Abstract
Meiosis is a process unique to the differentiation of germ cells and exhibits sex-specific in timing. Previous studies showed that retinoic acid (RA) as the vitamin A metabolite is crucial for controlling Stra8 (Stimulated by retinoic acid gene 8) expression in the gonad and to initiate meiosis; however, the mechanism by which retinoid-signaling acts has remained unclear. In the present study, we investigated the role of the enzyme retinaldehyde dehydrogenase 2 (RALDH2) which catalyzes RA synthesizes by initiating meiosis in chicken ovarian germ cells. Meiotic germ cells were first detected at day 15.5 in chicken embryo ovary when the expression of synaptonemal complex protein 3 (Scp3) and disrupted meiotic cDNA 1 homologue (Dmc1) became elevated, while Stra8 expression was specifically up-regulated at day 12.5 before meiosis onset. It was observed from the increase in Raldh2 mRNA expression levels and decreases in Cyp26b1 (the enzyme for RA catabolism) expression levels during meiosis that requirement for RA accumulation is essential to sustain meiosis. This was also revealed by RA stimulation of the cultured ovaries with the initiation of meiosis response, and the knocking down of the Raldh2 expression during meiosis, leading to abolishment of RA-dependent action. Altogether, these studies indicate that RA synthesis by the enzyme RALDH2 and signaling through its receptor is crucial for meiosis initiation in chicken embryonic ovary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meiosis is a process unique to the differentiation of germ cells and exhibits sex-specific property in timing during embryonic development (Hunt and Hassold 2002; Morelli and Cohen 2005). Entry into meiosis was proposed to be an intrinsic property of fetal germ cells, unless prevented by the meiosis-inhibiting factor produced in the fetal testis but not in the fetal ovary (McLaren and Southee 1997).
In the female mouse embryo, the germ cells initiate meiosis at E13.5 which continues to prophase I diplonema stage (Lin et al. 2008). Germ cells in the human fetal ovary, initially display a similar meiosis activity until around 10 weeks post-fertilization (wpf) (Bendsen et al. 2006; Gudas 1994). It has been reported that meiosis is initiated at day 15.5 in the female chicken (Smith et al. 2008), but the exact mechanism still remains unclear. In contrast to mammals, germ cell fate differs between the left and right gonads in chicken ovary. In the left gonad, the outer cortical layer becomes thick and germ cells accumulate there, while the right gonad fails to form a cortex and undergo regression later (Rodríguez-León et al. 2008). Therefore, chicken embryo represents a good model to study meiosis and ovary development.
As a bioactive metabolite of vitamin A, retinoic acid (RA) is a key molecule in developmental biology, regulating various events in cell growth and differentiation (Duester 2008; Gudas 1994). In particular, RA is a known differentiation factor, particularly with respect to germ cells (Bowles et al. 2006; Lin et al. 2008; Smith et al. 2008). Recently, it has been revealed that RA can act as a meiosis-inducing factor in the mouse gonad (Baltus et al. 2006; Bowles and Koopman 2007). The addition of exogenous RA has been shown to speed up the spontaneous initiation of meiosis that occurs in organ culture of mouse, while vitamin A deficiency in vivo has been reported to prevent meiosis initiation in the fetal rat ovary (Le Bouffant et al. 2010; Li and Clagett-Dame 2009). Previous studies also show that accumulation of RA triggers meiosis by activating RA receptor-dependent transcription of meiotic inducers, including Strs8 (Anderson et al. 2008; Zhou et al. 2008). Furthermore, Stra8-deficient mice demonstrated that Stra8 is required for meiotic initiation and meiotic process in both sexes (Bowles et al. 2006; Mark et al. 2008).
RA is generated by sequential oxidation of vitamin A through retinaldehyde dehydrogenase (RALDH), an enzyme accounting for most RA synthesis in the embryo (Ang et al. 1996). The levels of RA are strictly regulated in a spatiotemporal manner, through the balanced actions of synthesis (RALDH2) and catabolism (CYP26B1) enzymes. The biological effects of RA are mediated by the RA receptors (RARs) that modify many gene expressions in target cells (Chawla et al. 2001; de Lera et al. 2007; Mic et al. 2003) and similarly, the chemical inhibition of RAR function in embryonic ovaries led to the inhibition of Stra8 expression, and the arrest of entry into meiosis in vitro culture studies (Li and Clagett-Dame 2009).
In order to further define the molecular mechanism responsible for meiosis initiation in chicken embryonic ovary, we have employed an organ culture system, which provides optimized model to manipulate specific molecular constituents. In order to define the role of RA, we introduced short-hairpin RNA (shRNA) into germ cells of day 15.5 ovaries against Raldh2 transcripts. We found that RA has the capacity to induce Stra8 expression, but knock down of Raldh2 causes significant decrease in its expression. Thus, our results indicate the crucial role of RA synthesized by RALDH2 in regulating meiotic initiation of embryonic chicken ovary.
Materials and methods
Chicken embryos and organ culture
Fertilized Hyline chicken (Gallus gallus) eggs were incubated in an egg incubator (Victorial SRL, Italy) at 38.5 °C and 60 % humidity. Individual left ovaries without the mesonephros from 6.5, 10.5 and 12.5 days embryos were placed separately on Millicell membranes (Millipore, Bedford, MA) and taken for floating on 500 μl serum-free ITS culture medium (DMEM + 10 μg/ml insulin, 5 μg/ml transferrin and 3 × 10−8 M sodium selenium supplement). Before the ovaries were harvested until day 15.5 equivalently in vivo for RNA extraction or histological assessment, they were treated with the final concentration of RA (1 μM, Sigma-Aldrich, St. Louis, MO).
Immunofluorescence staining
Ovaries were fixed in 4 % paraformaldehyde and embedded in tissue-Tek OCT compound (Sakura Finetek, Japan). Cryosections were cut at a thickness of 10 μm to perform immunostaining. After blockade in TBS (containing 5 % goat serum and 0.1 % Triton X-100) for 30 min, sections were incubated with antibodies directed against γH2AX (1:1000, Abcam, UK) or SCP3 (1:1000, BD Biosciences, USA), then incubated with FITC or TRITC-conjugated goat anti-mouse IgG (1:1000) secondary antibody (KPL Inc., Maryland, USA). The nuclei were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI) and the images were visualized using fluorescence microscopy (TCS SP5, Leica, Germany).
RNA isolation and quantitative RT-PCR
RNA extraction and reverse transcription were performed as previously described (Yu et al. 2011). Real-time quantification of RNA targets was performed using SYBR green RT-PCR kit (Takara, Japan) on an ABI7300 Fast thermal cycler (Applied Biosystems) by following the manufacturer’s instructions. The expression level of meiosis-related genes was normalized to that of the Deleted in azoospermia-like (Dazl), a marker of germ cells, and β-actin for the other genes. Results were analyzed using the delta–delta Ct method. Primer sequences are listed in Table 1.
RNA interference assay
For knockdown of Raldh2, predesigned shRNA reagents were obtained from GenePharma Co. (Shanghai, China). The day 15.5 ovarian cells were grown to 70–80 % confluence in ITS medium, and then transfected with either shRNA specific for Raldh2, or a non-targeting shRNA as a negative control using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). After 24 h, transfection mixtures were replaced with regular medium. Four days following transfection, cells were collected and analyzed. The sequences of shRNA are listed in Table 2.
Statistical analysis
The results are expressed as the mean ± SD of at least three independent biological replicates. Images show a representative result for experiments that were all repeated at least three times. Data were analyzed by ANOVA and Duncan’s multiple range tests using the SAS 9.0 software. P < 0.05 was considered as significantly different.
Results
Immunostaining of meiosis markers in chicken embryo
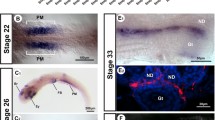
The profile of meiotic initiation was confirmed by immunofluorescent detection of SCP3, which is specially expressed during meiotic prophase, on day 14.5–18.5 in the chicken embryonic ovaries (Fig. 1a). In the female, SCP3 was first detected in very few germ cells in the developing cortex at day 15.5. Then positive cells were increased gradually and by day 18.5 the SCP3 expression was widespread (Fig. 1a). The SCP3 staining was corroborated by independent staining for γH2AX, which was phosphorylated in response to DNA double-strand breaks in the cell during meiotic recombination. Immunostaining for γH2AX (Fig. 1b) demonstrated a similar result with SCP3. In contrast, germ cells in males were negative for SCP3 or γH2AX immunostaining (data not shown), indicating that germ cells did not enter in meiosis in the male during this period.
Expression of SCP3 and γH2AX were detected by immunofluorescence in developing embryonic chicken gonads. Some SCP3 (a) positive cells (red) and γH2AX (b) positive cells (green) are first detectable in the cortex of the left ovary at day 15.5 (arrow), increasing in number up to day 18.5. The nuclei were counterstained with DAPI (blue). Scale bar 50 μm (color figure online)
Analysis of meiosis-related genes expression by qRT-PCR
For better characterization of sex-specific meiosis initiation, we analyzed the mRNA expression of several meiotic markers: Stra8, Scp3, and Dmc1 in chicken embryonic gonads ranging from day 12.5–18.5. In the female, Stra8 mRNA expression displayed a sharp increase at 12.5 day, while the expression of Scp3 and Dmc1 was dramatically up-regulated from day 15.5 (Fig. 2a–c). These results indicate that Stra8 is a premeiotic marker in the chicken, as in mouse. In addition, embryonic ovaries showed about 50 % decline in Cyp26b1 expression between day 12.5 and day 15.5, at which time increased expression of Raldh2 is believed to take over the accumulation of RA to actively promote meiosis in female germ cells (Fig. 3a). We also found that RARβ was abundantly expressed in endogenous female ovaries at day 12.5–18.5, and its expression level showed no major change though it tended to increase slightly from day 15.5 in the female (Fig. 3a). However, the expression levels of these genes displayed no obvious change and still maintained at low level in male gonads (Figs. 2, 3b).
Meiotic marker expression was assessed by measuring mRNA levels using qRT-PCR in embryonic chicken gonads. The expression of Stra8 (a), Scp3 (b) and Dmc1(c) increases from day 12.5 to 18.5 in female gonads, while sustains relatively low levels in male gonads during the time of meiosis. Data are representative of results in three independent experiments, and expressed as mean ± SD (N = 4) of three experiments for each condition normalized to Dazl. Bars with different letters are statistically different (P < 0.05)
RA synthesis is required for meiosis initiation in chicken ovary. The expression of RA synthesizing (Raldh2) and degrading enzyme (Cyp26b1) and RA receptor β (RARβ) was measured in fetal ovaries (a) and testes (b) by qRT-PCR. Data are representative of results in three independent experiments, and expressed as mean ± SD (N = 4) of three experiments for each condition normalized to β-actin. Bars with different letters are statistically different (P < 0.05)
RA promoted meiosis initiation in cultured gonads
Ovaries were carefully dissected from the embryonic mesonephros and cultured for upto day 15.5 (the equivalent in vivo stage) for testing the effects of RA on meiosis initiation in vitro. After 5 days culture, gonads explanted on day 10.5 without serum could spontaneously initiate meiosis in vitro (Fig. 4a). Similarly, meiosis initiation occurred in the 12.5 day ovary. We also found day 10.5 and 12.5 ovaries, co-cultured with RA possessed more meiosis cells than that of control (Fig. 4a). Addition of RA increased the percentage of meiotic germ cells form 10 to 15.2 % in day 10.5 ovary and 21.2 to 28.4 % in day 12.5 ovary, respectively (Fig. 4b). The ratio of germ cells entering meiosis gradually increased as gonads isolated progressively at later stages. But the percentage of germ cells in meiosis was slightly lower than day 15.5 ovary in vivo. Attempts were made to culture gonads from younger embryos (day 6.5) but no meiosis occurred.
Germ cells in female chicken embryonic ovaries undergo meiosis in vitro. Ovaries of day 10.5 and 12.5 were cultured to day 15.5, respectively, with or without RA. At the end of the culture, tissues were subjected to immunofluorescence. a Meiotic germ cells were identified by the detection of SCP3. Arrows indicate germ cells positive for SCP3; scale bar 50 μm. b The histograms show the percentage of meiotic germ cells. Bars with different letters are statistically different (P < 0.05)
The action of RA on meiotic initiation was further confirmed by the detection of mRNA expression in cultured ovaries by qRT-PCR (Fig. 5). We found that RA treatment directly induces Stra8 expression. We also showed that RA strongly up-regulated mRNA levels for all meiotic markers tested in day 10.5 and 12.5 ovaries (Fig. 5). While in day 6.5 cultured gonads, the expression of those genes was almost undetectable (Fig. 5). Together, these findings provide evidence that RA is required for induction of meiosis during ovary development.
Meiosis initiation in chicken ovary requires intrinsic RA synthesis
In order to further confirm the requirement of RA in meiosis initiation, we designed three pairs of shRNA, specific to Raldh2 to knock down its function. Among these shRNAs, shRNA2 produced the most significant inhibitory effect on Raldh2 mRNA expression (decreased about 70 %) (Fig. 6). shRNA interference of Raldh2 prevented the appearance of meiotic cells (Fig. 7a). Finally, as shown in Fig. 7b, Raldh2 shRNA2 remarkably inhibited the expression of Stra8, Scp3 and Dmc1. Indeed, the induced Stra8 expression was attenuated by small interfering RNA knockdown.
The effect of shRNA interference on Raldh2 expression. The cells of 15.5 day ovary were transfected for 24 h with either Raldh2 shRNAs (50 nM) or no targeting control shRNA (50 nM), and the expression of Raldh2 was detected by qRT-PCR. Data are representative of results in three independent experiments, and expressed as mean ± SD (N = 4) of three experiments for each condition. Bars with different letters are statistically different (P < 0.05)
The effect of Raldh2 shRNA interference on meiosis. After transfected with either Raldh2 shRNA2 (50 nM) or no targeting control shRNA (50 nM), the culture cells of 15.5 day ovary were subjected for immunofluorescence or qRT-PCR. a Meiotic germ cells were identified by the detection of SCP3. Arrows indicate germ cells positive for SCP3; scale bar 20 μm. b Meiotic marker genes expression was assessed by qRT-PCR. Data are representative of results in three independent experiments, and expressed as mean ± SD (N = 4) of three experiments for each condition. Bars with different letters are statistically different (P < 0.05)
Discussion
In mammals, there are dramatic differences between the sexes in the timing of meiosis regulation (Hunt and Hassold 2002; Koubova et al. 2006). Recent studies have shown that meiosis is indeed induced through RA signal in the mouse embryo (Bowles et al. 2006; Handel and Schimenti 2010; Wright 2010). RA is required for both the histological and molecular manifestations of meiotic prophase, including chromosomal cohesion, synaptonemal complex formation, and recombination (Wallacides et al. 2009).
RA is synthesized by the RALDH2 enzyme, controls the entry of the female germ cells into meiosis by inducing expression of a transcription factor, Stra8 (Anderson et al. 2008; Bowles et al. 2006). In contrast, in males, RA becomes degraded by the activity of the CYP26B1 enzyme, and the initiation of meiosis is inhibited (Baltus et al. 2006). Previous studies also demonstrated that males and females share an identical meiotic initiation pathway in which RA induces Stra8 expression in both embryonic ovaries and adult testes (Baltus et al. 2006; Le Bouffant et al. 2010). Thus, the expression of Stra8 appears to be a sensitive indicator of the availability of RA and entry of cells into meiosis. Other studies have shown that RA can induce Stra8 expression in cultured embryonic testes and ovaries and also in VAD adult testes (Li and Clagett-Dame 2009; Wright 2010; Zhou et al. 2008). The knockout of the Stra8 gene blocks entry into meiosis in both embryonic ovaries and pubertal testes (Mark et al. 2008; Wright 2010). To investigate the induction of Stra8 expression by RA in early chicken embryo germ cells, we examined expression of components for RA synthesis, metabolism and their downstream effectors in ovarian germ cells around the time of meiosis initiation in the chicken embryo gonads.
In present study, we found the expression of Raldh2 mRNA was upregulated at meiosis initiation, the critical time for RA to exert its function. Meanwhile, the expression of Cyp26b1 mRNA was decreased during meiosis to prevent degradation of RA. These are in agreement with previous reports that also placed meiotic initiation in human which occur after a short time of sex differentiation (Childs et al. 2011; Le Bouffant et al. 2010). But these data differ from those previously described in the mouse (Duester 2008). It seems therefore that though RA remains a key factor in meiosis initiation in chicken, the manner in which it is regulated in the embryonic gonad may vary in different species. However, the expression of Stra8 appears to be a conservative indicator of the accumulation of RA to induce meiosis of germ cells. And the action pattern of meiosis in chicken is similar to human, so chicken may be a good model to study meiosis for human.
However, besides RA signaling, Dazl as an intrinsic factor is also critical for meiosis, and RA is unable to trigger up regulation of Stra8 in the Dazl knockout model (Lin et al. 2008). In addition, Wnts as somatic cell signals in female germ line development have a role in regulating the initiation of meiosis by influencing the RA pathway (Naillat et al. 2010). Nanos2 which begins to be expressed in male germ cells at the point when Cyp26b1 is down-regulated is in turn critical for male germ line development (Suzuki and Saga 2008). Based on studies of knockout mice, they proposed that Nanos2 inhibits meiotic initiation in male germ cells via suppression of Stra8 expression (Suzuki and Saga 2008). If Nanos2 is ectopically expressed in the ovary, it prevents meiosis, following the male germ cell differentiation pathway (Suzuki and Saga 2008). Recently, Fibroblast growth factor 9 (Fgf9) expressed in the fetal testis has been proposed to be the candidate secreted factor, opposing RA effects as it inhibits meiosis entry presumably through increasing Nanos2 level (Barrios et al. 2010). These study show that an alternate form of the translation initiation machinery may be required for regulation and execution of key steps in male germ cell differentiation.
In this study, we showed the profile of meiosis in chicken embryo ovary and found the expression of Raldh2 increased sharply at day 15.5 in ovary, indicating the requirement of intrinsic synthesized RA to initiate meiosis. In our culture studies, meiosis was induced by the addition of exogenous RA that caused meiosis-associated gene expression, while the meiosis initiation was retarded by Raldh2 shRNA interference, that caused the expression of meiosis marker genes to decline. These data suggest that the intrinsic production of RA within the embryo ovary may be important in regulating the initiation of meiosis in the chicken embryonic ovary.
References
Anderson EL, Baltus AE, Roepers-Gajadien HL et al (2008) Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci USA 105:14976–14980
Ang HL, Deltour L, Hayamizu TF, Zgombic-Knight M, Duester G (1996) Retinoic acid synthesis in mouse embryos during gastrulation and craniofacial development linked to class IV alcohol dehydrogenase gene expression. J Biol Chem 271:9526–9534
Baltus AE, Menke DB, Hu YC et al (2006) In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 38:1430–1434
Barrios F, Filipponi D, Pellegrini M et al (2010) Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci 123:871–880
Bendsen E, Byskov AG, Andersen CY, Westergaard LG (2006) Number of germ cells and somatic cells in human fetal ovaries during the first weeks after sex differentiation. Hum Reprod 21:30–35
Bowles J, Koopman P (2007) Retinoic acid, meiosis and germ cell fate in mammals. Development 134:3401–3413
Bowles J, Knight D, Smith C et al (2006) Retinoid signaling determines germ cell fate in mice. Science 312:596–600
Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ (2001) Nuclear receptors and lipid physiology: opening the X-files. Science 294:866–870
Childs AJ, Cowan G, Kinnell HL, Anderson RA, Saunders PT (2011) Retinoic acid signaling and the control of meiotic entry in the human fetal gonad. PLoS One 6:20249
de Lera AR, Bourguet W, Altucci L, Gronemeyer H (2007) Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nat Rev Drug Discov 6:811–820
Duester G (2008) Retinoic acid synthesis and signaling during early organogenesis. Cell 134:921–931
Gudas LJ (1994) Retinoids and vertebrate development. J Biol Chem 269:15399–15402
Handel MA, Schimenti JC (2010) Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet 11:124–136
Hunt PA, Hassold TJ (2002) Sex matters in meiosis. Science 296:2181–2183
Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC (2006) Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA 103:2474–2479
Le Bouffant R, Guerquin MJ, Duquenne C et al (2010) Meiosis initiation in the human ovary requires intrinsic retinoic acid synthesis. Hum Reprod 25:2579–2590
Li H, Clagett-Dame M (2009) Vitamin A deficiency blocks the initiation of meiosis of germ cells in the developing rat ovary in vivo. Biol Reprod 81:996–1001
Lin Y, Gill ME, Koubova J, Page DC (2008) Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science 322:1685–1687
Mark M, Jacobs H, Oulad-Abdelghani M et al (2008) STRA8-deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensation. J Cell Sci 121:3233–3242
McLaren A, Southee D (1997) Entry of mouse embryonic germ cells into meiosis. Dev Biol 187:107–113
Mic FA, Molotkov A, Benbrook DM, Duester G (2003) Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proc Natl Acad Sci USA 100:7135–7140
Morelli MA, Cohen PE (2005) Not all germ cells are created equal: aspects of sexual dimorphism in mammalian meiosis. Reproduction 130:761–781
Naillat F, Prunskaite-Hyyryläinen R, Pietilä I et al (2010) Wnt4/5a signaling coordinates cell adhesion and entry into meiosis during presumptive ovarian follicle development. Hum Mol Genet 19:1539–1550
Rodríguez-León J, Rodríguez Esteban C, Martí M et al (2008) Pitx2 regulates gonad morphogenesis. Proc Natl Acad Sci USA 105:11242–11247
Smith CA, Roeszler KN, Bowles J, Koopman P, Sinclair AH (2008) Onset of meiosis in the chicken embryo; evidence of a role for retinoic acid. Dev Biol 8:85
Suzuki A, Saga Y (2008) Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev 22:430–435
Wallacides A, Chesnel A, Chardard D, Flament S, Dumond H (2009) Evidence for a conserved role of retinoic acid in urodele amphibian meiosis onset. Dev Dyn 238:1389–1398
Wright WW (2010) New insights into the regulation of gametogenesis by retinoic acid. Biol Reprod 83:890–892
Yu ML, Guan K, Zhang CQ (2011) The promoting effect of retinoic acid on proliferation of chicken primordial germ cells by increased expression of cadherin and catenins. Amino Acids 40:933–941
Zhou Q, Li Y, Nie R et al (2008) Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol Reprod 78:537–545
Acknowledgments
This study was supported by the Ph.D. Programs Foundation of Ministry of Education of China (20110101110099), Zhejiang Provincial Natural Science Foundation (Z3110115), the National Natural Science Foundation of China (30871843) and Chinese Universities Scientific Fund (2011XZZX006). We thank Weidong Zeng for the help in the experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, M., Yu, P., Leghari, I.H. et al. RALDH2, the enzyme for retinoic acid synthesis, mediates meiosis initiation in germ cells of the female embryonic chickens. Amino Acids 44, 405–412 (2013). https://doi.org/10.1007/s00726-012-1343-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1343-6