Abstract
The underlying pathophysiology of type 1 diabetes involves autoimmune-mediated islet inflammation, leading to dysfunction and death of insulin-secreting islet β cells. Recent studies have shown that polyamines, which are essential for mRNA translation, cellular replication, and the formation of the hypusine modification of eIF5A may play an important role in the progression of cellular inflammation. To test a role for polyamines in type 1 diabetes pathogenesis, we administered the ornithine decarboxylase inhibitor difluoromethylornithine to two mouse models—the low-dose streptozotocin model and the NOD model—to deplete intracellular polyamines, and administered streptozotocin to a third model, which was haploinsufficient for the gene encoding the hypusination enzyme deoxyhypusine synthase. Subsequent development of diabetes and/or glucose intolerance was monitored. In the low-dose streptozotocin mouse model, continuous difluoromethylornithine administration dose-dependently reduced the incidence of hyperglycemia and led to the preservation of β cell area, whereas in the NOD mouse model of autoimmune diabetes difluoromethylornithine reduced diabetes incidence by 50 %, preserved β cell area and insulin secretion, led to reductions in both islet inflammation and potentially diabetogenic Th17 cells in pancreatic lymph nodes. Difluoromethylornithine treatment reduced hypusinated eIF5A levels in both immune cells and islets. Animals haploinsufficient for the gene encoding deoxyhypusine synthase were partially protected from hyperglycemia induced by streptozotocin. Collectively, these studies suggest that interventions that interfere with polyamine biosynthesis and/or eIF5A hypusination may represent viable approaches in the treatment of diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 1 diabetes (T1D) is a disorder of glucose homeostasis that is characterized by an autoimmune reaction against islet β cells, coupled with an enhanced susceptibility of β cells to inflammatory dysfunction and death (Atkinson et al. 2011; Lehuen et al. 2010). In the non-obese diabetic (NOD) mouse model, pathogenesis of T1D begins early in life when the release of β cell antigens—possibly a result of normal neonatal β cell turnover—leads to accumulation and activation of antigen-presenting cells in the islet (Mathis et al. 2001). Subsequent presentation of these antigens to naïve T cells in the draining pancreatic lymph node leads to T cell differentiation and proliferation, whereby specific CD4+ effector T cell subtypes (most notably Th1, but possibly also Th17 cells) promote eventual β cell destruction. Regulatory T cells (Tregs) are thought to inhibit this process, and recent studies suggest that the balance between the effector T cells and Tregs may determine the overall susceptibility to β cell destruction (Cabrera et al. 2012). More recently, it has been suggested that the β cell itself may be an independent determinant in the progression of its own demise, such that specific genes or pathways in the β cell itself confer susceptibility or resistance to either initial antigen release or subsequent immune-mediated death (Atkinson et al. 2011; O’Sullivan-Murphy and Urano 2012). Whereas the mainstay of treatment for T1D is insulin replacement, several clinical trials have examined the potential for therapeutics that modulate immune tolerance or block the pathway beginning from antigen presentation to T cell differentiation and proliferation (Matthews et al. 2010). Although some of these clinical trials have shown initial preservation of insulin secretion (and, by inference, β cell mass and function) in new-onset diabetic subjects, none has resulted in durable preservation of insulin secretion or reversal of the disease process itself. Thus, there is an unmet need for the identification of new treatments that may impact T1D pathogenesis.
The native polyamines (putrescine, spermidine, and spermine) are polycationic aliphatic amines that influence nucleic acid structure and stability, modulate ion channel activity, govern mRNA translation rates, and are required for formation of the activating “hypusine” modification of the pro-inflammatory translational factor eIF5A (Igarashi and Kashiwagi 2010). Given their functions, alterations in polyamine accumulation might be expected to influence multiple aspects of T1D pathogenesis, including the production of autoantigens or formation of “neoantigens” in β cells, expression of costimulatory molecules, differentiation or proliferation of T cell subtypes, and the β cell inflammatory response (Brooks 2012). Polyamine accumulation in cells is controlled by three major processes: endogenous production, cellular uptake, and degradation. Production of polyamines is governed in part by the activity of the enzyme ornithine decarboxylase (ODC), which converts ornithine to putrescine (Igarashi and Kashiwagi 2010). Inhibition of ODC using the ornithine analog difluoromethylornithine (DFMO) has proved an attractive approach for depleting cellular polyamines in cells and in animals, and is clinically approved for use in humans. Although studies have shown that DFMO potently depletes putrescine and spermidine levels in islet β cells (Sjöholm et al. 1993), no study has directly addressed the effect of this reduction on the outcome of T1D in mouse models. As a prelude to potential studies in humans, here we undertook a study in three different mouse models of T1D to address the effect of polyamine depletion (by DFMO) and deoxyhypusine synthase (DHS) deficiency on diabetes development. Our results show that DFMO feeding of animals results in a dose-dependent decrease in T1D incidence and partial protection against diabetes under conditions of DHS deficiency. Our data suggest that the protective effects of polyamine depletion may be in part related to reductions in eIF5A hypusination.
Materials and methods
Animals and procedures
All procedures relating to mice were approved by the Indiana University Institutional Animal Care and Use Committee. 8-week-old male C57BL/6J and 5-week-old female NOD mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). The generation of mice heterozygous for deletion of the gene encoding DHS (Dhps) was described previously (Templin et al. 2011). Dhps+/− mice were maintained on a mixed C57BL6/129SvEv genetic background. Intraperitoneal glucose tolerance tests (GTTs) using 2 g/kg body weight glucose were performed as previously described (Evans-Molina et al. 2009). Islets and splenocytes were isolated also as described previously (Cabrera et al. 2013; Stull et al. 2012).
Diabetes induction and DFMO treatment
For the multiple low-dose streptozotocin (STZ) experiments, 8-week-old C57BL/6J mice were begun on difluoromethylornithine (DFMO, a gift from Dr. P. Woster) treatment at 0, 0.25, or 0.5 wt% added directly in the drinking water 3 days prior to STZ treatment, and then maintained on the respective dose of DFMO for the remainder of the study. STZ was administered intraperitoneally at a dose of 55 mg/kg body weight for 5 consecutive days as described previously (Maier et al. 2010). STZ experiments in Dhps+/− mice were performed similarly, except that mice did not receive DFMO. Blood glucose was monitored twice weekly using a hand-held glucometer and a GTT was performed at the end of the study (4 weeks following the start of DFMO treatment) (Evans-Molina et al. 2009).
For T1D prevention studies, female NOD mice were treated continuously with 0, 0.25, or 1.0 wt% DFMO in drinking water between 6 and 10 weeks of age. No differences in water intake between control and DFMO-treated animals were observed. Blood glucose was monitored weekly using a hand-held glucometer and diabetes was diagnosed when two consecutive blood glucose values exceeded 250 mg/dL.
Immunoblots, immunohistochemistry, β cell area, and insulitis scoring
Immunoblots from islet and splenocytes extracts were performed as described previously (Maier et al. 2010) using antibodies against eIF5A (BD Biosciences) and hypusinated eIF5A, and image quantifications were performed using Li-Cor software (Li-Cor Biosciences, Lincoln, NE, USA). For immunohistochemistry experiments, pancreata from mice were fixed, sectioned and stained for insulin as described (Evans-Molina et al. 2009). Images were acquired using a Zeiss AxioObserver Z1 equipped with a high-resolution color camera (Carl Zeiss, Thornwood, NY, USA). Islet β cell area was calculated as described previously (Maier et al. 2010) using three sections per pancreas (75 μm apart) from at least three mice per group. For insulitis scoring, three pancreas sections at least 75 μm apart from five animals per group were graded by two independent observers (one of whom was blinded to sample identity) using the following scheme: grade 1, no islet-associated mononuclear cell infiltrates; grade 2, peri-insulitis affecting <50 % of the circumference of the islet without evidence of islet invasion; grade 3, peri-insulitis affecting greater than 50 % of the circumference of the islet without evidence of islet invasion; grade 4, islet invasion.
Flow cytometric analysis
At the time of diabetes diagnosis or when mice aged to 25 weeks (whichever occurred first), single cell suspensions of pancreatic lymph node cells were prepared for flow cytometry as described (Cabrera et al. 2013). For analysis of Treg cell populations, equal volumes of the single cell suspensions were stained using anti-CD4 (clone RM4-5) and anti-CD25 (PC61.5) antibodies and fixed overnight before being permeabilized and stained with anti-Foxp3 (FJK-16s) antibody according to the manufacturer’s instructions (eBioscience, San Diego, CA, USA). For analysis of Th1 and Th17 cell populations, equal volumes of the single cell suspensions were first incubated with 1× Cell Stimulation Cocktail (eBioscience) for 4 h prior to staining with anti-CD4 antibody; cells were fixed overnight then permeabilized and stained for IL-17A (eBio17B7) and IFNγ (XMG1.2) according to manufacturer’s instructions (eBioscience). Cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) and FlowJo software (TreeStar, Ashland, OR, USA).
Serum measurements
Serum insulin levels were measured using the Ultra Sensitive Mouse Insulin ELISA kit (Crystal Chem, Downers Grove, IL, USA). The unmethylation index was determined using a modification of a PCR-based assay described previously (Husseiny et al. 2012).
Statistical analysis
All data are presented as the mean ± SEM. One-way ANOVA (with Bonferroni post-test) was used for comparisons involving more than two conditions, and a two-tailed Student t test was used for comparisons involving two conditions. Prism 5 software (GraphPad, La Jolla, CA, USA) was used for all statistical analyses. Statistical significance was assumed at P < 0.05.
Results
Polyamine depletion protects against diabetes development in the low-dose STZ model
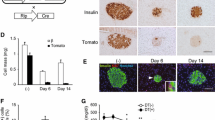
To determine the effect of polyamine depletion on the development of diabetes in mice, we employed a multiple low-dose STZ model (55 mg/kg body weight STZ × 5 days) of inflammatory T1D, in which animals develop a T1D-like phenotype with local islet inflammation and resultant hyperglycemia over time (Calderon et al. 2008; Lukic et al. 1998). Male C57BL/6J mice that were fed DFMO were compared to control animals in this STZ model. No differences were observed in water consumption between control and DFMO-fed mice (data not shown). As shown in Fig. 1a, mice fed 0.5 wt% DFMO exhibited a substantially reduced incidence of diabetes (defined as random blood glucoses >250 mg/dl on two consecutive measurements), with 80 % of animals remaining diabetes-free at the end of the study. By contrast, 0–10 % of control animals and those fed 0.25 wt% DFMO remained diabetes-free at the end of the study. We also examined the severity of hyperglycemia, as shown in Fig. 1b. Whereas blood glucoses of untreated and STZ-treated mice diverged by day 17 after initiation of the experiments, mice fed 0.5 wt% DFMO in the drinking water concurrent with STZ treatment remained normoglycemic throughout the course of the study. Mice fed a lower dose of 0.25 wt% DFMO displayed a less severe hyperglycemic phenotype (Fig. 1b), suggesting a dose-dependent effect of DFMO on diabetes development in this model. Likewise, STZ-treated control mice had significantly worsened glucose tolerance as assessed by GTT at 4 weeks post-STZ, whereas 0.5 wt% DFMO-fed mice displayed glucose tolerance indistinguishable from non-STZ-treated controls, and 0.25 % DFMO-fed mice showed an intermediate tolerance (Fig. 1c, d). Whereas control mice and mice fed 0.25 wt% DFMO exhibited significantly reduced β cell area percentages in their pancreas relative to non-STZ-treated mice, mice fed 0.5 wt% DFMO showed statistically indistinguishable β cell area percentage compared to non-STZ-treated mice (Fig. 1e). Analysis of paraffin-embedded pancreatic sections also showed substantially greater insulin staining in 0.5 wt% DFMO-fed mice compared to STZ-treated control mice (Fig. 1f). Collectively, the data in Fig. 1 suggest that polyamine depletion using DFMO protects islet β cells from inflammation-induced dysfunction and destruction.
Polyamine depletion protects against low-dose STZ-induced diabetes. Male C57BL/6J mice (N = 10 per group) were fed DFMO in drinking water at the indicated dose for 3 days prior to 5 daily intraperitoneal injections of STZ (55 mg/kg). a Survival curve showing incidence of diabetes. Arrows indicate timing of STZ injections. b Weekly blood glucose values. Arrows indicate timing of STZ injections. c GTTs at 30 days post-start of STZ injections. d Area under the curve corresponding to the GTTs in c. e β cell area as a percent of pancreatic area in treated mice and controls. f Images of representative pancreata immunostained for insulin (brown) and counterstained for hematoxylin (blue). Scale bar 200 μM. * indicates value for Control STZ is signficantly different (P < 0.05) compared to Control (no STZ). # indicates value for 0.25 wt% DFMO is signficantly different (P < 0.05) compared to Control (no STZ) (color figure online)
Polyamine depletion delays the onset of T1D in NOD mice
To test the effects of polyamine depletion in an autoimmune mouse model of T1D, we conducted DFMO feeding studies in female non-obese diabetic (NOD) mice. Female NOD mice develop insulitis as early as 4 weeks of age and display increasing islet endoplasmic reticulum (ER) stress, inflammation, and β cell dysfunction until 10–12 weeks of age, after which β cell mass declines and diabetes ensues (Sherry et al. 2006; Tersey et al. 2012). As shown in Fig. 2a, >90 % of female NOD mice developed diabetes between 13 and 25 weeks of age in our vivarium. Based on the studies in Fig. 1, we hypothesized that depletion of polyamines during the inflammatory/ER stress phase of the disease (between 6 and 10 weeks of age in NOD mice) would reduce islet β cell stress and subsequently delay or prevent T1D. As shown in Fig. 2a, whereas NOD mice that were fed 0.25 wt% DFMO between 6 and 10 weeks of age showed a similar rate of diabetes incidence as control mice, mice treated with 1.0 wt% DFMO showed a 50 % decrease in the incidence of diabetes. Serum insulin levels measured at the time of euthanasia (regardless of whether animals had diabetes or not) were significantly higher in mice fed 1.0 wt% DFMO compared to either controls or mice fed 0.25 wt% DFMO (Fig. 2b). Consistent with these increased serum insulin levels, the relative β cell area in pancreata of 1.0 wt% DFMO-fed mice was tenfold higher than control mice (Fig. 2c). Consistent with the observation of greater β cell area, mice fed 1.0 wt% DFMO exhibited a significantly reduced index of unmethylated preproinsulin in the serum (a biomarker that directly correlates with β cell death (Akirav et al. 2011; Husseiny et al. 2012), suggesting lower β cell death in these animals (Fig. 2d).
Polyamine depletion delays T1D incidence in female NOD mice. Female NOD mice (N = 10 per group) were fed DFMO in the drinking water at the indicated dose between 6 and 10 weeks of age. a Survival curve showing incidence of diabetes. b Serum insulin concentration at diabetes occurrence or at 25 weeks of age, whichever occurred first. c β cell area as a percentage of total pancreatic area. d Serum unmethylation index (from N = 5 mice per group). e Images of representative pancreata immunostained for insulin (brown) and counterstained for hematoxylin (blue). Black arrows indicate insulin immunostaining, red arrows indicate insulitis. Scale bar 200 μM. f Average insulitis score. *P < 0.05 compared to control (no DFMO) (color figure online)
Polyamine depletion in NOD mice correlated with reduced insulitis, increased pancreatic lymph node Treg cells, and reduced pancreatic lymph node Th17 cells
NOD mice were next evaluated for insulitis by examining tissue sections from DFMO-fed and control animals. To quantitate insulitis, we performed morphometric scoring of pancreas sections, with higher scores reflecting greater insulitis (Cabrera et al. 2013). As shown in Fig. 2e, 1.0 wt% DFMO-treated NOD animals had a significantly lower insulitis score at the time of euthanasia compared to control animals. Figure 2f shows representative images of islets from fixed pancreas sections, with 1.0 wt% DFMO-fed mice showing less insulitis compared to controls. DFMO-fed NOD mice had islets with either mild insulitis and robust insulin staining (Fig. 2f, middle panel) or a significant amount of infiltrate while retaining robust insulin staining (Fig. 2f, right panel), suggesting that DFMO may reduce or change the makeup of infiltrate that surrounds the islet.
Whereas the reduction in overall insulitis in DFMO-fed animals may be a sufficient explanation for the delay in diabetes development in these animals, it is also possible that the composition of the cell types that make up the insulitis might also provide important information. Because immune cells in insulitis are thought to arise from local pancreatic lymph nodes (Mathis et al. 2001), we examined CD4+ T cell subsets in pancreatic lymph nodes by flow cytometry as an assessment of T cell populations appearing in insulitis. Figure 3a, b show gating strategies that were used to identify appropriate CD4+ T cells. As shown in Fig. 3c, no differences were observed in total CD4+ T cells compared to total cells in the pancreatic lymph nodes. There were also no differences in Th1 cells (as a percentage of total CD4+ T cells) observed in the pancreatic lymph nodes of 1.0 wt% DFMO-fed mice compared to lymph nodes of control mice (Fig. 3d). Notably, however, pancreatic lymph nodes of 1.0 wt% DFMO-fed NOD mice exhibited significantly increased immune-tolerogenic Treg cells (as a percentage of total CD4+ T cells) compared to controls (Fig. 3e). Potentially pathogenic Th17 cells were reduced in the pancreatic lymph nodes of 1.0 wt% DFMO-fed mice compared to controls (Fig. 3f).
Polyamine depletion in NOD mice alters T cell populations. Female NOD mice were fed DFMO in drinking water at the indicated dose between 6 and 10 weeks of age. At diabetes development or 25 weeks of age (whichever occurred first), pancreatic lymph nodes from N = 5 mice per group were harvested and flow cytometry was performed. a Contour plots showing gating of CD4+ cells (upper panels), followed by separation by CD25 and Foxp3 staining intensities (lower panels). b Contour plots showing gating of CD4+ cells (upper panels), followed by separation by IFNγ and IL-17a staining intensities (lower panels). c Percent of total CD4+ cells in pancreatic lymph node. d CD4+IFNγ+ (Th1) cells as a percent of total CD4+ cells. e CD4+CD25+Foxp3+ (Treg) cells as a percent of total CD4+ cells. f CD4+IL17A+ (Th17) cells as a percentage of total CD4+ cells. *P < 0.05 compared to 0 wt% control
DFMO treatment reduces hypusination of eIF5A in splenocytes and islets
Polyamine depletion might be expected to reduce hypusinated eIF5A (eIF5A-Hyp) via the reduction in intracellular spermidine content. To determine if DFMO reduces eIF5A-Hyp in immune cells, splenocytes from C57BL/6J mice were stimulated in vitro with anti-CD3, anti-CD28, and IL-2 (with and without 50 μM DFMO) to mimic the nature of T cell activation occurring in T1D (Tang et al. 2004). As shown in Fig. 4a, activated splenocytes showed an increase in total eIF5A levels as well as an increase in eIF5A-Hyp levels (as judged by the eIF5A-Hyp/total eIF5A ratio), consistent with prior observations that activation of immune cell populations correlates with increases in hypusination rates (Kruse et al. 2000). Concurrent DFMO treatment substantially blunted the increase in eIF5A-Hyp levels upon stimulation (Fig. 4a). One possible interpretation of these results could be that reduced eIF5A hypusination consequent to DFMO treatment causes a reduction in insulitis observed in our NOD experiments.
Polyamine depletion with DFMO reduces eIF5A-Hyp formation. a Splenocytes from C57BL/6J mice were stimulated in vitro with anti-CD3, anti-CD28, and IL-2 (with and without 50 μM DFMO) and total protein was subjected to immunoblotting for eIF5A and eIF5A-Hyp. b Total protein from islets from mice treated for 3 days with 0, 0.25 or 1.0 wt% DFMO was subjected to immunoblotting for eIF5A and eIF5A-Hyp. Quantified band intensity ratios as indicated are shown below each panel
Hypusination of eIF5A has been shown to promote cytokine-induced inflammatory responses in pancreatic islets (Maier et al. 2010; Nishiki et al. 2013). To determine if DFMO feeding to mice reduces levels of eIF5A-Hyp in islets, we fed C57BL/6J mice ad lib for 3 days with 0, 0.25 or 1.0 wt% DFMO in the drinking water, and subsequently isolated their islets. As shown in Fig. 4b, 1.0 wt% DFMO feeding reduced islet eIF5A-Hyp levels relative to actin by almost twofold, accompanied by slight reductions in total eIF5A levels. 0.25 wt% DFMO feeding resulted in a roughly 30 % reduction in levels of eIF5A-Hyp relative to actin. These data suggest that one benefit of polyamine depletion in our NOD experiments might accrue from the reduction in inflammation-promoting eIF5A-Hyp.
Dhps heterozygosity protects against multiple low-dose STZ-induced diabetes
The data in Fig. 4a, b suggest that polyamine depletion overall might protect NOD mice against T1D development via the reduction in eIF5A-Hyp levels. To test this possibility, we subjected mice heterozygous for a targeted deletion of the gene encoding DHS (Dhps+/− mice) to multiple low-dose STZ and followed animals for the development of hyperglycemia. In prior studies, we showed that Dhps−/− mice are embryonic lethal and Dhps+/− mice exhibited approximately 50 % reduced rates of eIF5A hypusination (Templin et al. 2011). As shown in Fig. 5a, islets from Dhps+/− mice exhibited reduced levels of DHS protein by immunoblot. As shown in Fig. 5b, Dhps+/− mice began exhibiting significantly improved blood glucose levels compared to wild-type littermates 12 days following STZ injections, although complete protection against hyperglycemia as observed in the 1.0 wt% DFMO-fed mice did not occur in the Dhps+/− mice. Dhps+/− mice exhibited a tendency to increased β cell area compared to wild-type littermates (Fig. 5b), although this difference did not reach statistical significance.
Dhps heterozygosity protects against low-dose STZ-induced diabetes. 8 week-old Dhps+/− male mice and wild-type littermates (N = 5–10 per group) were treated with five daily intraperitoneal injections of STZ (55 mg/kg, IP). a Islet extracts immunoblotted for DHS and total eIF5A protein. b Weekly blood glucose values. Arrows indicate timing of STZ injections. c β cell area as a percentage of total pancreatic area. *P < 0.05 compared to wild-type control
Discussion
T1D is an autoimmune disease that is characterized in both mice and humans by a prodrome that includes β cell dysfunction and dysglycemia (Ferrannini et al. 2010; Ize-Ludlow et al. 2011; Tersey et al. 2012). Both the immune activation cascade and inflammation-stressed islet β cells are prominent targets for experimental therapies for T1D. However, such therapies to date have proved inadequate to fully remit the disease (Matthews et al. 2010), there is a need for identification of new molecular pathways that would serve as targets for future therapies. Polyamines are polycationic aliphatic amines that have been implicated in a variety of cellular functions that may directly impact inflammatory responses, including stabilization of nucleic acids during transcription and replication, promoting mRNA translation in response to inflammation, and alteration of cellular ion channel activity [for a review, see ref. (Brooks 2012)]. In this study, we examined the potential of targeting ODC, a key enzyme in the polyamine biosynthetic pathway, with the aim to mitigate disease incidence in T1D in two different mouse models: the multiple low-dose STZ model and the NOD model. We show that treatment with the ODC inhibitor DFMO results in increases in β cell area and insulin secretion, with improved blood glucose control and reductions in the incidence of T1D. Our studies raise the possibility that targeting the polyamine biosynthesis through small molecule inhibitors could favorably alter the course of T1D.
The multiple low-dose STZ model is often used to mimic inflammatory responses seen in T1D. STZ is a DNA alkylating agent that is taken up selectively by β cells through membrane GLUT2 transporters and leads to the formation of superoxide radicals and the liberation of toxic amounts of nitric oxide (Szkudelski 2001). In the low-dose STZ mouse model, STZ is thought to cause low-level β cell death, which leads to the influx of macrophages/dendritic cells that subsequently release inflammatory cytokines such as IL-1β and TNF-α (Calderon et al. 2008; Maier et al. 2010). Pro-inflammatory cytokines lead to β cell inflammation, dysfunction, and eventual apoptosis/necrosis (Steer et al. 2006). In this study, we show that continual feeding of 1.0 wt% DFMO in the drinking water prevents apparent β cell loss and the development of hyperglycemia in response to low-dose STZ. In this respect, polyamine accumulation may contribute to cellular oxidative stress and apoptosis when they undergo oxidative deamination by polyamine oxidase to generate H2O2 and aldehydes (acrolein) (Poulin et al. 1995; Seiler and Raul 2005); reduction of polyamine levels by use of DFMO has been demonstrated in cell systems to protect against apoptotic cell death (Bhattacharya et al. 2003; Ray et al. 2000). Indeed, among cells in the pancreas, β cells have perhaps the highest intracellular polyamine levels, which are enhanced further upon stimulation of cells with glucose (via stimulation of ODC activity) (Hougaard et al. 1986). Given that islet β cells also have strikingly low antioxidant capacity (Grankvist et al. 1981), it is tempting to speculate that sensitivity of β cells to oxidative destruction by STZ could be enhanced by the presence of polyamines, and as such, depletion of polyamines using DFMO allows for a greater capacity of β cells to resist the effects of STZ. Moreover, oxidative stress is closely linked to ER stress (Scheuner and Kaufman 2008) and, as such, reduction of oxidative stress may also reduce β cell ER stress.
Few studies have directly addressed a role for polyamines in the pathogenesis of autoimmune T1D. Using a mouse model of autoimmune T1D, we show that 1.0 wt% DFMO administration in the drinking water to mice between the age of 6 and 10 weeks resulted in delayed incidence of T1D. Although we did not directly measure polyamine levels in islets following DFMO, several previous studies have shown that DFMO administration to mice results in tissue polyamine depletion (Sunkara and Rosenberger 1987; Thomas and Messner 1991). In prior studies, we showed that the period between 6 and 10 weeks of age in NOD mice represents a time of increasing insulitis and β cell ER stress (Tersey et al. 2012). We therefore hypothesized that administration of DFMO during this time period might have two key effects that would lead to delayed incidence of T1D: (1) to reduce T cell proliferation/differentiation, and/or (2) to reduce β cell ER and oxidative stress. With regard to the former, prior studies have shown that depletion of polyamines using DFMO significantly inhibited the T cell proliferative responses to a variety of known stimuli (concavalin A, alloantigens) in vitro, and that this effect was reversed by supplementation with polyamines (Singh et al. 1992). In agreement with this report, we demonstrate here that 1.0 wt% DFMO administration leads to a reduction in insulitis in NOD mice. Moreover, we show that the relative proportion of Tregs are increased and effector Th17 cells are reduced in pancreatic lymph nodes of DFMO-fed NOD mice, a finding suggestive of an effect of polyamine depletion that biases the differentiation of T cell subsets toward a more immune-tolerizing balance. With respect to effects of polyamine depletion on β cell function, our studies show that insulin secretion is significantly enhanced in NOD mice fed 1.0 wt% DFMO in the drinking water. Although this effect might be secondary to the reduced insulitis in these animals (and therefore reduced β cell inflammation), we cannot rule out an independent effect of polyamine depletion on β cell function. Studies from the 1980s and 1990s attempted to examine the effect of DFMO-induced polyamine depletion on β cell function using primary rodent islets and rodent-derived β cell lines. DFMO was shown to diminish putrescine and spermidine levels in these cells, but in some studies insulin release was impaired (Sjöholm 1996; Welsh and Sjöholm 1988) and in others it was enhanced (Sjöholm et al. 1993). Whereas differences might lie in the nature of cells used in these studies (cell lines vs. primary islets), the implications with respect to whole animal glucose homeostasis was not directly studied. Also, in none of these studies was the effect of polyamine depletion studied with respect to the kind of ER or oxidative stress observed in NOD mouse islets, thereby leaving open the possibility that an effect of polyamines in β cells must be studied in appropriate context.
Recent studies suggest that the more downstream effect of polyamines on eIF5A hypusination may be particularly important. The enzyme DHS transfers the aminobutyl moiety of spermidine to the ε-amino group of Lys50 of eIF5A, forming the hypusine residue (Park et al. 2010). In the absence of hypusine formation (a result of either spermidine depletion or DHS inhibition), the RNA binding and translational functions of eIF5A are impaired. In antigen-presenting cells, studies of Hauber and colleagues showed that hypusine formation is required for the nucleocytoplasmic shuttling and translation of the mRNA encoding the maturation marker CD83, without which antigen-presenting cells are impaired in their ability to activate T cells (Kruse et al. 2000). Recent data from our group have shown that hypusine is also required in part for the production of the IL2 receptor α chain (CD25) in murine T cells, without which T proliferation is impaired (unpublished observations). Other recent studies by our group support a role for hypusine in facilitating the β cell inflammatory response to pro-inflammatory cytokines, particularly with respect to the translation of the mRNA encoding inducible nitric oxide synthase (Maier et al. 2010; Nishiki et al. 2013). In this respect, we show that DFMO feeding to mice leads to a reduction in hypusine formation in both activated splenocytes and islets. Furthermore, mice heterozygous for the Dhps gene show resistance to low-dose STZ-induced diabetes in a manner similar to DFMO feeding. These studies raise the intriguing possibility that the effects observed with polyamine depletion in our studies may result from a reduction in hypusine formation.
Taken together, our studies provide evidence that polyamine depletion might represent a novel approach in the treatment of T1D. Although no studies to date have shown altered levels of polyamines in individuals with T1D, the elevations in polyamine levels observed in other autoimmune diseases such as lupus and rheumatoid arthritis suggest the possibility that polyamines may be contributing to disease pathogenesis in autoimmune disorders [for a review, see (Brooks 2012)]. Nevertheless, an elevation in polyamine oxidase activity in the sera of children with T1D compared to control subjects (Bjelakovic et al. 2010) suggests the possibility that oxidative stress might be exacerbated in such individuals if polyamine levels were to be elevated. The use of antioxidants in diabetes has proven variably efficacious in mouse models (Haskins et al. 2003; Padgett et al. 2013), although to date no specific therapies have been approved for use in humans. Our studies represent the first step toward the elucidation of a role for polyamines in T1D and suggest a possible role for polyamine depletion therapy as a means to control disease prevention or progression. Finally, it has recently been suggested that the gut microbiome may contribute to triggering T1D in susceptible individuals (Boerner and Sarvetnick 2011). Therefore, even in the absence of elevated polyamine in diabetic individuals, it is possible that polyamine depletion may alter the gut microbiome (Barry et al. 2011) and thereby provide some protection against diabetes development. Future studies will include analysis of preclinical animal models in which ornithine decarboxylase is mutated to ascertain the role of polyamines intrinsic to the immune system and the β cell.
References
Akirav EM, Lebastchi J, Galvan EM et al (2011) Detection of beta cell death in diabetes using differentially methylated circulating DNA. Proc Natl Acad Sci USA 108:19018–19023. doi:10.1073/pnas.1111008108
Atkinson MA, Bluestone JA, Eisenbarth GS et al (2011) How does type 1 diabetes develop?: the notion of homicide or β-cell suicide revisited. Diabetes 60:1370–1379. doi:10.2337/db10-1797
Barry DP, Asim M, Leiman DA et al (2011) Difluoromethylornithine is a novel inhibitor of Helicobacter pylori growth, CagA translocation, and interleukin-8 induction. PLoS ONE 6:e17510. doi:10.1371/journal.pone.0017510
Bhattacharya S, Ray RM, Viar MJ, Johnson LR (2003) Polyamines are required for activation of c-Jun NH2-terminal kinase and apoptosis in response to TNF-alpha in IEC-6 cells. Am J Physiol Gastrointest Liver Physiol 285:G980–G991. doi:10.1152/ajpgi.00206.2003
Bjelakovic G, Beninati S, Bjelakovic B et al (2010) Does polyamine oxidase activity influence the oxidative metabolism of children who suffer of diabetes mellitus? Mol Cell Biochem 341:79–85. doi:10.1007/s11010-010-0439-0
Boerner BP, Sarvetnick NE (2011) Type 1 diabetes: role of intestinal microbiome in humans and mice. Ann N Y Acad Sci 1243:103–118. doi:10.1111/j.1749-6632.2011.06340.x
Brooks WH (2012) Autoimmune diseases and polyamines. Clin Rev Allergy Immunol 42:58–70. doi:10.1007/s12016-011-8290-y
Cabrera SM, Rigby MR, Mirmira RG (2012) Targeting regulatory T Cells in the treatment of type 1 diabetes mellitus. Curr Mol Med 12:1261–1272. doi:10.2174/156652412803833634
Cabrera SM, Colvin SC, Tersey SA et al (2013) Effects of combination therapy with dipeptidyl peptidase-IV and histone deacetylase inhibitors in the NOD mouse model of type 1 diabetes. Clin Exp Immunol 172:375–382. doi:10.1111/cei.12068
Calderon B, Suri A, Miller MJ, Unanue ER (2008) Dendritic cells in islets of Langerhans constitutively present beta cell-derived peptides bound to their class II MHC molecules. Proc Natl Acad Sci USA 105:6121–6126. doi:10.1073/pnas.0801973105
Evans-Molina C, Robbins RD, Kono T et al (2009) PPAR-γ activation restores islet function in diabetic mice through reduction of ER stress and maintenance of euchromatin structure. Mol Cell Biol 29:2053–2067. doi:10.1128/MCB.01179-08
Ferrannini E, Mari A, Nofrate V et al (2010) Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes 59:679–685. doi:10.2337/db09-1378
Grankvist K, Marklund SL, Taljedal IB (1981) CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J 199:393–398
Haskins K, Bradley B, Powers K et al (2003) Oxidative stress in type 1 diabetes. Ann N Y Acad Sci 1005:43–54
Hougaard DM, Nielsen JH, Larsson LI (1986) Localization and biosynthesis of polyamines in insulin-producing cells. Biochem J 238:43–47
Husseiny MI, Kuroda A, Kaye AN et al (2012) Development of a quantitative methylation-specific polymerase chain reaction method for monitoring beta cell death in type 1 diabetes. PLoS ONE 7:e47942. doi:10.1371/journal.pone.0047942
Igarashi K, Kashiwagi K (2010) Modulation of cellular function by polyamines. Int J Biochem Cell Biol 42:39–51. doi:10.1016/j.biocel.2009.07.009
Ize-Ludlow D, Lightfoot YL, Parker M et al (2011) Progressive erosion of β-cell function precedes the onset of hyperglycemia in the NOD mouse model of type 1 diabetes. Diabetes 60:2086–2091. doi:10.2337/db11-0373
Kruse M, Rosorius O, Kratzer F et al (2000) Inhibition of CD83 cell surface expression during dendritic cell maturation by interference with nuclear export of CD83 mRNA. J Exp Med 191:1581–1590
Lehuen A, Diana J, Zaccone P, Cooke A (2010) Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol 10:501–513. doi:10.1038/nri2787
Lukic ML, Stosic-Grujicic S, Shahin A (1998) Effector mechanisms in low-dose streptozotocin-induced diabetes. Dev Immunol 6:119–128
Maier B, Ogihara T, Trace AP et al (2010) The unique hypusine modification of eIF5A promotes islet beta cell inflammation and dysfunction in mice. J Clin Invest 120:2156–2170. doi:10.1172/JCI38924
Mathis D, Vence L, Benoist C (2001) beta-Cell death during progression to diabetes. Nature 414:792–798. doi:10.1038/414792a
Matthews JB, Staeva TP, Bernstein PL et al (2010) Developing combination immunotherapies for type 1 diabetes: recommendations from the ITN-JDRF Type 1 Diabetes Combination Therapy Assessment Group. Clin Exp Immunol 160:176–184. doi:10.1111/j.1365-2249.2010.04153.x
Nishiki Y, Adewola A, Hatanaka M et al (2013) Translational control of inducible nitric oxide synthase by p38 MAPK in islet beta-cells. Mol Endocrinol 27:336–349. doi:10.1210/me.2012-1230
O’Sullivan-Murphy B, Urano F (2012) ER stress as a trigger for β-cell dysfunction and autoimmunity in type 1 diabetes. Diabetes 61:780–781. doi:10.2337/db12-0091
Padgett LE, Broniowska KA, Hansen PA et al (2013) The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann N Y Acad Sci 1281:16–35. doi:10.1111/j.1749-6632.2012.06826.x
Park MH, Nishimura K, Zanelli CF, Valentini SR (2010) Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids 38:491–500. doi:10.1007/s00726-009-0408-7
Poulin R, Pelletier G, Pegg AE (1995) Induction of apoptosis by excessive polyamine accumulation in ornithine decarboxylase-overproducing L1210 cells. Biochem J 311(Pt 3):723–727
Ray RM, Viar MJ, Yuan Q, Johnson LR (2000) Polyamine depletion delays apoptosis of rat intestinal epithelial cells. Am J Physiol Cell Physiol 278:C480–C489
Scheuner D, Kaufman RJ (2008) The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev 29:317–333
Seiler N, Raul F (2005) Polyamines and apoptosis. J Cell Mol Med 9:623–642. doi:10.1111/j.1582-4934.2005.tb00493.x
Sherry NA, Kushner JA, Glandt M et al (2006) Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes 55:3238–3245. doi:10.2337/db05-1034
Singh AB, Thomas TJ, Thomas T et al (1992) Differential effects of polyamine homologues on the prevention of DL-alpha-difluoromethylornithine-mediated inhibition of malignant cell growth and normal immune response. Cancer Res 52:1840–1847
Sjöholm A (1996) Effects of secretagogues on insulin biosynthesis and secretion in polyamine-depleted pancreatic beta-cells. Am J Physiol 270:C1105–C1110
Sjöholm A, Arkhammar P, Welsh N et al (1993) Enhanced stimulus-secretion coupling in polyamine-depleted rat insulinoma cells. An effect involving increased cytoplasmic Ca2+, inositol phosphate generation, and phorbol ester sensitivity. J Clin Invest 92:1910–1917. doi:10.1172/JCI116784
Steer SA, Scarim AL, Chambers KT, Corbett JA (2006) Interleukin-1 stimulates beta-cell necrosis and release of the immunological adjuvant HMGB1. PLoS Med 3:e17. doi:10.1371/journal.pmed.0030017
Stull ND, Breite A, McCarthy RC et al (2012) Mouse islet of langerhans isolation using a combination of purified collagenase and neutral protease. J Vis Exp 67:e4137. doi:10.3791/4137
Sunkara PS, Rosenberger AL (1987) Antimetastatic activity of DL-alpha-difluoromethylornithine, an inhibitor of polyamine biosynthesis, in mice. Cancer Res 47:933–935
Szkudelski T (2001) The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50:537–546
Tang Q, Henriksen KJ, Bi M et al (2004) In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med 199:1455–1465. doi:10.1084/jem.20040139
Templin AT, Maier B, Nishiki Y et al (2011) Deoxyhypusine synthase haploinsufficiency attenuates acute cytokine signaling. Cell Cycle 10:1–7. doi:10.4161/cc.10.7.15206
Tersey SA, Nishiki Y, Templin AT et al (2012) Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes 61:818–827. doi:10.2337/db11-1293
Thomas TJ, Messner RP (1991) Difluoromethylornithine therapy of female NZB/W mice. J Rheumatol 18:215–222
Welsh N, Sjöholm A (1988) Polyamines and insulin production in isolated mouse pancreatic islets. Biochem J 252:701–707
Acknowledgments
The authors wish to acknowledge Ms. S. Burley for her assistance in these studies. The authors also wish to acknowledge assistance from the Indiana Diabetes Research Center Rodent Core. This study was supported by a grant from the Juvenile Diabetes Research Foundation (to R.G.M.), Grant R01 DK083583 (to R.G.M.) from the National Institutes of Health, and Grant KL2 TR000163 (to S.A.T.) from the National Institutes of Health.
Conflict of interest
The authors declare that they have no conflicts of interest relative to the work presented in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tersey, S.A., Colvin, S.C., Maier, B. et al. Protective effects of polyamine depletion in mouse models of type 1 diabetes: implications for therapy. Amino Acids 46, 633–642 (2014). https://doi.org/10.1007/s00726-013-1560-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-013-1560-7