Abstract
Sympathoadrenergic pathways are crucial to the communication between the nervous system and the immune system. The present review addresses emerging issues in the adrenergic modulation of immune cells, including: the specific pattern of adrenoceptor expression on immune cells and their role and changes upon cell differentiation and activation; the production and utilization of noradrenaline and adrenaline by immune cells themselves; the dysregulation of adrenergic immune mechanisms in disease and their potential as novel therapeutic targets. A wide array of sympathoadrenergic therapeutics is currently used for non-immune indications, and could represent an attractive source of non-conventional immunomodulating agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Physiopharmacology of noradrenaline and adrenaline
Noradrenaline and adrenaline are catecholamines, i.e. chemical compounds with a 3,4-dihydroxyphenyl group (catechol) and an amine function. Together with dopamine, they represent the main catecholamines in humans and are all produced from the non-essential amino acid tyrosine. The enzyme dopamine β-hydroxylase synthesizes noradrenaline from dopamine, and phenylethanolamine N-methyltransferase converts noradrenaline to adrenaline (Fig. 1). The natural stereoisomers are l-(-)-(R)-noradrenaline and adrenaline. Adrenaline was isolated as pure crystalline base in 1900 by Jokichi Takamine, while noradrenaline was synthesized a few years later (the prefix ‘nor’ is the acronym of nitrogen öhne radikal, indicating the absence of a methyl group) and in 1949 it was proved by Ulf von Euler in Stockholm to be the main sympathomimetic neurotransmitter in humans (Sneader 2005).
Noradrenaline and, to a lesser extent, adrenaline act as neurotransmitters in the central and peripheral nervous systems, and as neurohormones in chromaffin cells in medulla of adrenal glands. The most important noradrenergic nucleus in the central nervous system is the locus coeruleus (LC), which axons project rostrally to hippocampus, septum, hypothalamus and thalamus, cortex and amygdala, dorsally to cerebellum, and caudally to spinal cord, affecting attention, arousal and vigilance to salient and relevant external stimuli, and regulating hunger and feeding behavior. Adrenaline in the central nervous system acts as neurotransmitter in the medullary reticular formation, possibly contributing to coordination of eating and in visceral activities such as blood pressure regulation. Outside the central nervous system, noradrenaline is the main transmitter of most autonomic sympathetic postganglionic fibers. Peripheral actions of noradrenaline and adrenaline include: contraction of certain smooth muscles (blood vessels supplying skin, kidney, and mucous membranes), stimulation of exocrine glands (e.g., salivary and sweat glands), relaxation of certain other smooth muscles (gut wall, bronchi, blood vessels supplying skeletal muscle), increases of heart rate and force of contraction, metabolic actions (increased glycogenolysis in liver and muscle, lipolysis in adipose tissue), and endocrine actions (e.g., modulation of the secretion of insulin and renin). Detailed discussion of noradrenaline and adrenaline neurochemistry, anatomy and physiology can be found in Feldman et al. (1997).
The effects of noradrenaline and adrenaline are mediated by adrenergic receptors or ‘adrenoceptors’ (ARs), 7-transmembrane G protein-coupled receptors expressed in the central nervous system and in virtually all peripheral tissues (Table 1). ARs involved in the control of blood pressure, myocardial contractile rate and force, airway reactivity, as well as a variety of metabolic and central nervous system functions. ARs include three major types—α1, α2 and β—each further divided into three subtypes (Bylund et al. 2011). ARs can be either pre- or postsynaptic. Presynaptic ARs are mainly of the α2 type and mediate inhibition of neurotransmitter release, while postsynaptic receptors include all AR types. In peripheral tissues, α-ARs are expressed in smooth muscle cells and usually mediate contraction, while β-ARs can be found in heart (mainly β1, which mediate contraction), in smooth muscle cells (mainly β2, inducing relaxation), and in skeletal muscle (β2, possibly inducing hypertrophy). Virtually all normal human cells express β2-ARs. Usually, α1- and β1-ARs are located nearby sympathoadrenergic terminals and are therefore the main target of noradrenaline released upon nerve stimulation. On the contrary, α2- and β2-ARs are preferentially extrajunctional receptors and are acted upon by circulating noradrenaline and adrenaline. ARs are also widely distributed in the brain. AR agonists and antagonists are currently used to treat a variety of diseases, including hypertension, angina pectoris, congestive heart failure, asthma, depression, benign prostatic hypertrophy, and glaucoma. Additional conditions where these agents proved useful include shock, premature labor and opioid withdrawal, and as adjunct medications in general anesthesia.
Both primary (bone marrow and thymus) and secondary (spleen and lymph nodes) lymphoid organs are innervated mainly by sympathoadrenergic nerve fibers. Together with the hypothalamic–pituitary–adrenal axis, the sympathetic nervous system represents the major pathway involved in the cross-talk between the brain and the immune system (reviewed in Elenkov et al. 2000). The origin, pattern of distribution and targets of sympathetic nerves in lymphoid organs, and their neurochemical signaling have been the subject of several excellent reviews (see e.g. Felten 1991; Straub 2004), as well as the occurrence of age-induced changes in sympathetic-immune interactions (Bellinger et al. 1992; Friedman and Irwin 1997; Madden et al. 1998) and the consequences of dysregulated sympathetic nervous system in stress responses (Irwin 1994; Sloan et al. 2008) and in the development and progression of immune-mediated diseases (Madden et al. 1995; Friedman and Irwin 1997; Marshall and Agarwal 2000; Frohman et al. 2001; Wrona 2006; Straub et al. 2006; Bellinger et al. 2008; Benarroch 2009).
Adrenergic modulation of immune cells
Usually, β2-ARs are the most expressed on immune cells and as a consequence they are regarded as the main mediators of the immune effects of noradrenaline and adrenaline. Evidence is, however, increasing regarding the occurrence of other ARs, in particular the α1-AR subtype (reviewed by Kavelaars 2002). Different immune cell populations express distinct patterns of ARs, which in turn undergo up/downregulation upon cell development and/or response to activating stimuli. Hereafter, current knowledge will be summarized regarding ARs on well-defined cell types (and not, e.g., peripheral blood mononuclear cell preparations), with particular regard to results obtained in human samples. Several reviews have been dedicated to the sympathoadrenergic modulation of the immune response (e.g., Elenkov et al. 2000; Kohm and Sanders 2001; Nance and Sanders 2007; Flierl et al. 2008) and to the sympathoadrenergic control of thymic function (Leposavić et al. 2008).
T and B lymphocytes
It is well established that T cells express less β-AR than B cells (Bidart et al. 1983; Paietta and Schwarzmeier 1983). Among T cells, Khan et al. (1986) reported the following rank order of β-AR (likely β2-AR) density: T suppressor > T cytolytic > T helper. Karaszewski et al. (1990) confirmed that B lymphocytes had the greatest number of β-ARs (12.1 ± 1.8 fmol/106) cells, followed by CD8+ T lymphocytes (3.4 ± 0.4 fmol/106) and CD4+ T lymphocytes (1.2 ± 0.1 fmol/106), and subsequently reported that CD8+CD28− (suppressor) T cells express more (2.8 ± 0.3 fmol/106 cells) than CD8+CD28+ (cytotoxic) T cells (1.4 ± 0.4 fmol/106 cells) (Karaszewski et al. 1991). Human CD4+CD25+ regulatory T lymphocytes express mRNA not only for β2- but also for β1-ARs (Cosentino et al. 2007), and it was recently suggested that these receptors may mediate the effects of stress on T regulatory cells (Freier et al. 2010). Expression of β3-AR mRNA was reported in only one study in concanavalin A (Con A)-stimulated human T lymphocytes, however, without correlations with specific functional responses (Borger et al. 1998). Only a few data exist regarding α-ARs. Ligand binding studies suggest the occurrence of α2-ARs in human CD4+ and CD8+ T lymphocytes, as well as their upregulation after in vivo administration of adrenaline (Jetschmann et al. 1997), while it was shown that the T lymphocyte-preferring mitogen phytohaemagglutinin (PHA) can induce α1-ARs in stimulated peripheral blood mononuclear cells, suggesting that their expression may occur in particular on activated T cells (Rouppe van der Voort et al. 2000). The density and functional responsiveness of β2-ARs on human T lymphocytes is increased by interleukin-2 (IL-2) and after activation with PHA (Korichneva and Tkachuk 1990; Carlson et al. 1994). IL-2 upregulates β2-AR expression CD8+ T lymphocytes, but not on CD4+ T cells, while IL-1β has no effect (Wahle et al. 2001b). In vivo, the expression of β2-ARs is increased in both T and B cells after physical stress and returns to normal after 30 min rest (Ratge et al. 1988).
Stimulation of β-ARs usually inhibits lymphocyte responses, e.g. IL-2 receptor expression (Feldman et al. 1987), proliferation of T lymphocytes (Elliott et al. 1992) as well as of CD4+, CD8+, or CD45RO+ lymphocyte subsets and production of IL-2 (Bartik et al. 1993), IFN-γ, GM-CSF, and IL-3 (Borger et al. 1998). Polarized T helper (Th) cells, however, may be less sensitive to β2-AR-induced inhibition of both Th1 (IFN-γ) and Th2 (IL-4, IL-5) cytokine production (Heijink et al. 2003).
Activation of β-ARs on human lymphocytes may also result in stimulation of immunity (Sanders et al. 1997; Kohm and Sanders 1999). Noradrenaline may indeed promote IL-12-mediated differentiation of naive CD4+ T cells into Th1 effector cells, and increase the amount of IFN-γ produced by Th1 cells (Swanson et al. 2001). With regard to B lymphocytes, IgG1 and IgE production increases when noradrenaline is added either during antigen processing or within the first 12 h of culture with Th2 cells (Kasprowicz et al. 2000), while in plasma cells noradrenaline decreases antibody production (Melmon et al. 1974).
Natural killer cells
Binding assays in lymphocyte subsets showed that CD16+CD56+ natural killer cells express the highest number of β-ARs (1,934 ± 122 sites/cell), which after physical exercise increased (to 2,617 ± 289 sites/cell) (Maisel et al. 1990). Activation of β-AR (likely β2-AR) pathways decrease NK cell cytotoxicity (Whalen and Bankhurst 1990; Takamoto et al. 1991) and reduce adhesion to endothelial cells (Benschop et al. 1994, 1997). In human subjects, infusion of either adrenaline or noradrenaline modulates the migratory capacity of human NK cells via β2-AR mechanism (Schedlowski et al. 1996; Benschop et al. 1997).
Human NK cells express also α-ARs. In CD16+ lymphocytes, ligand binding studies indeed showed the presence of β2-, α1-, α2- but not β1-AR, and infusion of adrenaline (but not noradrenaline) significantly decreased their numbers (Jetschmann et al. 1997). The functional relevance of α-AR pathways in human NK cells, however, still awaits investigation.
Monocytes/macrophages
In classical binding experiments, β-AR density on human circulating monocytes was about 2,400 binding sites/cell, thus higher than that in lymphocytes, and further increased to 3,220 binding sites/cell after physical exercise (Ratge et al. 1988). Changes in receptor number can be, however, regulated with different states of cell maturation and function (Radojcic et al. 1991). Indeed, during the maturation of human monocytes to macrophages in vitro β-AR responsiveness may be lost (Baker and Fuller 1995), thus these cells may become insensitive to the inhibitory effects of β-AR agonists (Ezeamuzie et al. 2011). Activation of β-ARs (possibly, β2-ARs) on human monocytes usually result in anti-inflammatory effects, e.g. inhibition of oxygen radicals production (Schopf and Lemmel 1983), upregulation of TNF receptors and inhibition of TNF (Guirao et al. 1997), reduction of the phagocytosis of C. albicans (Borda et al. 1998), and inhibition of LPS-induced macrophage inflammatory protein-1 α (MIP-1 α), which has an important role in the development of inflammatory responses during infection by regulating leukocyte trafficking and function (Li et al. 2003). Selective activation of β2-ARs may down-regulate IL-18-induced intercellular adhesion molecule (ICAM)-1 expression and IL-12, TNF-α and IFN-γ production (Takahashi et al. 2003) as well as LPS-induced IL-18 and IL-12 production in human monocytes, an effect which might be beneficial in the treatment of sepsis (Mizuno et al. 2005).
Evidence exists, however, that under certain conditions, monocyte β-AR stimulation may lead to proinflammatory responses. Indeed, β2-AR activation in unstimulated monocytes may increase the production of IL-18 (Takahashi et al. 2004), while in LPS- or IL-1-stimulated cells it increased the production not only of the antiinflammatory cytokine IL-10 but also of the pro-inflammatory chemokine IL-8 (Kavelaars et al. 1997). In vitro, adrenaline may upregulate the surface expression of L-selectin on human monocytes possibly through a partial contribution of β-ARs (Rainer et al. 1999), and noradrenaline and adrenaline, possibly through the activation of β2-ARs, up-regulate MMP-1 and potentiate LPS-induced expression of MMP-1 in peripheral blood monocytes and monocyte-derived macrophages (Speidl et al. 2004). Isoprenaline treatment was reported to increase phorbol ester-induced production of TNF-α, IL-12, and nitric oxide, while it decreased inflammatory mediator production in combination with LPS stimulation (Szelenyi et al. 2006). Isoprenaline also increased LPS-induced production of IL-1β in human monocytes, however, this effect—according to pharmacological evidence—was due to the activation of β1-ARs, which were directly observed in the monocytic cell line THP-1 by immunoblot techniques as well as by radioligand binding studies (Grisanti et al. 2010).
Under certain circumstances, human monocytes may also express functional α-ARs, e.g. after culture with dexamethasone or the β2-AR agonist terbutaline, when both α1B- and α1D-AR mRNA can be detected (Rouppe van der Voort et al. 1999). In the human THP-1 monocytic cell line, α1B- and α1D-AR mRNA are, respectively, upregulated and reduced by the proinflammatory cytokines TNF-α and IL-1β (but not by IL-6 or IL-8) (Heijnen et al. 2002). With regard to the functional relevance of α-AR in human monocytes, in a recent study it was shown that the selective α1-AR agonist phenylephrine synergistically increased LPS-induced IL-1β production and this effect was blocked in the presence of a selective α1-AR antagonist as well as of inhibitors of protein kinase C (PKC) (Grisanti et al. 2011). Pharmacological evidence might also suggest the occurrence of α2-AR-mediated effects in human monocytes (Maes et al. 2000); however, the actual expression of functional α2-ARs on these cells remains to be established.
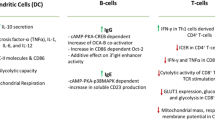
Dendritic cells
Extensive evidence exists for the presence and the functional relevance on murine dendritic cells (DCs) of ARs, which mediate sympathetic nervous system influence on DC–T cell interactions thus contributing to the shaping of the appropriate adaptive immune response (Maestroni 2005, 2006; Manni and Maestroni 2008; Yanagawa et al. 2010; Manni et al. 2011). On the contrary, very few information is available regarding human DCs. Activation of β2-ARs in human dendritic cells stimulated via CD40 results in inhibited IL-12 production via increased cAMP, finally blunting Th1 and promoting Th2 differentiation (Panina-Bordignon et al. 1997), while in human DCs obtained by differentiating human cord blood CD34+ precursor cells β2-ARs inhibit LPS-stimulated production of IL-23, IL-12 p40, TNF-α and IL-6 (Goyarts et al. 2008). This response pattern is similar to that obtained in mouse skin DCs (Maestroni 2005, 2006), thus suggesting that noradrenaline may regulate human skin DC function resulting in decreased Th1 differentiation of CD4+ T cells.
Granulocytes
Expression of β-ARs on human polymorphonuclear cells (PMN) has been investigated only by means of ligand binding assays, which indicated the existence on average of 1,700–2,200 binding sites per cell (Pohl et al. 1991; Schwab et al. 1993) or 39–61 fmol/mg of protein (Boreus et al. 1986; Gurguis et al. 1999). In one study, the possible existence of α2-AR was excluded based on both ligand binding and functional experiments in either PMN and differentiated HL-60 cells, a promyelocytic cell line frequently used as a model for neutrophils (Musgrave and Seifert 1994). The functional relevance of β-AR on human PMN is a matter of debate. Low concentration of the β-AR agonist isoprenaline inhibited the respiratory burst induced by various stimuli (Nielson 1987), however, in another study adrenaline reduced only slightly and only at very high concentrations PMN production of IL-8 and expression of the adhesion molecules CD15, CD44, and CD54. It should be, however, considered that β-AR desensitization has been reported after activation of PMN respiratory burst (Vago et al. 1990).
Expression of β-AR on circulating PMN was found decreased in essential hypertension (Corradi et al. 1981), juvenile type I diabetes mellitus (Schwab et al. 1993), and increased in post-traumatic stress disorder (Gurguis et al. 1999a). Physical exercise may decrease PMN β-AR, however, only after acute heavy resistance exercises (Ratge et al. 1988; Fragala et al. 2011).
Microglia
In the central nervous system (CNS), microglial cells are involved in phagocytosis and neuroinflammatory responses, triggering or amplifying both innate and acquired immune responses and in particular contributing to T-cell activation within the CNS. Microglia are therefore usually considered the CNS mononuclear phagocytes (Ghorpade et al. 2008). Adrenergic mechanisms in microglia have been so far investigated only in non-human cells. Hertz et al. (2010) showed the occurrence in murine microglia of β2-ARs and possibly of β1-ARs and α2A-ARs. Activation of β-ARs in these cells results in increased IL-1β, TNF-α and IL-6 expression through signal transduction mechanisms involving cAMP and cAMP-dependent protein kinase (Tomozawa et al. 1995) as well as ERK1/2 and P38 MAPK (Wang et al. 2010). Interestingly, however, noradrenaline acting on β-ARs has been shown to induce also IL-1ra and IL-1 type II receptor expression in murine microglia enriched cultures and to protect cortical neurons against IL-1 β-induced neurotoxicity (McNamee et al. 2010). In agreement with these observations, exposure to both β1- and β2-AR agonists decreased the levels of secreted TNF-α, IL-6 and monocyte chemoattractant protein-1, prevented microglia activation and was antiinflammatory and neuroprotective in LPS-treated murine hippocampal slices (Markus et al. 2010). Notably, it has been recently shown that, in a rat model of monoarthritis, spinal glia, as well as dorsal root ganglion primary afferent neurons, express α2-AR and the α2-AR agonist dexmedetomidine exerted analgesic effects involving the blockade of spinal glial activation (Xu et al. 2010).
Astrocytes
Astrocytes provide mechanical and functional support for neurons, there is, however, also evidence that they contribute to neuroinflammation upon severe challenges by releasing pro-inflammatory molecules (e.g., TNF-α, IL-1, IL-6) and possibly by contributing to antigen presentation under autoimmune response, although this latter function needs further investigation (Jana et al. 2008). Human astrocytes express mainly β2-AR, which play a key role in glycogen metabolism, regulation of immune responses, release of neurotrophic factors, as well as in the astrogliosis that occurs in response to neuronal injury. Accordingly, downregulation of the astrocytic β2-AR-pathway might be involved in a number of neurological conditions such as multiple sclerosis, Alzheimer’s disease, human immunodeficiency virus encephalitis, stroke and hepatic encephalopathy (reviewed in Hertz et al. 2004 and Laureys et al. 2010). Stimulation of β-ARs together with TNF-receptor triggering in astrocytes can, however, also induce synergistic expression of IL-6, which may be involved in neurodegeneration and glioma development (Spooren et al. 2011). Circumstantial evidence also exists regarding the occurrence of α1-AR, e.g. in astrocytes from human optic nerves (Mantyh et al. 1995).
Production and utilization of endogenous noradrenaline and adrenaline by immune cells
Traditional criteria to assess the role of a substance as neurotransmitter in the nervous system usually include (Purves et al. 2001): (a) presence of the substance within the cell (either synthesized by the cell or taken up from other cells that release it); (b) stimulus-dependent release; (c) mechanisms for removal (i.e., by degradation or reuptake); and (d) action on target cells through specific receptors (effects mimicked by exogenous application of the substance in appropriate amounts). Evidence for the presence and the functional relevance of ARs on immune cells has been already discussed (see previous section).
Presence and synthesis
Consistent amounts of noradrenaline and adrenaline (as well as dopamine, the direct precursor of noradrenaline, and of their major metabolites) can be found in murine lymphocytes (Josefsson et al. 1996), peritoneal macrophages (Spengler et al. 1994), bone marrow-derived mast cells (Freeman et al. 2001), human PBMC (Musso et al. 1996; Bergquist and Silberring 1998; Marino et al. 1999; Cosentino et al. 2002a), various lymphocyte subsets (Bergquist et al. 1994; Cosentino et al. 2000), including CD4+CD25+ regulatory T lymphocytes (Cosentino et al. 2007), granulocytes (Cosentino et al. 1999), as well as in hematopoietic cell lines (Cosentino et al. 2000). Intracellular levels of noradrenaline and adrenaline (as well as of dopamine) sharply increase in human stimulated PBMC (Cosentino et al. 2002a). Similar results have been published also in rodent lymphocytes, and by use of immunohistochemistry it was shown that TH-positive cells in rodent lymphoid organs have highest density in lymph nodes and lowest density in thymus (Qiu et al. 2004). Mitogen-stimulated increase of intracellular catecholamines is in line with the reported upregulation of ARs occurring in lymphocytes following mitogen, glucocorticoid or proinflammatory cytokine treatment (see e.g. Zoukos et al. 1994; Rouppe van der Voort et al. 2000a) and suggests a preferential involvement of intracellular catecholamine-operated pathways in activated immune cells. According to pharmacological evidence, endogenous synthesis of catecholamines occurs through protein kinase C (PKC) activation and the contribution of intracellular Ca2+-dependent mechanisms (Cosentino et al. 2002a).
The existence of a classical pathway (Fig. 1) for the synthesis of noradrenaline and adrenaline in immune cells is suggested by the expression of the enzyme tyrosine hydroxylase (TH, EC 1.14.16.2), the first and rate-limiting enzyme in the synthesis of catecholamines, which undergoes upregulation following cell stimulation, as well as by the ability of the TH inhibitor α-methyl-p-tyrosine to prevent intracellular enhancement of catecholamine levels (Cosentino et al. 2002a, b; Reguzzoni et al. 2002). Circumstantial evidence also exists regarding the presence in immune cells of phenylethanolamine N-methyltransferase (Andreassi et al. 1998; Ziegler et al. 2002) as well as of dopamine β-hydroxylase (Giubilei et al. 2004).
In human PBMC stimulated in vitro with PHA, TH mRNA expression and catecholamine production occur only in T and B lymphocytes (but not in monocytes) and are reduced by dopamine (but not by noradrenaline or adrenaline) acting through dopaminergic D1-like receptors (Ferrari et al. 2004), and by the proinflammatory cytokine IFN-γ, which in turn is counteracted by IFN-β (Cosentino et al. 2005). TH expression and catecholamine production are on the contrary enhanced by agents which induce catecholamine release (see next section).
Storage and release
In human lymphocytes, catecholamines are stored in reserpine-sensitive compartments (Marino et al. 1999; Cosentino et al. 2000, 2007), possibly through vesicular monoamine transporters (VMAT) similar to those expressed in neural and neuroendocrine cells (Henry et al. 1994). VMAT-1 and 2 indeed have been reported in rat thymus and spleen (Mignini et al. 2009) and possibly also in human peripheral blood lymphocytes (Amenta et al. 2001). In human activated lymphocytes, catecholamine release can be effectively induced by biological agents such as IFN-β (Cosentino et al. 2005) or by physiological stimuli such as elevation of extracellular K+ concentrations ([K+]e) (Cosentino et al. 2003). High [K+]e is characteristic of various pathological conditions and is per se a sufficient stimulus to activate integrin-mediated adhesion and migration of T cells (reviewed in Levite 2001). An excess [K+]e may thus both assist the recruitment of lymphocytes to an injured tissue and lead to local increase of catecholamines, which in turn may act upon lymphocytes themselves and/or upon neighboring cells. Release of catecholamines is usually associated with increased TH mRNA levels and catecholamine production (Cosentino et al. 2000, 2005).
Mechanisms for removal
Signal termination of noradrenaline and adrenaline as neurotransmitters and hormones is the result of reuptake through specific membrane transporters and/or of degradation, mainly through monoamine oxidase (MAO)- and catechol-O-methyl transferase (COMT)-mediated pathways (Fig. 1). In the synapse of noradrenergic neurons, termination of the action of noradrenaline is brought about by NET (norepinephrine transporter) (see e.g. Mandela and Ordway 2006). In immune cells, however, only few indirect evidence exists for the presence of NET (Audus and Gordon 1982). More convincing evidence, however, for the expression and the functional relevance of DAT (dopamine transporter) (reviewed in Marazziti et al. 2010), which has for noradrenaline the same affinity as NET (see e.g. PDSP Ki database—http://pdsp.med.unc.edu/pdsp.php). Incubation of human PBMC with the NET inhibitor desipramine or with the DAT inhibitor GBR 12909 increases the extracellular levels of both dopamine and noradrenaline (Marino et al. 1999), an observation which is compatible with the occurrence of both transporters on the human lymphocyte membrane. Whether the immunomodulating effects of monoamine uptake inhibitors (see e.g. Berkeley et al. 1994) can be attributed to a direct effect on NET and/or DAT remain, however, to be established.
Occurrence in human immune cells of both MAO and COMT is supported by functional assays and pharmacological experiments. Investigations on COMT activity were so far very limited (Bidart et al. 1981) and its eventual connections with modulation of immune response were never examined. On the contrary, MAO expression and activity in immune cells has received significantly more attention, not only as a marker of neurodegenerative and neuropsychiatric disease (Tsavaris et al. 1995; Jiang et al. 2006). MAO activity occurs in both human granulocytes and lymphocytes and it is predominantly of the B type (Pintar and Breakefield 1982; Thorpe et al. 1987; Balsa et al. 1989), and the MAO inhibitor pargyline leads to increased catecholamine levels in concanavalin A-stimulated rodent lymphocytes (Qiu et al. 2005) as well as in human peripheral blood mononuclear cells (Marino et al. 1999) and granulocytes (Cosentino et al. 1999). Recently, evidence has been provided that MAO type A is expressed in human monocytes in particular after incubation with IL-4, and it may contribute to switch naive monocytes into a resolving phenotype (Chaitidis et al. 2004, 2005).
Functional relevance
Possible strategies to study the role and the functional relevance of endogenous noradrenaline and adrenaline production in immune cells include: effect of AR antagonists; interference with synthesis/degradation; and interference with intracellular storage/release/uptake. The first evidence that endogenous noradrenaline and adrenaline may subserve autocrine/paracrine regulatory loops in immune cells was obtained by Spengler et al. (1994), who showed that in mouse peritoneal macrophages stimulated with LPS, the β-AR selective antagonist propranolol increased and the α2-AR selective antagonist idazoxan decreased TNF-α production, which—together with the presence of intracellular noradrenaline in these cells—was taken as an evidence of the existence of an adrenergic autocrine loop, even more pronounced in macrophages obtained from rats with streptococcal cell wall-induced arthritis (Chou et al. 1998).
Involvement of endogenous catecholamines and α2-ARs in the regulation of innate immunity was further demonstrated showing that exposure of rodent phagocytes to LPS led to catecholamine release together with induction of catecholamine-generating and degrading enzymes, and blockade of α2-ARs or pharmacological inhibition of catecholamine synthesis suppressed (while α2-AR agonism or inhibition of catecholamine-degrading enzymes enhanced) lung inflammation in two rodent models of acute lung injury (Flierl et al. 2007, 2009).
Experimental evidence for the functional relevance of endogenous noradrenaline and adrenaline also exists in rodent lymphocytes. Qiu et al. (2004, 2005) reported that stimulation with concanavalin A markedly increased both TH expression and catecholamine content in lymphocytes and that inhibition of TH with α-methyl-p-tyrosine significantly facilitated concanavalin A-induced IL-2 production and proliferation.
In human immune cells, indirect evidence for a functional role of endogenous noradrenaline and adrenaline was provided by Knudsen et al. (1996), who showed that their intracellular levels in circulating lymphocytes from healthy subjects were strongly correlated with both basal and isoprenaline-stimulated cAMP. More direct evidence came a few years later from studies in human PBMC, where stimulation with PHA induces the synthesis of catecholamines through induction of TH and its pharmacological inhibition with α-methyl-p-tyrosine results in decreased activation-induced apoptosis (Cosentino et al. 2002a). Similar findings were subsequently obtained in rodent lymphocytes activated with concanavalin A (Jiang et al. 2007). In separate experiments, by use of a pharmacological approach, it was shown that the effects of pargyline-increased endogenous catecholamine levels on rodent lymphocyte apoptosis were mediated by cAMP–PKA- and PLC–PKC-linked CREB-Smac/DIABLO pathways coupled to α1- and β2-ARs (Jiang et al. 2009).
Relevance for immune-mediated disease
Multiple sclerosis
Multiple sclerosis (MS) is an organ-specific autoimmune disorder characterized by inflammation, demyelination and axonal loss in the CNS (Noseworthy et al. 2000; Frohman et al. 2006). Several lines of evidence indicate that adrenergic pathways contribute to MS in immune system cells as well as in glial cells. Indeed, membrane expression of β2-ARs in PBMC is upregulated in MS (Arnason et al. 1988; Karaszewski et al. 1990, 1993; Zoukos et al. 1992) possibly in relation to disease activity (Zoukos et al. 1994). Increased expression of β2-ARs seems specific for CD8+CD28-T lymphocytes (Karaszewski et al. 1990, 1993). In circulating PBMC, gene expression of β2-ARs (and of other G protein-coupled receptors like dopaminergic receptors D5) and responsiveness to the β-AR agonist isoprenaline are on the contrary downregulated in untreated patients (suggesting the occurrence of receptor uncoupling) but restored in IFN-β-treated subjects (Giorelli et al. 2004). Intracellular levels of catecholamines are also affected in lymphocytes of MS patients. Peripheral blood lymphocyte levels of adrenaline may be higher in first-attack MS whilst noradrenaline levels are lower during remissions (Rajda et al. 2002). In stimulated lymphocytes from MS patients, no difference was observed in noradrenaline or adrenaline levels in comparison to cells from healthy controls, however, cells from patients with chronic-progressive MS or relapsing-remitting MS in relapse produced less dopamine (Cosentino et al. 2002a). In view of the role of stimulation-induced increase of endogenous catecholamines in activation-induced apoptosis of lymphocytes (Cosentino et al. 2002a), this finding was tentatively linked to the occurrence of impaired apoptotic mechanisms, which in MS can contribute to survival of autoreactive cells (Pender 1998; Macchi et al. 1999; Comi et al. 2000; Sharief et al. 2002).
Additional evidence for the relevance of adrenergic (and dopaminergic) lymphocyte-related mechanisms come from the observation that in vitro IFN-β increases lymphocyte production and release of catecholamines and, while IFN-γ profoundly decreases catecholamines production as well as TH mRNA expression, and coincubation with both IFNs prevents the inhibitory effect of IFN-γ, as well as the enhancing/releasing effect of IFN-β (Cosentino et al. 2005), and in MS patients IFN-β treatment enhances the ability of lymphocytes to produce CA, and restores the efficiency of β2-AR- (and dopaminergic receptor-) operated pathways (Zaffaroni et al. 2008), which could result in reduced T-cell proliferation and IFN-γ secretion (Giorelli et al. 2004).
With regard to glial cells, consistent evidence indicates that astrocytes of MS patients are deficient in β2-AR, both in normal-appearing white matter as well as in chronic active and inactive plaques (De Keyser et al. 1999; Zeinstra et al. 2000), resulting in failure of β2-AR-mediated suppression of class II major histocompatibility complex molecules and subsequent enhancement of antigen-presenting functions of these cells in MS (De Keyser et al. 2003). Astrocyte β2-AR dysregulation, however, may contribute to MS pathogenesis and progression through several other mechanisms, including: deficient suppression of nitric oxide and proinflammatory cytokine production and glutamate uptake, deficient glycogenolysis and production of trophic factors (De Keyser et al. 2004) and reduced perfusion of normal-appearing white matter (De Keyser et al. 2008).
Astrocytes as therapeutic targets in MS were recently challenged in a proof of concept study in MS subjects by use of fluoxetine, which activates protein kinase (PK) A in astrocytes. PKA is physiologically activated by β2-AR-mediated cAMP increase and in turn suppresses coactivator class II transactivator (CIITA), which regulates major histocompatibility (MHC) class II molecule transcription (De Keyser et al. 2010). Direct activation of PKA could in principle bypass the functional deficiency of β2-ARs in astrocytes, however, preliminary results need to be confirmed and extended in larger, randomized studies.
Indeed, β2-ARs have been already regarded as a promising target in the pharmacotherapy of MS and in particular salbutamol has been proposed as add-on therapy in patients with MS (Makhlouf et al. 2002). In-depth understanding of the complex (dys)regulation of β2-AR pathways in lymphocytes as well in the central nervous system in MS patients also in relation to treatment with immunomodulating agents could allow better exploitation of the potential benefits of drugs acting on β2-ARs.
Rheumatoid arthritis
Several studies over the last two decades reported decreased density and affinity of β2-ARs on PBMC from rheumatoid arthritis (RA) patients, in particular on CD8+ T lymphocytes and in CD19+ B lymphocytes, showing negative correlation with disease activity (Baerwald et al. 1992, 1997; Wahle et al. 2001a) and serum IL-2R levels (Krause et al. 1992). Reduction of β2-ARs may be even more pronounced in synovial fluid lymphocytes, with impairment of the suppressive effect of catecholamines on anti-CD3-induced lymphocyte proliferation (Baerwald et al. 1997, 1999). In RA patients with high disease activity, a shift to α1-AR-mediated catecholamine effects on PBMC reactivity could also be observed (Wahle et al. 1999). Indeed, noradrenaline and adrenaline fail to shift anti-CD3/anti-CD28-induced T-cell cytokine responses toward a Th2 profile and in particular CD8+ T cells are responsible for the impaired adrenergic control of IFN-γ production (Wahle et al. 2006). At least one study, however, found no changes in β2-ARs on RA lymphocytes, while showing a significant decrease in G protein-coupled receptor kinase (GRK) activity, with reduced protein expression of GRK-2 and GRK-6, possibly as a result of increased cell exposure to proinflammatory cytokines. RA lymphocytes showed a significantly increased cAMP production and inhibition of TNF-α production after β2-AR stimulation (Lombardi et al. 1999).
Evidence for dysregulated sympathoadrenergic modulation of the immune response is available also in juvenile RA (JRA), a subset of arthritis occurring in children, which may be transient or chronic. Patients with JRA have an altered function of the autonomic nervous system associated with increased central noradrenergic outflow and decreased β2-AR response of leukocytes (Kuis et al. 1996). Exposure of JRA patients (but not healthy controls) to a noradrenergic stressor results in enhanced LPS-induced IL-6 production by peripheral blood cells. In addition, PBMC of patients with JRA express mRNA encoding α1-ARs, predominantly of the α1D-AR subtype, which on the contrary are undetectable in cells from healthy subjects (Rouppe van der Voort et al. 2000) and which mediate noradrenaline-induced production of the proinflammatory cytokine IL-6 (Heijnen et al. 1996).
Dysregulation of the sympathoadrenergic tuning of the immune response in RA occurs also at the local level, in synovial tissue, where sympathetic innervation is reduced while sensory innervation is increased (Miller et al. 2000). Local noradrenaline production is maintained by TH+ cells, mainly synovial macrophages, in direct correlation with the degree of inflammation and with spontaneous secretion of IL-6, IL-8, and MMP-3 (Miller et al. 2002). In vitro, in human synoviocytes noradrenaline inhibits IL-8 and TNF production (Miller et al. 2002), as well as the production of the proinflammatory bactericidal alpha-defensins human neutrophil peptides 1-3 (HNP1-3) (Riepl et al. 2010), suggesting that the loss of sympathetic nerve fibers with low resting noradrenaline levels is crucial for the development of the inflammatory process, possibly through a shift from β-to-α adrenergic signaling (β-to-α adrenergic shift) (reviewed by Straub and Härle 2005). Noradrenaline secreted by TH+ cells occurring in synovial tissue during RA would thus represent an antiinflammatory mechanism acting together with systemic secretion of cortisol to counteract local inflammation (Straub et al. 2002a), while on the contrary systemic infusion of adrenaline (e.g. during a typical stress reaction) may result in lowered endogenous cortisol production and consequently increased inflammation (Straub et al. 2002b). Increased catecholamine release induced after blockade of vesicular monoamine transporter 2 (VMAT2) results in strong reduction of TNF and amelioration of inflammation in an animal model of RA (Capellino et al. 2010). Local catecholamine-producing cells may thus represent a novel target for the pharmacotherapy of RA, possibly in the context of neuroimmunopharmacological strategies aimed at restoring the global autonomic balance (Koopman et al. 2011).
In summary, extensive experimental and clinical evidence support the occurrence of dysregulated immune system responses to the hypothalamic–pituitary–adrenal axis and the sympathetic nervous system in RA, and targeting the neuroimmune network is increasingly regarded as an attractive therapeutic strategy (Baerwald et al. 2000; Wahle et al. 2002; Lorton et al. 2003; Straub et al. 2005).
Cancer
In animal models, activation of the sympathoadrenergic system through either stressful events or direct stimulation of β-ARs usually leads to compromised resistance to tumor development and metastasis (Stefanski and Ben-Eliyahu 1996; Shakhar and Ben-Eliyahu 1998). Enhancement of tumor progression is usually ascribed to β-AR-mediated decrease of NK activity (Shakhar and Ben-Eliyahu 1998; Ben-Eliyahu et al. 2000), although several lines of evidence suggest that noradrenaline and adrenaline may also directly affect tumor proliferation, angiogenesis, invasion, migration and metastasis (reviewed by Chakroborty et al. 2009; Li and Cho 2011). Impairment of NK activity and reduced antitumor resistance following stress and β-AR stimulation seem to be affected by age (Page and Ben-Eliyahu 2000) as well as by gender (Page et al. 2008). Recently, the prophylactic use of pharmacological blockade of β-ARs in association with type-C CpG oligodeoxynucleotides and COX inhibition has been proposed as a potential approach to limit postoperative immunosuppression and metastatic progression through enhancement of NK activity (Goldfarb et al. 2011).
AR modulation of immune response may be relevant also to cancer vaccine strategies. Indeed, Botta and Maestroni (2008) found that β2-AR antagonism along with TLR2 activation at the site of intradermal cancer vaccination may either enhance the resulting antitumor response or be tolerogenic in dependence of the maturation state of the transferred DCs, suggesting that manipulation of β2-ARs expressed in the site of DCs inoculation may influence the efficacy of the antitumor response.
Epidemiological studies support the hypothesis that exposure to β2-AR antagonists (beta blockers) may indeed reduce cancer progression and mortality, e.g. in melanoma (De Giorgi et al. 2011) and in breast cancer (Powe et al. 2010; Barron et al. 2011), although conflicting results have also been reported (Shah et al. 2011). Larger epidemiological studies as well as well-designed randomised clinical trials are needed for several cancer types to establish the potential of AR manipulation as antitumor therapy.
A role for ARs has been proposed long time ago also in the proliferation of hematological malignancies. Very low β-AR density and loss of adenylate cyclase activity are well-characterized features of pathologic cells from acute and chronic lymphocytic leukemia (CLL) (Sheppard et al. 1977; Paietta and Schwarzmeier 1983). Impaired β-AR expression in circulating cells from patients with CLL was shown to be specific for β2-ARs and to be associated with disease progression (Kamp et al. 1997). Whether activation of β-ARs may represent a therapeutic strategy in leukemias remains, however, to be established. Indeed, although accumulation of cAMP has been shown to increase the chemosensitivity of CLL cells, the proapoptotic effect of the long acting β2-AR agonists salmeterol and formoterol in these cells has been shown to be independent from β2-AR activation (Mamani-Matsuda et al. 2004). On the other side, endogenous adrenaline together with prostaglandins has been recently shown to mediate the promoting effects of stress on leukemia progression at least in animal models through suppression of NK activity, thus providing the rationale to explore the therapeutic potential of β-AR blockers and COX inhibitors also in hematological malignancies (Inbar et al. 2011).
Other diseases
The density of β2-ARs on circulating lymphocytes is decreased in several other chronic inflammatory and autoimmune diseases, including Crohn’s disease (Krause et al. 1992), systemic lupus erythematosus (del Rey and Besedovsky 2008), and myasthenia gravis (Xu et al. 1998). In lymphocytes from Alzheimer’s disease (AD) subjects, isoprenaline-induced cAMP increase may be reduced (Garlind et al. 1997) and GRK2 expression may be increased (Leosco et al. 2007), and α2C-AR gene expression is altered (Kálmán et al. 2005). Lymphocytes of patients with asthma as well have reduced β-AR binding capacity (Hataoka et al. 1993), however, sympathoadrenergic modulation of immunity in asthma and allergy received so far limited attention, despite extensive evidence on the role of neuroimmune mechanisms in such conditions (Marshall 2004).
Reduced lymphocyte β-AR density has been reported in patients with cardiac disease such as: congestive ischemic disease and coronary artery bypass grafting (Chello et al. 1995) and chronic severe heart failure of various origins (Townend et al. 1993; Wu et al. 1996; Dzimiri et al. 1995). Reduced β-AR on lymphocytes during cardiac disease results in impaired β-adrenergic control of lymphocyte activation (Werner et al. 2001), and should be addressed in the light of chronic low-intensity inflammation occurring in critical heart disease (see e.g. Barnes and Ackland 2010; Arslan et al. 2011).
Noradrenaline and adrenaline exert extensive effects on innate immunity which may be highly relevant for bacterial infections and sepsis (reviewed by Flierl et al. 2008). The therapeutic potential of α2-AR antagonism or pharmacological inhibition of catecholamine synthesis in rodent models of acute lung injury, as well as the beneficial effects of the β-AR antagonist propranolol in reducing the risk of opportunistic infections in severely burned patients have been already discussed (see previous section). Viral infections (e.g., herpes simplex virus type-1, cytomegalovirus and varicella zoster virus) can be promoted by stressful life events through activation of the sympathetic nervous system (Prösch et al. 2000). Noradrenaline may also accelerate human immunodeficiency virus (HIV) replication in infected lymphocytes via β-ARs (Cole et al. 1998), while in the central nervous system, HIV coat protein gp120 may disrupt β-AR-mediated regulation of astrocytes and microglia eventually promoting neuroinflammation (Levi et al. 1993).
Finally, decreased β-ARs on circulating lymphocytes have been reported in subjects with psychic disturbances, including depression (reviewed by Werstiuk et al. 1990), panic disorder (Brown et al. 1988), and even in normal subjects with increased tension and anxiety traits (Yu et al. 1999). The significance of these findings should be, however, assessed in the context of dysregulated immunity occurring in depression (reviewed by Blume et al. 2011).
Concluding remarks
Sympathoadrenergic mechanisms represent the main channel of communication between the nervous system and the immune system, however, much remains to be elucidated before AR-mediated pathways can be fully exploited as pharmacotherapeutic targets. Open issues include: (1) the existence of multiple subtypes of ARs which are expressed on immune cells with specific patterns in each cell subset, where each of them is involved in the control of well-defined functions; (2) receptor dysregulations occurring in disease states is not only specific for the receptor type but also for the cell subset(s); (3) receptors may be acted upon not only by exogenous but also by endogenous catecholamines directly produced by immune cells; (4) dynamic changes occur to receptor expression and responsiveness (and to endogenous catecholamine production) during treatment with immunomodulatory drugs. A wide array of sympathoadrenergic agents is currently used for various indications with a usually favorable therapeutic index, and represent therefore an attractive source of potentially novel immunomodulating agents with significant therapeutic potential.
References
Amenta F, Bronzetti E, Cantalamessa F, El-Assouad D, Felici L, Ricci A, Tayebati SK (2001) Identification of dopamine plasma membrane and vesicular transporters in human peripheral blood lymphocytes. J Neuroimmunol 117:133–142
Andreassi JL 2nd, Eggleston WB, Stewart JK (1998) Phenylethanolamine N-methyltransferase mRNA in rat spleen and thymus. Neurosci Lett 241:75–78
Arnason BG, Brown M, Maselli R, Karaszewski J, Reder A (1988) Blood lymphocyte beta-adrenergic receptors in multiple sclerosis. Ann NY Acad Sci 540:585–588
Arslan F, de Kleijn DP, Pasterkamp G (2011) Innate immune signaling in cardiac ischemia. Nat Rev Cardiol 8:292–300
Audus KL, Gordon MA (1982) Characteristics of tricyclic antidepressant binding sites associated with murine lymphocytes from spleen. J Immunopharmacol 4:1–12
Baerwald C, Graefe C, von Wichert P, Krause A (1992) Decreased density of beta-adrenergic receptors on peripheral blood mononuclear cells in patients with rheumatoid arthritis. J Rheumatol 19:204–210
Baerwald CG, Laufenberg M, Specht T, von Wichert P, Burmester GR, Krause A (1997) Impaired sympathetic influence on the immune response in patients with rheumatoid arthritis due to lymphocyte subset-specific modulation of beta 2-adrenergic receptors. Br J Rheumatol 36:1262–1269
Baerwald CG, Wahle M, Ulrichs T, Jonas D, von Bierbrauer A, von Wichert P, Burmester GR, Krause A (1999) Reduced catecholamine response of lymphocytes from patients with rheumatoid arthritis. Immunobiology 200:77–91
Baerwald CG, Burmester GR, Krause A (2000) Interactions of autonomic nervous, neuroendocrine, and immune systems in rheumatoid arthritis. Rheum Dis Clin North Am 26:841–857
Baker AJ, Fuller RW (1995) Loss of response to beta-adrenoceptor agonists during the maturation of human monocytes to macrophages in vitro. J Leukoc Biol 57:395–400
Balsa MD, Gómez N, Unzeta M (1989) Characterization of monoamine oxidase activity present in human granulocytes and lymphocytes. Biochim Biophys Acta 992:140–144
Barnes SJ, Ackland GL (2010) Beta-adrenoreceptor modulation of metabolic, endocrine and immunologic function during critical illness. Endocr Metab Immune Disord Drug Targets 10:292–300
Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K (2011) Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol 29:2635–2644
Bartik MM, Brooks WH, Roszman TL (1993) Modulation of T cell proliferation by stimulation of the beta-adrenergic receptor: lack of correlation between inhibition of T cell proliferation and cAMP accumulation. Cell Immunol 148:408–421
Bellinger DL, Lorton D, Felten SY, Felten DL (1992) Innervation of lymphoid organs and implications in development, aging, and autoimmunity. Int J Immunopharmacol 14:329–344
Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, Molinaro C, Lubahn C, Lorton D (2008) Sympathetic modulation of immunity: relevance to disease. Cell Immunol 252:27–56
Benarroch EE (2009) Autonomic-mediated immunomodulation and potential clinical relevance. Neurology 73:236–242
Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K (2000) Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation 8:154–164
Benschop RJ, Nijkamp FP, Ballieux RE, Heijnen CJ (1994) The effects of beta-adrenoceptor stimulation on adhesion of human natural killer cells to cultured endothelium. Br J Pharmacol 113:1311–1316
Benschop RJ, Schedlowski M, Wienecke H, Jacobs R, Schmidt RE (1997) Adrenergic control of natural killer cell circulation and adhesion. Brain Behav Immun 11:321–332
Bergquist J, Silberring J (1998) Identification of catecholamines in the immune system by electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 12:683–688
Bergquist J, Tarkowski A, Ekman R, Ewing A (1994) Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proc Natl Acad Sci USA 91:12912–12916
Berkeley MB, Daussin S, Hernandez MC, Bayer BM (1994) In vitro effects of cocaine, lidocaine and monoamine uptake inhibitors on lymphocyte proliferative responses. Immunopharmacol Immunotoxicol 16:165–178
Bidart JM, Assicot M, Bohuon C (1981) Catechol-O-methyl transferase activity in human mononuclear cells. Res. Commun. Chem Pathol Pharmacol 34:47–54
Bidart JM, Motte P, Assicot M, Bohuon C, Bellet D (1983) Catechol-O-methyltransferase activity and aminergic binding sites distribution in human peripheral blood lymphocyte subpopulations. Clin Immunol Immunopathol 26:1–9
Blume J, Douglas SD, Evans DL (2011) Immune suppression and immune activation in depression. Brain Behav Immun 25:221–229
Borda ES, Tenenbaum A, Sales ME, Rumi L, Sterin-Borda L (1998) Role of arachidonic acid metabolites in the action of a beta adrenergic agonist on human monocyte phagocytosis. Prostaglandins Leukot Essent Fatty Acids 58:85–90
Boreus LO, Hjemdahl P, Lagercrantz H, Martinsson A, Yao AC (1986) Beta-adrenoceptor function in white blood cells from newborn infants: no relation to plasma catecholamine levels. Pediatr Res 20:1152–1155
Borger P, Hoekstra Y, Esselink MT, Postma DS, Zaagsma J, Vellenga E, Kauffman HF (1998) Beta-adrenoceptor-mediated inhibition of IFN-gamma, IL-3, and GM-CSF mRNA accumulation in activated human T lymphocytes is solely mediated by the beta2-adrenoceptor subtype. Am J Respir Cell Mol Biol 19:400–407
Botta F, Maestroni GJ (2008) Adrenergic modulation of dendritic cell cancer vaccine in a mouse model: role of dendritic cell maturation. J Immunother 31:263–270
Brown SL, Charney DS, Woods SW, Heninger GL, Tallman J (1988) Lymphocyte beta-adrenergic receptor binding in panic disorder. Psychopharmacology (Berl) 94:24–28
Bylund DB, Bond RA, Eikenburg DC, Hieble JP, Hills R, Minneman KP, Parra S (2010) Adrenoceptors. Last modified 10 Feb 2011. IUPHAR database (IUPHAR-DB). http://www.iuphar-db.org/DATABASE/FamilyMenuForward?familyId=4. Accessed 24 Aug 2011
Capellino S, Cosentino M, Wolff C, Schmidt M, Grifka J, Straub RH (2010) Catecholamine-producing cells in the synovial tissue during arthritis: modulation of sympathetic neurotransmitters as new therapeutic target. Ann Rheum Dis 69:1853–1860
Carlson SL, Trauth K, Brooks WH, Roszman TL (1994) Enhancement of beta-adrenergic-induced cAMP accumulation in activated T-cells. J Cell Physiol 161:39–48
Chaitidis P, Billett EE, O’Donnell VB, Fajardo AB, Fitzgerald J, Kuban RJ, Ungethuem U, Kühn H (2004) Th2 response of human peripheral monocytes involves isoform-specific induction of monoamine oxidase-A. J Immunol 173:4821–4827
Chaitidis P, O’Donnell V, Kuban RJ, Bermudez-Fajardo A, Ungethuem U, Kühn H (2005) Gene expression alterations of human peripheral blood monocytes induced by medium-term treatment with the TH2-cytokines interleukin-4 and -13. Cytokine 30:366–377
Chakroborty D, Sarkar C, Basu B, Dasgupta PS, Basu S (2009) Catecholamines regulate tumor angiogenesis. Cancer Res 69:3727–3730
Chello M, Mastroroberto P, Romano R, Cirillo F, Marchese AR (1995) Improved beta-adrenergic receptor function after coronary artery bypass grafting in patients with congestive heart failure. Coron Artery Dis 6:957–963
Chou RC, Dong XL, Noble BK, Knight PR, Spengler RN (1998) Adrenergic regulation of macrophage-derived tumor necrosis factor-alpha generation during a chronic polyarthritis pain model. J Neuroimmunol 82:140–148
Cole SW, Korin YD, Fahey JL, Zack JA (1998) Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. J Immunol 161:610–616
Comi C, Leone M, Bonissoni S, DeFranco S, Bottarel F, Mezzatesta C, Chiocchetti A, Perla F, Monaco F, Dianzani U (2000) Defective T cell Fas function in patients with multiple sclerosis. Neurology 55:921–927
Corradi L, Negri F, Parini A, Partesana N, Finardi G (1981) Decreased beta-adrenoceptors in polymorphonucleates in essential hypertension. Boll Soc Ital Biol Sper 57:1766–1770
Cosentino M, Marino F, Bombelli R, Ferrari M, Lecchini S, Frigo G (1999) Endogenous catecholamine synthesis, metabolism, storage and uptake in human neutrophils. Life Sci 64:975–981
Cosentino M, Bombelli R, Ferrari M, Marino F, Rasini E, Maestroni GJM, Conti A, Boveri M, Lecchini S, Frigo G (2000) HPLC-ED measurement of endogenous catecholamines in human immune cells and hematopoietic cell lines. Life Sci 68:283–295
Cosentino M, Marino F, Bombelli R, Ferrari M, Rasini E, Lecchini S, Frigo G (2002a) Stimulation with phytohaemagglutinin induces the synthesis of catecholamines in human peripheral blood mononuclear cells: role of protein kinase C and contribution of intracellular calcium. J Neuroimmunol 125:125–133
Cosentino M, Zaffaroni M, Marino F, Bombelli R, Ferrari M, Rasini E, Lecchini S, Ghezzi A, Frigo GM (2002b) Catecholamine production and tyrosine hydroxylase expression in peripheral blood mononuclear cells from multiple sclerosis patients: effect of cell stimulation and possible relevance for activation-induced apoptosis. J Neuroimmunol 133:233–240
Cosentino M, Marino F, Bombelli R, Ferrari M, Lecchini S, Frigo G (2003) Unravelling dopamine (and catecholamine) physiopharmacology in lymphocytes: open questions. Trends Immunol 24:581–582
Cosentino M, Zaffaroni M, Ferrari M, Marino F, Bombelli R, Rasini E, Frigo G, Ghezzi A, Comi G, Lecchini S (2005) Interferon-γ and interferon-β affect endogenous catecholamines in human peripheral blood mononuclear cells: implications for multiple sclerosis. J Neuroimmunol 162:112–121
Cosentino M, Fietta AM, Ferrari M, Rasini E, Bombelli R, Carcano E, Saporiti F, Meloni F, Marino F, Lecchini S (2007) Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood 109:632–642
De Giorgi V, Grazzini M, Gandini S, Benemei S, Lotti T, Marchionni N, Geppetti P (2011) Treatment with β-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med 171:779–781
De Keyser J, Wilczak N, Leta R, Streetland C (1999) Astrocytes in multiple sclerosis lack beta-2 adrenergic receptors. Neurology 53:1628–1633
De Keyser J, Zeinstra E, Frohman E (2003) Are astrocytes central players in the pathophysiology of multiple sclerosis? Arch Neurol 60:132–136
De Keyser J, Zeinstra E, Wilczak N (2004) Astrocytic beta2-adrenergic receptors and multiple sclerosis. Neurobiol Dis 15:331–339
De Keyser J, Steen C, Mostert JP, Koch MW (2008) Hypoperfusion of the cerebral white matter in multiple sclerosis: possible mechanisms and pathophysiological significance. J Cereb Blood Flow Metab 28:1645–1651
De Keyser J, Laureys G, Demol F, Wilczak N, Mostert J, Clinckers R (2010) Astrocytes as potential targets to suppress inflammatory demyelinating lesions in multiple sclerosis. Neurochem Int 57:446–450
del Rey A, Besedovsky HO (2008) Sympathetic nervous system-immune interactions in autoimmune lymphoproliferative diseases. Neuroimmunomodulation 15:29–36
Dzimiri N, Hussain S, Moorji A, Prabhakar G, Bakr S, Kumar M, Almotrefi AA, Halees Z (1995) Characterization of lymphocyte beta-adrenoceptor activity and Gs-protein in patients with rheumatic heart valvular disease. Fundam Clin Pharmacol 9:372–380
Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES (2000) The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 52:595–638
Elliott L, Brooks W, Roszman T (1992) Inhibition of anti-CD3 monoclonal antibody-induced T-cell proliferation by dexamethasone, isoproterenol, or prostaglandin E2 either alone or in combination. Cell Mol Neurobiol 12:411–427
Ezeamuzie CI, Shihab PK, Al-Radwan R (2011) Loss of surface beta-2 adrenoceptors accounts for the insensitivity of cultured human monocytes to beta-2 adrenoceptor agonists. Int Immunopharmacol [Epub ahead of print]
Feldman RD, Hunninghake GW, McArdle WL (1987) Beta-adrenergic-receptor-mediated suppression of interleukin 2 receptors in human lymphocytes. J Immunol 139:3355–3359
Feldman RS, Meyer JS, Quenzer LF (1997) Catecholamines. In: Principles of neuropsychopharmacology. Sinauer Associates Inc., Sunderland, Massachusets, pp 277–344
Felten DL (1991) Neurotransmitter signaling of cells of the immune system: important progress, major gaps. Brain Behav Immun 5:2–8
Ferrari M, Cosentino M, Marino F, Bombelli R, Rasini E, Lecchini S, Frigo G (2004) Dopaminergic D1-like receptor-dependent inhibition of tyrosine hydroxylase mRNA expression and catecholamine production in human lymphocytes. Biochem Pharmacol 67:865–873
Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, McGuire SR, List RP, Day DE, Hoesel LM, Gao H, Van Rooijen N, Huber-Lang MS, Neubig RR, Ward PA (2007) Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature 449:721–725
Flierl MA, Rittirsch D, Huber-Lang M, Sarma JV, Ward PA (2008) Catecholamines—Crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening Pandora’s box? Mol Med 14:195–204
Flierl MA, Rittirsch D, Nadeau BA, Sarma JV, Day DE, Lentsch AB, Huber-Lang MS, Ward PA (2009) Upregulation of phagocyte-derived catecholamines augments the acute inflammatory response. PLoS One 4:e4414
Fragala MS, Kraemer WJ, Mastro AM, Denegar CR, Volek JS, Häkkinen K, Anderson JM, Lee E, Maresh CM (2011) Leukocyte β2-adrenergic receptor expression in response to resistance. Exerc Med Sci Sports Exerc 43:1422–1432
Freeman JG, Ryan JJ, Shelburne CP, Bailey DP, Bouton LA, Narasimhachari N, Domen J, Simeon N, Couderc F, Stewart JK (2001) Catecholamines in murine bone marrow derived mast cells. J Neuroimmunol 119:231–238
Freier E, Weber CS, Nowottne U, Horn C, Bartels K, Meyer S, Hildebrandt Y, Luetkens T, Cao Y, Pabst C, Muzzulini J, Schnee B, Brunner-Weinzierl MC, Marangolo M, Bokemeyer C, Deter HC, Atanackovic D (2010) Decrease of CD4(+)FOXP3(+) T regulatory cells in the peripheral blood of human subjects undergoing a mental stressor. Psychoneuroendocrinology 35:663–673
Friedman EM, Irwin MR (1997) Modulation of immune cell function by the autonomic nervous system. Pharmacol Ther 74:27–38
Frohman EM, Monson NL, Lovett-Racke AE, Racke MK (2001) Autonomic regulation of neuroimmunological responses: implications for multiple sclerosis. J Clin Immunol 21:61–73
Frohman EM, Racke MK, Raine CS (2006) Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med 354:942–955
Garlind A, Johnston JA, Algotsson A, Winblad B, Cowburn RF (1997) Decreased beta-adrenoceptor-stimulated adenylyl cyclase activity in lymphocytes from Alzheimer’s disease patients. Neurosci Lett 226:37–40
Ghorpade A, Gendelman HE, Kipnis J (2008) Macrophages, microglia and dendritic cells. In: Ikezu T, Gendelman HE (eds) Neuroimmune pharmacology. Springer, Berlin, pp 89–104
Giorelli M, Livrea P, Trojano M (2004) Post-receptorial mechanisms underlie functional disregulation of beta2-adrenergic receptors in lymphocytes from Multiple Sclerosis patients. J Neuroimmunol 155:143–149
Giubilei F, Calderaro C, Antonini G, Sepe-Monti M, Tisei P, Brunetti E, Marchione F, Caronti B, Pontieri FE (2004) Increased lymphocyte dopamine beta-hydroxylase immunoreactivity in Alzheimer’s disease: compensatory response to cholinergic deficit? Dement Geriatr Cogn Disord 18:338–341
Goldfarb Y, Sorski L, Benish M, Levi B, Melamed R, Ben-Eliyahu S (2011) Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg 253:798–810
Goyarts E, Matsui M, Mammone T, Bender AM, Wagner JA, Maes D, Granstein RD (2008) Norepinephrine modulates human dendritic cell activation by altering cytokine release. Exp Dermatol 17:188–196
Grisanti LA, Evanson J, Marchus E, Jorissen H, Woster AP, DeKrey W, Sauter ER, Combs CK, Porter JE (2010) Pro-inflammatory responses in human monocytes are beta1-adrenergic receptor subtype dependent. Mol Immunol 47:1244–1254
Grisanti LA, Woster AP, Dahlman J, Sauter ER, Combs CK, Porter JE (2011) Alpha-1 adrenergic receptors positively regulate toll-like receptor cytokine production from human monocytes and macrophages. J Pharmacol Exp Ther 338:648–657
Guirao X, Kumar A, Katz J, Smith M, Lin E, Keogh C, Calvano SE, Lowry SF (1997) Catecholamines increase monocyte TNF receptors and inhibit TNF through beta 2-adrenoreceptor activation. Am J Physiol 273:E1203–E1208
Gurguis GN, Andrews R, Antai-Otong D, Vo SP, Blakeley JE, Orsulak PJ, Rush AJ (1999a) Neutrophil beta2-adrenergic receptor coupling efficiency to Gs protein in subjects with post-traumatic stress disorder and normal controls. Psychopharmacology (Berl) 143:131–140
Gurguis GN, Vo SP, Griffith JM, Rush AJ (1999b) Neutrophil beta(2)-adrenoceptor function in major depression: G(s) coupling, effects of imipramine and relationship to treatment outcome. Eur J Pharmacol 386:135–144
Hataoka I, Okayama M, Sugi M, Inoue H, Takishima T, Shirato K (1993) Decrease in beta-adrenergic receptors of lymphocytes in spontaneously occurring acute asthma. Chest 104:508–514
Heijink IH, Vellenga E, Borger P, Postma DS, Monchy JG, Kauffman HF (2003) Polarized Th1 and Th2 cells are less responsive to negative feedback by receptors coupled to the AC/cAMP system compared to freshly isolated T cells. Br J Pharmacol 138:1441–1450
Heijnen CJ, Rouppe van der Voort C, Wulffraat N, van der Net J, Kuis W, Kavelaars A (1996) Functional alpha 1-adrenergic receptors on leukocytes of patients with polyarticular juvenile rheumatoid arthritis. J Neuroimmunol 71:223–226
Heijnen CJ, Rouppe van der Voort C, van de Pol M, Kavelaars A (2002) Cytokines regulate alpha(1)-adrenergic receptor mRNA expression in human monocytic cells and endothelial cells. J Neuroimmunol 125:66–72
Henry JP, Botton D, Sagne C, Isambert MF, Desnos C, Blanchard V, Raisman-Vozari R, Krejci E, Massoulie J, Gasnier B (1994) Biochemistry and molecular biology of the vesicular monoamine transporter from chromaffin granules. J Exp Biol 196:251–262
Hertz L, Chen Y, Gibbs ME, Zang P, Peng L (2004) Astrocytic adrenoceptors: a major drug target in neurological and psychiatric disorders? Curr Drug Targets CNS Neurol Disord 3:239–267
Hertz L, Lovatt D, Goldman SA, Nedergaard M (2010) Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca(2+)]i. Neurochem Int 57:411–420
Inbar S, Neeman E, Avraham R, Benish M, Rosenne E, Ben-Eliyahu S (2011) Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E2 through reduced NK activity. PLoS One 6:e19246
Irwin M (1994) Stress-induced immune suppression: role of brain corticotropin releasing hormone and autonomic nervous system mechanisms. Adv Neuroimmunol 4:29–47
Jana M, Dasgupta S, Ghorpade A, Pahan K (2008) Astrocytes, oligodendrocytes, and Schwann cells. In: Ikezu T, Gendelman HE (eds) Neuroimmune pharmacology. Springer, Berlin, pp 69–88
Jetschmann JU, Benschop RJ, Jacobs R, Kemper A, Oberbeck R, Schmidt RE, Schedlowski M (1997) Expression and in vivo modulation of alpha- and beta-adrenoceptors on human natural killer (CD16+) cells. J Neuroimmunol 74:159–164
Jiang H, Jiang Q, Liu W, Feng J (2006) Parkin suppresses the expression of monoamine oxidases. J Biol Chem 281:8591–8599
Jiang JL, Peng YP, Qiu YH, Wang JJ (2007) Effect of endogenous catecholamines on apoptosis of Con A-activated lymphocytes of rats. J Neuroimmunol 192:79–88
Jiang JL, Peng YP, Qiu YH, Wang JJ (2009) Adrenoreceptor-coupled signal-transduction mechanisms mediating lymphocyte apoptosis induced by endogenous catecholamines. J Neuroimmunol 213:100–111
Josefsson E, Bergquist J, Ekman R, Tarkowski A (1996) Catecholamines are synthesized by mouse lymphocytes and regulate function of these cells by induction of apoptosis. Immunology 88:140–146
Kálmán J, Kitajka K, Pákáski M, Zvara A, Juhász A, Vincze G, Janka Z, Puskás LG (2005) Gene expression profile analysis of lymphocytes from Alzheimer’s patients. Psychiatr Genet 15:1–6
Kamp T, Liebl B, Haen E, Emmerich B, Hallek M (1997) Defects of beta 2-adrenergic signal transduction in chronic lymphocytic leukaemia: relationship to disease progression. Eur J Clin Invest 27:121–127
Karaszewski JW, Reder AT, Maselli R, Brown M, Arnason BG (1990) Sympathetic skin responses are decreased and lymphocyte beta-adrenergic receptors are increased in progressive multiple sclerosis. Ann Neurol 27:366–372
Karaszewski JW, Reder AT, Anlar B, Kim WC, Arnason BG (1991) Increased lymphocyte beta-adrenergic receptor density in progressive multiple sclerosis is specific for the CD8+, CD28− suppressor cell. Ann Neurol 30:42–47
Karaszewski JW, Reder AT, Anlar B, Arnason GW (1993) Increased high affinity beta-adrenergic receptor densities and cyclic AMP responses of CD8 cells in multiple sclerosis. J Neuroimmunol 43:1–7
Kasprowicz DJ, Kohm AP, Berton MT, Chruscinski AJ, Sharpe A, Sanders VM (2000) Stimulation of the B cell receptor, CD86 (B7-2), and the beta 2-adrenergic receptor intrinsically modulates the level of IgG1 and IgE produced per B cell. J Immunol 165:680–690
Kavelaars A, van de Pol M, Zijlstra J, Heijnen CJ (1997) Beta 2-adrenergic activation enhances interleukin-8 production by human monocytes. J Neuroimmunol 77:211–216
Khan MM, Sansoni P, Silverman ED, Engleman EG, Melmon KL (1986) Beta-adrenergic receptors on human suppressor, helper, and cytolytic lymphocytes. Biochem Pharmacol 35:1137–1142
Knudsen JH, Christensen NJ, Bratholm P (1996) Lymphocyte norepinephrine and epinephrine, but not plasma catecholamines predict lymphocyte cAMP production. Life Sci 59:639–647
Kohm AP, Sanders VM (1999) Suppression of antigen-specific Th2 cell-dependent IgM and IgG1 production following norepinephrine depletion in vivo. J Immunol 162:5299–5308
Kohm AP, Sanders VM (2001) Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev 53:487–525
Koopman FA, Stoof SP, Straub RH, Van Maanen MA, Vervoordeldonk MJ, Tak PP (2011) Restoring the balance of the autonomic nervous system as an innovative approach to the treatment of rheumatoid arthritis. Mol Med. doi:10.2119/molmed.2011.00065 [Epub ahead of print]
Korichneva IL, Tkachuk VA (1990) Alterations in beta-adrenoceptor density on T-lymphocytes upon activation with interleukin-2 and phytohaemagglutinin. Biomed Sci 1:84–88
Krause A, Henrich A, Beckh KH, Von Wichert P, Baerwald C (1992) Correlation between density of beta 2-adrenergic receptors on peripheral blood mononuclear cells and serum levels of soluble interleukin-2 receptors in patients with chronic inflammatory diseases. Eur J Clin Invest 22(Suppl 1):47–51
Kuis W, van Steenwijk C, Sinnema G, Kavelaars A, Prakken B, Helders PJ, Heijnen CJ (1996) The autonomic nervous system and the immune system in juvenile rheumatoid arthritis. Brain Behav Immun 10:387–398
Laureys G, Clinckers R, Gerlo S, Spooren A, Wilczak N, Kooijman R, Smolders I, Michotte Y, De Keyser J (2010) Astrocytic beta(2)-adrenergic receptors: from physiology to pathology. Prog Neurobiol 91:189–199
Leosco D, Fortunato F, Rengo G, Iaccarino G, Sanzari E, Golino L, Zincarelli C, Canonico V, Marchese M, Koch WJ, Rengo F (2007) Lymphocyte G-protein-coupled receptor kinase-2 is upregulated in patients with Alzheimer’s disease. Neurosci Lett 415:279–282
Leposavić G, Pilipović I, Radojević K, Pesić V, Perisić M, Kosec D (2008) Catecholamines as immunomodulators: a role for adrenoceptor-mediated mechanisms in fine tuning of T-cell development. Auton Neurosci 144:1–12
Levi G, Patrizio M, Bernardo A, Petrucci TC, Agresti C (1993) Human immunodeficiency virus coat protein gp120 inhibits the beta-adrenergic regulation of astroglial and microglial functions. Proc Natl Acad Sci USA 90:1541–1545
Levite M (2001) Nervous immunity: neurotransmitters, extracellular K+ and T-cell function. Trends Immunol 22:2–5
Li ZJ, Cho CH (2011) Neurotransmitters, more than meets the eye—neurotransmitters and their perspectives in cancer development and therapy. Eur J Pharmacol 667:17–22
Li CY, Chou TC, Lee CH, Tsai CS, Loh SH, Wong CS (2003) Adrenaline inhibits lipopolysaccharide-induced macrophage inflammatory protein-1 alpha in human monocytes: the role of beta-adrenergic receptors. Anesth Analg 96:518–523
Lombardi MS, Kavelaars A, Schedlowski M, Bijlsma JW, Okihara KL, Van de Pol M, Ochsmann S, Pawlak C, Schmidt RE, Heijnen CJ (1999) Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. FASEB J 13:715–725
Lorton D, Lubahn C, Bellinger DL (2003) Potential use of drugs that target neural-immune pathways in the treatment of rheumatoid arthritis and other autoimmune diseases. Curr Drug Targets Inflamm Allergy 2:1–30
Macchi B, Matteucci C, Nocentini U, Caltagirone C, Mastino A (1999) Impaired apoptosis in mitogen-stimulated lymphocytes of patients with multiple sclerosis. NeuroReport 10:399–402
Madden KS, Sanders VM, Felten DL (1995) Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu Rev Pharmacol Toxicol 35:417–448
Madden KS, Thyagarajan S, Felten DL (1998) Alterations in sympathetic noradrenergic innervation in lymphoid organs with age. Ann NY Acad Sci 840:262–268
Maes M, Lin A, Kenis G, Egyed B, Bosmans E (2000) The effects of noradrenaline and alpha-2 adrenoceptor agents on the production of monocytic products. Psychiatry Res 96:245–253
Maestroni GJ (2005) Adrenergic modulation of dendritic cells function: relevance for the immune homeostasis. Curr Neurovasc Res 2:169–173
Maestroni GJ (2006) Sympathetic nervous system influence on the innate immune response. Ann NY Acad Sci 1069:195–207
Maisel AS, Harris T, Rearden CA, Michel MC (1990) Beta-adrenergic receptors in lymphocyte subsets after exercise. Alterations in normal individuals and patients with congestive heart failure. Circulation 82:2003–2010
Makhlouf K, Weiner HL, Khoury SJ (2002) Potential of beta2-adrenoceptor agonists as add-on therapy for multiple sclerosis: focus on salbutamol (albuterol). CNS Drugs 16:1–8
Mamani-Matsuda M, Moynet D, Molimard M, Ferry-Dumazet H, Marit G, Reiffers J, Mossalayi MD (2004) Long-acting beta2-adrenergic formoterol and salmeterol induce the apoptosis of B-chronic lymphocytic leukaemia cells. Br J Haematol 124:141–150
Mandela P, Ordway GA (2006) The norepinephrine transporter and its regulation. J Neurochem 97:310–333
Manni M, Maestroni GJ (2008) Sympathetic nervous modulation of the skin innate and adaptive immune response to peptidoglycan but not lipopolysaccharide: involvement of beta-adrenoceptors and relevance in inflammatory diseases. Brain Behav Immun 22:80–88
Manni M, Granstein RD, Maestroni G (2011) β2-Adrenergic agonists bias TLR-2 and NOD2 activated dendritic cells towards inducing an IL-17 immune response. Cytokine 55:380–386
Mantyh PW, Rogers SD, Allen CJ, Catton MD, Ghilardi JR, Levin LA, Maggio JE, Vigna SR (1995) Beta 2-adrenergic receptors are expressed by glia in vivo in the normal and injured central nervous system in the rat, rabbit, and human. J Neurosci 15:152–164
Marazziti D, Consoli G, Masala I, Catena Dell’Osso M, Baroni S (2010) Latest advancements on serotonin and dopamine transporters in lymphocytes. Mini Rev Med Chem 10:32–40
Marino F, Cosentino M, Bombelli R, Ferrari M, Lecchini S, Frigo G (1999) Endogenous catecholamine synthesis, metabolism storage, and uptake in human peripheral blood mononuclear cells. Exp Hematol 27:489–495
Markus T, Hansson SR, Cronberg T, Cilio C, Wieloch T, Ley D (2010) β-Adrenoceptor activation depresses brain inflammation and is neuroprotective in lipopolysaccharide-induced sensitization to oxygen-glucose deprivation in organotypic hippocampal slices. J Neuroinflammation 7:94
Marshall GD (2004) Neuroendocrine mechanisms of immune dysregulation: applications to allergy and asthma. Ann Allergy Asthma Immunol 93(2 Suppl 1):S11–S17
Marshall GD Jr, Agarwal SK (2000) Stress, immune regulation, and immunity: applications for asthma. Allergy Asthma Proc 21:241–246
McNamee EN, Ryan KM, Kilroy D, Connor TJ (2010) Noradrenaline induces IL-1ra and IL-1 type II receptor expression in primary glial cells and protects against IL-1beta-induced neurotoxicity. Eur J Pharmacol 626:219–228
Melmon KL, Bourne HR, Weinstein Y, Shearer GM, Kram J, Bauminger S (1974) Hemolytic plaque formation by leukocytes in vitro. Control by vasoactive hormones. J Clin Invest 53:13–21
Mignini F, Tomassoni D, Traini E, Amenta F (2009) Dopamine, vesicular transporters and dopamine receptor expression and localization in rat thymus and spleen. J Neuroimmunol 206:5–13
Miller LE, Jüsten HP, Schölmerich J, Straub RH (2000) The loss of sympathetic nerve fibers in the synovial tissue of patients with rheumatoid arthritis is accompanied by increased norepinephrine release from synovial macrophages. FASEB J 14:2097–2107
Miller LE, Grifka J, Schölmerich J, Straub RH (2002) Norepinephrine from synovial tyrosine hydroxylase positive cells is a strong indicator of synovial inflammation in rheumatoid arthritis. J Rheumatol 29:427–435
Mizuno K, Takahashi HK, Iwagaki H, Katsuno G, Kamurul HA, Ohtani S, Mori S, Yoshino T, Nishibori M, Tanaka N (2005) Beta2-adrenergic receptor stimulation inhibits LPS-induced IL-18 and IL-12 production in monocytes. Immunol Lett 101:168–172
Musgrave IF, Seifert R (1994) Human neutrophils and HL-60 cells do not possess alpha 2-adrenoceptors. Biochem Pharmacol 47:233–239
Musso NR, Brenci S, Setti M, Indiveri F, Lotti G (1996) Catecholamine content and in vitro catecholamine synthesis in peripheral human lymphocytes. J Clin Endocrinol Metab 81:3553–3557
Nance DM, Sanders VM (2007) Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun 21:736–745
Nielson CP (1987) Beta-adrenergic modulation of the polymorphonuclear leukocyte respiratory burst is dependent upon the mechanism of cell activation. J Immunol 139:2392–2397
Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG (2000) Multiple sclerosis. N Engl J Med 343:938–952
Page GG, Ben-Eliyahu S (2000) Natural killer cell activity and resistance to tumor metastasis in prepubescent rats: deficient baselines, but invulnerability to stress and beta-adrenergic stimulation. Neuroimmunomodulation 7:160–168
Page GG, Fennelly AM, Littleton-Kearney MT, Ben-Eliyahu S (2008) Male-female differences in the impact of beta-adrenoceptor stimulation on resistance to experimental metastasis: exploring the effects of age and gonadal hormone involvement. J Neuroimmunol 193:113–119
Paietta E, Schwarzmeier JD (1983) Differences in beta-adrenergic receptor density and adenylate cyclase activity between normal and leukaemic leukocytes. Eur J Clin Invest 13:339–346
Panina-Bordignon P, Mazzeo D, Lucia PD, D’Ambrosio D, Lang R, Fabbri L, Self C, Sinigaglia F (1997) Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. J Clin Invest 100:1513–1519
Pender MP (1998) Genetically determined failure of activation-induced apoptosis of autoreactive T cells as a cause of multiple sclerosis. Lancet 351:978–981
Pintar JE, Breakefield XO (1982) Monoamine oxidase (MAO) activity as a determinant in human neurophysiology. Behav Genet 12:53–68
Pohl A, Otto J, Urbanek R (1991) Beta-2-adrenoceptors of polymorphonuclear leukocytes in children with atopic dermatitis. Their number and affinity to the radioligand [125I]-cyanopindolol. Int Arch Allergy Appl Immunol 95:261–265
Powe DG, Voss MJ, Zänker KS, Habashy HO, Green AR, Ellis IO, Entschladen F (2010) Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 1:628–638
Prösch S, Wendt CE, Reinke P, Priemer C, Oppert M, Krüger DH, Volk HD, Döcke WD (2000) A novel link between stress and human cytomegalovirus (HCMV) infection: sympathetic hyperactivity stimulates HCMV activation. Virology 272:357–365
Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia A-S, McNamara JO, Williams SM (eds) (2001) Criteria that define a neurotransmitter. In: Neuroscience, 2nd ed. Sinauer Associates, Sunderland. http://www.ncbi.nlm.nih.gov/books/NBK10957/?rendertype=box&id=A377. Accessed 24 Aug 2011
Qiu YH, Peng YP, Jiang JM, Wang JJ (2004) Expression of tyrosine hydroxylase in lymphocytes and effect of endogenous catecholamines on lymphocyte function. Neuroimmunomodulation 11:75–83
Qiu YH, Cheng C, Dai L, Peng YP (2005) Effect of endogenous catecholamines in lymphocytes on lymphocyte function. J Neuroimmunol 167:45–52
Radojcic T, Baird S, Darko D, Smith D, Bulloch K (1991) Changes in beta-adrenergic receptor distribution on immunocytes during differentiation: an analysis of T cells and macrophages. J Neurosci Res 30:328–335
Rainer TH, Lam N, Cocks RA (1999) Adrenaline upregulates monocyte L-selectin in vitro. Resuscitation 43:47–55
Rajda C, Bencsik K, Vecsei LL, Bergquist J (2002) Catecholamine levels in peripheral blood lymphocytes from multiple sclerosis patients. J Neuroimmunol 124:93–100
Ratge D, Wiedemann A, Kohse KP, Wisser H (1988) Alterations of beta-adrenoceptors on human leukocyte subsets induced by dynamic exercise: effect of prednisone. Clin Exp Pharmacol Physiol 15:43–53
Reguzzoni M, Cosentino M, Rasini E, Marino F, Ferrari M, Bombelli R, Congiu T, Protasoni M, Quacci D, Lecchini S, Raspanti M, Frigo G (2002) Ultrastructural localization of tyrosine hydroxylase in human peripheral blood mononuclear cells: effect of stimulation with phytohaemagglutinin. Cell Tissue Res 310:297–304
Riepl B, Grässel S, Wiest R, Fleck M, Straub RH (2010) Tumor necrosis factor and norepinephrine lower the levels of human neutrophil peptides 1–3 secretion by mixed synovial tissue cultures in osteoarthritis and rheumatoid arthritis. Arthr Res Ther 12:R110
Rouppe van der Voort C, Kavelaars A, van de Pol M, Heijnen CJ (1999) Neuroendocrine mediators up-regulate alpha1b- and alpha1d-adrenergic receptor subtypes in human monocytes. J Neuroimmunol 95:165–173
Rouppe van der Voort C, Heijnen CJ, Wulffraat N, Kuis W, Kavelaars A (2000a) Stress induces increases in IL-6 production by leucocytes of patients with the chronic inflammatory disease juvenile rheumatoid arthritis: a putative role for alpha(1)-adrenergic receptors. J Neuroimmunol 110:223–229
Rouppe van der Voort C, Kavelaars A, van de Pol M, Heijnen CJ (2000b) Noradrenaline induces the phosphorylation of ERK-2 in human peripheral blood mononuclear cells after induction of α1-adrenergic receptors. J Neuroimmunol 108:82–91
Sanders VM, Baker RA, Ramer-Quinn DS, Kasprowicz DJ, Fuchs BA, Street NE (1997) Differential expression of the beta-2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J Immunol 158:4200–4210
Schedlowski M, Hosch W, Oberbeck R, Benschop RJ, Jacobs R, Raab HR, Schmidt RE (1996) Catecholamines modulate human NK cell circulation and function via spleen-independent beta 2-adrenergic mechanisms. J Immunol 156:93–99
Schopf RE, Lemmel EM (1983) Control of the production of oxygen intermediates of human polymorphonuclear leukocytes and monocytes by beta-adrenergic receptors. J Immunopharmacol 5:203–216
Schwab KO, Bartels H, Martin C, Leichtenschlag EM (1993) Decreased beta 2-adrenoceptor density and decreased isoproterenol induced c-AMP increase in juvenile type I diabetes mellitus: an additional cause of severe hypoglycaemia in childhood diabetes? Eur J Pediatr 152:797–801
Shah SM, Carey IM, Owen CG, Harris T, Dewilde S, Cook DG (2011) Does β-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br J Clin Pharmacol 72:157–161
Shakhar G, Ben-Eliyahu S (1998) In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol 160:3251–3258
Sharief MK, Douglas M, Noori M, Semra YK (2002) The expression of pro- and anti-apoptosis Bcl-2 family proteins in lymphocytes from patients with multiple sclerosis. J Neuroimmunol 125:155–162
Sheppard JR, Gormus R, Moldow CF (1977) Catecholamine hormone receptors are reduced on chronic lymphocytic leukaemic lymphocytes. Nature 269:693–695
Sloan EK, Capitanio JP, Cole SW (2008) Stress-induced remodeling of lymphoid innervation. Brain Behav Immun 22:15–21
Sneader W (2005) Drug discovery: a history. John, Chichester, pp 155–157
Speidl WS, Toller WG, Kaun C, Weiss TW, Pfaffenberger S, Kastl SP, Furnkranz A, Maurer G, Huber K, Metzler H, Wojta J (2004) Catecholamines potentiate LPS-induced expression of MMP-1 and MMP-9 in human monocytes and in the human monocytic cell line U937: possible implications for peri-operative plaque instability. FASEB J 18:603–605
Spengler RN, Chensue SW, Giacherio DA, Blenk N, Kunkel SL (1994) Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J Immunol 152:3024–3031
Spooren A, Mestdagh P, Rondou P, Kolmus K, Haegeman G, Gerlo S (2011) IL-1β potently stabilizes IL-6 mRNA in human astrocytes. Biochem Pharmacol 81:1004–1015
Stefanski V, Ben-Eliyahu S (1996) Social confrontation and tumor metastasis in rats: defeat and beta-adrenergic mechanisms. Physiol Behav 60:277–282
Straub RH (2004) Complexity of the bi-directional neuroimmune junction in the spleen. Trends Pharmacol Sci 25:640–646
Straub RH, Härle P (2005) Sympathetic neurotransmitters in joint inflammation. Rheum Dis Clin North Am 31:43–59, viii
Straub RH, Günzler C, Miller LE, Cutolo M, Schölmerich J, Schill S (2002a) Anti-inflammatory cooperativity of corticosteroids and norepinephrine in rheumatoid arthritis synovial tissue in vivo and in vitro. FASEB J 16:993–1000
Straub RH, Kittner JM, Heijnen C, Schedlowski M, Schmidt RE, Jacobs R (2002b) Infusion of epinephrine decreases serum levels of cortisol and 17-hydroxyprogesterone in patients with rheumatoid arthritis. J Rheumatol 29:1659–1664
Straub RH, Dhabhar FS, Bijlsma JW, Cutolo M (2005) How psychological stress via hormones and nerve fibers may exacerbate rheumatoid arthritis. Arthritis Rheum 52:16–26
Straub RH, Wiest R, Strauch UG, Härle P, Schölmerich J (2006) The role of the sympathetic nervous system in intestinal inflammation. Gut 55:1640–1649
Swanson MA, Lee WT, Sanders VM (2001) IFN-gamma production by Th1 cells generated from naive CD4+ T cells exposed to norepinephrine. J Immunol 166:232–240
Szelenyi J, Selmeczy Z, Brozik A, Medgyesi D, Magocsi M (2006) Dual beta-adrenergic modulation in the immune system: stimulus-dependent effect of isoproterenol on MAPK activation and inflammatory mediator production in macrophages. Neurochem Int 49:94–103
Takahashi HK, Morichika T, Iwagaki H, Yoshino T, Tamura R, Saito S, Mori S, Akagi T, Tanaka N, Nishibori M (2003) Effect of beta 2-adrenergic receptor stimulation on interleukin-18-induced intercellular adhesion molecule-1 expression and cytokine production. J Pharmacol Exp Ther 304:634–642
Takahashi HK, Iwagaki H, Mori S, Yoshino T, Tanaka N, Nishibori M (2004) Beta 2-adrenergic receptor agonist induces IL-18 production without IL-12 production. J Neuroimmunol 151:137–147
Takamoto T, Hori Y, Koga Y, Toshima H, Hara A, Yokoyama MM (1991) Norepinephrine inhibits human natural killer cell activity in vitro. Int J Neurosci 58:127–131
Thorpe LW, Westlund KN, Kochersperger LM, Abell CW, Denney RM (1987) Immunocytochemical localization of monoamine oxidases A and B in human peripheral tissues and brain. J Histochem Cytochem 35:23–32
Tomozawa Y, Yabuuchi K, Inoue T, Satoh M (1995) Participation of cAMP and cAMP-dependent protein kinase in beta-adrenoceptor-mediated interleukin-1 beta mRNA induction in cultured microglia. Neurosci Res 22:399–409
Townend JN, Virk SJ, Qiang FX, Lawson N, Bain RJ, Davies MK (1993) Lymphocyte beta adrenoceptor upregulation and improved cardiac response to adrenergic stimulation following converting enzyme inhibition in congestive heart failure. Eur Heart J 14:243–250
Tsavaris N, Konstantopoulos K, Vaidakis S, Koumakis K, Pangalis G (1995) Cytochemical determination of monoamine oxidase activity in lymphocytes and neutrophils of schizophrenic patients. Haematologia (Budap) 26:143–146
Vago T, Norbiato G, Baldi G, Chebat E, Bertora P, Bevilacqua M (1990) Respiratory-burst stimulants desensitize beta-2 adrenoceptors on human polymorphonuclear leukocytes. Int J Tissue React 12:53–58
Wahle M, Krause A, Ulrichs T, Jonas D, von Wichert P, Burmester GR, Baerwald CG (1999) Disease activity related catecholamine response of lymphocytes from patients with rheumatoid arthritis. Ann NY Acad Sci 876:287–296
Wahle M, Kölker S, Krause A, Burmester GR, Baerwald CG (2001a) Impaired catecholaminergic signalling of B lymphocytes in patients with chronic rheumatic diseases. Ann Rheum Dis 60:505–510
Wahle M, Stachetzki U, Krause A, Pierer M, Häntzschel H, Baerwald CG (2001b) Regulation of beta2-adrenergic receptors on CD4 and CD8 positive lymphocytes by cytokines in vitro. Cytokine 16:205–209
Wahle M, Krause A, Pierer M, Hantzschel H, Baerwald CG (2002) Immunopathogenesis of rheumatic diseases in the context of neuroendocrine interactions. Ann NY Acad Sci 966:355–364
Wahle M, Hanefeld G, Brunn S, Straub RH, Wagner U, Krause A, Häntzschel H, Baerwald CG (2006) Failure of catecholamines to shift T-cell cytokine responses toward a Th2 profile in patients with rheumatoid arthritis. Arthr Res Ther 8:R138
Wang J, Li J, Sheng X, Zhao H, Cao XD, Wang YQ, Wu GC (2010) Beta-adrenoceptor mediated surgery-induced production of pro-inflammatory cytokines in rat microglia cells. J Neuroimmunol 223:77–83
Werner C, Werdan K, Pönicke K, Brodde OE (2001) Impaired beta-adrenergic control of immune function in patients with chronic heart failure: reversal by beta1-blocker treatment. Basic Res Cardiol 96:290–298
Werstiuk ES, Steiner M, Burns T (1990) Studies on leukocyte beta-adrenergic receptors in depression: a critical appraisal. Life Sci 47:85–105
Whalen MM, Bankhurst AD (1990) Effects of beta-adrenergic receptor activation, cholera toxin and forskolin on human natural killer cell function. Biochem J 272:327–331
Wrona D (2006) Neural-immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J Neuroimmunol 172:38–58
Wu JR, Chang HR, Huang TY, Chiang CH, Chen SS (1996) Reduction in lymphocyte beta-adrenergic receptor density in infants and children with heart failure secondary to congenital heart disease. Am J Cardiol 77:170–174
Xu BY, Pirskanen R, Lefvert AK (1998) Antibodies against beta1 and beta2 adrenergic receptors in myasthenia gravis. J Neuroimmunol 91:82–88
Xu B, Zhang WS, Yang JL, Lû N, Deng XM, Xu H, Zhang YQ (2010) Evidence for suppression of spinal glial activation by dexmedetomidine in a rat model of monoarthritis. Clin Exp Pharmacol Physiol 37:e158–e166
Yanagawa Y, Matsumoto M, Togashi H (2010) Enhanced dendritic cell antigen uptake via alpha2 adrenoceptor-mediated PI3K activation following brief exposure to noradrenaline. J Immunol 185:5762–5768
Yu BH, Dimsdale JE, Mills PJ (1999) Psychological states and lymphocyte beta-adrenergic receptor responsiveness. Neuropsychopharmacology 21:147–152
Zaffaroni M, Marino F, Bombelli R, Rasini E, Monti M, Ferrari M, Ghezzi A, Comi G, Lecchini S, Cosentino M (2008) Therapy with interferon-beta modulates endogenous catecholamines in lymphocytes of patients with multiple sclerosis. Exp Neurol 214:315–321
Zeinstra E, Wilczak N, De Keyser J (2000) [3H]dihydroalprenolol binding to beta adrenergic receptors in multiple sclerosis brain. Neurosci Lett 289:75–77
Ziegler MG, Bao X, Kennedy BP, Joyner A, Enns R (2002) Location, development, control, and function of extraadrenal phenylethanolamine N-methyltransferase. Ann NY Acad Sci 971:76–82
Zoukos Y, Leonard JP, Thomaides T, Thompson AJ, Cuzner ML (1992) Beta-Adrenergic receptor density and function of peripheral blood mononuclear cells are increased in multiple sclerosis: a regulatory role for cortisol and interleukin-1. Ann Neurol 31:657–662
Zoukos Y, Kidd D, Woodroofe MN, Kendall BE, Thompson AJ, Cuzner ML (1994) Increased expression of high affinity IL-2 receptors and β-adrenoceptors on peripheral blood mononuclear cells is associated with clinical and MRI activity in multiple sclerosis. Brain 117:307–315
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marino, F., Cosentino, M. Adrenergic modulation of immune cells: an update. Amino Acids 45, 55–71 (2013). https://doi.org/10.1007/s00726-011-1186-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-1186-6