (Yuan et al. 2007, 2010) and taurine supplementation increased bone mineral density in rats (Choi and DiMarco 2009; Choi and Chang 2013). However, the mechanism of taurine regulation of bone metabolism has not been clarified.
In the present study, we detected the effect of taurine on viability of MSCs. We found that taurine did not affect the viability of hMSCs, indicating taurine was not cytotoxic to hMSCs.
ALP degrades pyrophosphate to generate phosphate, which reacts with calcium to form hydroxyapatite (Harrison et al. 1995). Hypophosphatasia, a rare inherited disorder, is characterized by hypomineralization of hard tissues (Whyte 2010), and primary osteoblasts isolated from ALP knockout mice were not able to initiate mineralization (Wennberg et al. 2000). Therefore, ALP plays an important role in bone mineralization and is an early marker of osteoblast differentiation. Our results indicated that taurine significantly increased the mRNA expression and activity of ALP. Taurine also enhanced calcium deposits in dose-dependent manner, suggesting that taurine accelerated mineralization of hMSCs. These results showed that taurine promoted the osteogenic differentiation of hMSCs in early and late stage.
The runt family transcription factor Runx2 and Osx is two pivotal transcription regulators in osteoblast differentiation. Intramembranous and endochondral ossification are completely blocked in Runx2 and Osx null mice (Ducy et al. 1997; Nakashima et al. 2002), indicating that Runx2 and Osx are required for osteoblastic differentiation. Osteopontin is an extracellular matrix protein and a marker of osteoblastic differentiation (Yao et al. 1994). Real-time PCR analysis showed that mRNA levels of ALP, OPN, Runx2 and Osx were up-regulated by taurine. The results indicated that taurine enhanced osteogenic differentiation of MSCs through mediating transcription factors of Runx2 and Osx.
A study by Park et al. (2001) showed that taurine increased alkaline phosphatase activity and collagen synthesis via ERK2 activation in osteoblast-like UMR-106 cells. Taurine also increased cell proliferation by activating ERK signal pathway (Jeon et al. 2007). However, studies found that taurine suppressed osteoblastic differentiation in vascular smooth muscle and aortic valve interstitial cells via ERK pathway (Feng et al. 2012; Liao et al. 2008). The discrepancy may be due to differences in cell types and culture condition. Thus, we explored the role of ERK1/2 in the taurine-mediated regulation of osteogenic differentiation in hMSCs. Our results demonstrated that taurine significantly increased the ERK1/2 phosphorylation. Furthermore, treatment with U1206 could efficiently inhibit the effect of taurine on ALP activity, mRNA expression of OSX, Runx2, OPN, and mineralized nodule formation in hMSCs. These data indicated that ERK1/2 pathway was involved in the taurine-mediated regulation of osteogenic differentiation of hMSCs.
In conclusion, the present data demonstrated that taurine promoted osteogenesis of hMSCs through activating ERK1/2 signaling pathways. This may be one of the mechanisms by which taurine increased bone mineral density. In addition, taurine is very safe in terms of tissue toxicity and is used as a supplement in baby milk. Thus, taurine might be useful in preventing osteoporosis and further investigations are needed to explore antiosteoporotic effect of taurine in osteoporotic patients.
References
Benisch P, Schilling T, Klein-Hitpass L et al (2012) The transcriptional profile of mesenchymal stem cell populations in primary osteoporosis is distinct and shows overexpression of osteogenic inhibitors. PLoS One 7(9):e45142
Cao L, Liu G, Gan Y et al (2012) The use of autologous enriched bone marrow MSCs to enhance osteoporotic bone defect repair in long-term estrogen deficient goats. Biomaterials 33(20):5076–5084
Choi MJ, Chang KJ (2013) Effect of dietary taurine and arginine supplementation on bone mineral density in growing female rats. Adv Exp Med Biol 776:335–345
Choi MJ, DiMarco NM (2009) The effects of dietary taurine supplementation on bone mineral density in ovariectomized rats. Adv Exp Med Biol 643:341–349
Dalle Carbonare L, Valenti MT, Zanatta M et al (2009) Circulating mesenchymal stem cells with abnormal osteogenic differentiation in patients with osteoporosis. Arthritis Rheum 60(11):3356–3365
Deal C (2009) Potential new drug targets for osteoporosis. Nat Clin Pract Rheumatol 5(1):20–27
Ducy P, Zhang R, Geoffroy V et al (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89(5):747–754
Feng X, Li JM, Liao XB et al (2012) Taurine suppresses osteoblastic differentiation of aortic valve interstitial cells induced by beta-glycerophosphate disodium, dexamethasone and ascorbic acid via the ERK pathway. Amino Acids 43(4):1697–1704
Ge C, Xiao G, Jiang D et al (2007) Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J Cell Biol 176(5):709–718
Guan M, Yao W, Liu R et al (2012) Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med 18(3):456–462
Harrison G, Shapiro IM, Golub EE (1995) The phosphatidylinositol-glycolipid anchor on alkaline phosphatase facilitates mineralization initiation in vitro. J Bone Miner Res 10(4):568–573
Jeon SH, Lee MY, Kim SJ et al (2007) Taurine increases cell proliferation and generates an increase in [Mg2+]i accompanied by ERK 1/2 activation in human osteoblast cells. FEBS Lett 581(30):5929–5934
Kanis JA, McCloskey EV, Johansson H et al (2009) Approaches to the targeting of treatment for osteoporosis. Nat Rev Rheumatol 5(8):425–431
Ke HZ, Richards WG, Li X et al (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33(5):747–783
Lai CF, Chaudhary L, Fausto A et al (2001) Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J Biol Chem 276(17):14443–14450
Liao XB, Zhou XM, Li JM et al (2008) Taurine inhibits osteoblastic differentiation of vascular smooth muscle cells via the ERK pathway. Amino Acids 34(4):525–530
Nakashima K, Zhou X, Kunkel G et al (2002) The novel zinc finger-containing transcription factor Osterix is required for osteoblast differentiation and bone formation. Cell 108(1):17–29
Park S, Kim H, Kim SJ (2001) Stimulation of ERK2 by taurine with enhanced alkaline phosphatase activity and collagen synthesis in osteoblast-like UMR-106 cells. Biochem Pharmacol 62(8):1107–1111
Pino AM, Rosen CJ, Rodríguez JP (2012) In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis. Biol Res 45(3):279–287
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Prall WC, Haasters F, Heggebö J et al (2013) Mesenchymal stem cells from osteoporotic patients feature impaired signal transduction but sustained osteoinduction in response to BMP-2 stimulation. Biochem Biophys Res Commun 440(4):617–622
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377(9773):1276–1287
Rahman MM, Park HM, Kim SJ et al (2011) Taurine prevents hypertension and increases exercise capacity in rats with fructose-induced hypertension. Am J Hypertens 24(5):574–581
Schaffer SW, Jong CJ, Ramila KC et al (2010) Physiological roles of taurine in heart and muscle. J Biomed Sci 17(Suppl 1):S2
Terauchi A, Nakazaw A, Johkura K et al (1998) Immunohistochemical localization of taurine in various tissues of the mouse. Amino Acids 15(1–2):151–160
Wang X, Chi D, Su G et al (2011) Determination of taurine in biological samples by high-performance liquid chromatography using 4-fluoro-7-nitrobenzofurazan as a derivatizing agent. Biomed Environ Sci 24(5):537–542
Wang X, Wang Y, Gou W et al (2013) Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int Orthop 37(12):2491–2498
Wennberg C, Hessle L, Lundberg P et al (2000) Functional characterization of osteoblasts and osteoclasts from alkaline phosphatase knockout mice. J Bone Miner Res 15(10):1879–1888
Whyte MP (2010) Physiological role of alkaline phosphatase explored in hypophosphatasia. Ann N Y Acad Sci 1192:190–200
Yao KL, Todescan R Jr, Sodek J (1994) Temporal changes in matrix protein synthesis and mRNA expression during mineralized tissue formation by adult rat bone marrow cells in culture. J Bone Miner Res 9:231–240
Yao D, Xie XH, Wang XL et al (2012) Icaritin, an exogenous phytomolecule, enhances osteogenesis but not angiogenesis—an in vitro efficacy study. PLoS One 7(8):e41264
Yuan LQ, Xie H, Luo XH et al (2006) Taurine transporter is expressed in osteoblasts. Amino Acids 31(2):157–163
Yuan LQ, Lu Y, Luo XH et al (2007) Taurine promotes connective tissue growth factor (CTGF) expression in osteoblasts through the ERK signal pathway. Amino Acids 32(3):425–430
Yuan LQ, Liu W, Cui RR et al (2010) Taurine inhibits osteoclastogenesis through the taurine transporter. Amino Acids 39(1):89–99
Acknowledgments
The authors thank financial support from National Natural Science Foundation of China (30772768), Natural Science Foundation of Guangdong Province (10152402301000000), Science and Technology Planning Project of Dongguan (2011108102019) and Science and Technology Innovation Fund of Guangdong Medical College (STIF201104).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Zhou and X. Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhou, C., Zhang, X., Xu, L. et al. Taurine promotes human mesenchymal stem cells to differentiate into osteoblast through the ERK pathway. Amino Acids 46, 1673–1680 (2014). https://doi.org/10.1007/s00726-014-1729-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1729-8