Abstract
Phage display technology could provide a rapid means for the discovery of novel peptides. To find peptide ligands specific for the brain vascular receptors, we performed a modified phage display method. Phages were recovered from mice brain parenchyma after administrated with a random 7-mer peptide library intravenously. A longer circulation time was arranged according to the biodistributive brain/blood ratios of phage particles. Following sequential rounds of isolation, a number of phages were sequenced and a peptide sequence (CTSTSAPYC, denoted as PepC7) was identified. Clone 7-1, which encodes PepC7, exhibited translocation efficiency about 41-fold higher than the random library phage. Immunofluorescence analysis revealed that Clone 7-1 had a significant superiority on transport efficiency into the brain compared with native M13 phage. Clone 7-1 was inhibited from homing to the brain in a dose-dependent fashion when cyclic peptides of the same sequence were present in a competition assay. Interestingly, the linear peptide (ATSTSAPYA, Pep7) and a scrambled control peptide PepSC7 (CSPATSYTC) did not compete with the phage at the same tested concentration (0.2–200 pg). Labeled by Cy5.5, PepC7 exhibited significant brain-targeting capability in in vivo optical imaging analysis. The cyclic conformation of PepC7 formed by disulfide bond, and the correct structure itself play a critical role in maintaining the selectivity and affinity for the brain. In conclusion, PepC7 is a promising brain-target motif never been reported before and it could be applied to targeted drug delivery into the brain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Therapeutic strategies to deliver drugs into the CNS are limited by the restrictive tight junctions at the endothelial cells of the blood–brain barrier (BBB). BBB is formed by endothelial cells and tight junctions that form the walls of the capillaries (Abbott et al. 2010). The BBB is a homeostatic-defense-mechanism of the brain against pathogens and toxins (Praveen et al. 2004). Naturally, ideal drug candidates which are small, lipophilic, hydrophobic and compact are easy to cross the BBB (Roney et al. 2005; Abbott 2005). However, 98% of small-molecule drugs and 100% of large-molecule drugs, including peptides, recombinant proteins, monoclonal antibodies, genes and short interfering RNAs cannot cross the BBB.
To solve the problem of drug delivery across the BBB, quite a few CNS delivery strategies have been developed (Begley 2004; Liu and Chen 2005; Gabathuler 2010; Costantino et al. 2009), among which the most promising approach is the receptor-mediated transport (RMT) (Béduneau et al. 2007). Some receptors are highly expressed on the endothelial cells forming the BBB, such as the insulin receptor, transferrin receptor, low-density lipoprotein receptor (LDLR) and its related protein (LRP), and others (Pardridge 2005; Huwyler et al. 1996; Zhang and Pardridge 2005; Hu et al. 2009; Demeule et al. 2008), which can be called “vascular addresses”. Specific ligands, modified ligand and antibodies can target to these vascular addresses, thus facilitating therapeutic compounds to cross the BBB. Ulbrich et al. demonstrated that nanoparticles modified with transferrin or transferrin receptor monoclonal antibodies (OX26 or R17217) are able to transport drugs across the BBB (Ulbrich et al. 2009). Re et al. reported that nanoliposomes (NLs) functionalized by apolipoprotein E- derived peptides (residues 141–150) had the ability to enhance the transport of drug through a RBE4 brain capillary endothelial cell monolayer (Re et al. 2011). Angiopeps is a new family of peptides derived from proteins that efficiently cross the BBB using LRP. Angiopep-2 which is one of the Angiopeps family could efficiently transport therapeutic agents to the brain parenchyma. The brain uptake of Angiopep-2 conjugated with paclitaxel was 4.5-fold greater than that of free paclitaxel (Régina et al. 2008). Finding peptide ligand which can recognize such vascular addresses may help make drugs more selective, thus providing higher therapeutic efficiency while simultaneously decreasing systemic toxicity.
Phage display was established by Smith et al on the surface of filamentous M13-derived bacteriophage in 1985 (Smith 1985; Pande et al. 2010). M13 is a filamentous bacteriophage that consists of a single-stranded DNA core surrounded by a proteinaceous coat. M13 phage has been by far the most commonly used display system. Its biology has been reviewed before (Sidhu 2001; Kehoe and Kay 2005; Cabilly 1999). A gene of interest can be fused to a phage coat protein, resulting in phage particles that display the encoded protein on the surface and contain its gene. The principle underlying phage display technology is the ability to physically link phenotypes of polypeptides displayed on the coat protein to their corresponding genotype (Barbas et al. 2001). This allows phage libraries to be subjected to a selection step, and recovered clones to be identified by sequencing. Phage display has developed to be a technology used to screen peptides that bind to specific receptors (Lee et al. 2001; Murai et al. 2003), tumor cells (Lee et al. 2004), or tumor vessels (Arap et al. 1988; Joyce et al. 2003). Strategies for panning cells in vitro (Barry et al. 1996) or tissues in vivo (Pasqualini et al. 1997; Pasqualini et al. 2000) with phage libraries have been described to yield peptide ligands with organ- or tumor-binding specificity. There are examples using the in vivo screening method to search for tissue-homing peptides (Arap et al. 2002; Kolonin et al. 2006). Wan et al (2009) applied a C7C phage display library intranasally to rats and recovered phage from the brain tissue and finally gained a peptide sequence (ACTTPHAWLCG) that can bypass the BBB through the nasal-to-brain passage. Rooy et al (2010) selected two 15 amino acid peptides (GLA and GYR) that can bind to the murine brain in an in situ brain perfusion model.
Phage display library expressing peptides or proteins is suitable to be screened for peptide ligand that could recognize the target on the BBB. The aim of our study is to identify peptides that could traverse the BBB from the system circulation. Thus, we employed a cyclic 7-mer peptide library based on M13 bacteriophage for the ligand that target the “vascular addresses”. This M13 library, representing 1.2 × 109 unique genotypes encoding random 7-mer disulphide constrained peptides, genomically fused to the pIII coat protein of the filamentous phage M13. During the in vivo phage display selection process, library phages are intravenously administrated to the mice. The library phage are able to circulate for a certain period of time to allow distribution of the clones in the vascular system followed by perfusion and washing away the unbound phage, and recovering the specifically bound phage by host Escherichia coli infection from the brain. The recovered phage is then amplified by infecting E. coli and taken through additional binding/amplification cycles to enrich the pool in favor of binding sequences. Usually after three to four rounds of selection, phage clone with high affinity to the brain could be obtained. Screening phage-displayed peptide libraries in vivo would, therefore, be a direct and rapid method of identifying novel peptide sequences for drug-targeted delivery.
Materials and methods
Materials
The Ph.D.-C7C™ Phage Display Peptide Library Kit, constructed based on M13 bacteriophage, was purchased from New England Biolabs (Beverly, MA, USA). The kit also includes E. coli ER2738 (a robust F+ strain) which was used for M13 phage propagation. The displayed peptides were fused at the N terminus of the minor coat protein pIII. The complexity of the library is ~1.2 × 109 transformants. Mouse anti-M13 monoclonal antibody was obtained from GE Healthcare (Piscataway, NJ, USA); Cy3 labeled goat anti-mouse IgG and 5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside (Xgal) were purchased from Sigma–Aldrich (St. Louis, MO, USA); 4,6-diamidino-2-phenylindole (DAPI) was purchased from Molecular Probes (Eugene, OR, USA); Isopropyl-beta-d-thiogalactoside (IPTG) was purchased from Merck (Germany); Optimal Cutting Temperature-compound ‘Tissue-Tek’ (O.C.T. compound) was from Miles Laboratories Inc. (USA). Deionized water (Millipore, Bedford, MA) was used throughout the study.
Screening of phage libraries in vivo
Adult male ICR mice (18–22 g) were obtained from the Sino-British Sippr/BK Lab. Animals were maintained at 22 ± 2°C on a 12 h light–dark cycle with access to food and water ad libitum. The animal experiments were carried out in accordance with the protocols evaluated and approved by the Ethical Committee of Fudan University.
To obtain brain-homing phage-displayed peptides, the appropriate time to recover phages from the brain is determined according to the brain/blood ratio. The brain/blood ratio was calculated by dividing the phage titer (in TU/g tissue, TU is transducing unit) in the brain at a given time by the phage titer in blood (in TU/ml) at that time (Zou et al. 2004). Mice were injected in the tail vein with 1011 plaque forming units (pfu) of Ph.D.-C7C™ Phage Display Library in 100 μl TBS. The phages were allowed to circulate in the mice for 0.25, 0.5, 1, 2, 4, 8, 12, 16, 20, 24, 30 and 36 h. The mice were then killed by cervical dislocation. Blood was collected and the brain was withdrawn and weighed.
A total of four rounds of screening were performed with adult male ICR mice (18–22 g). For the first round, ICR mice (n = 3) were injected intravenously (i.v.) with 1011 pfu of Ph.D.-C7C™ Phage Display Library in 100 μl TBS (50 mM Tris–HCl, 150 mM NaCl, pH 7.5). Phages were allowed to circulate for a period of time in vivo. Then mice were anesthetized using 5% chloral hydrate (0.4 g/kg) and perfused through the heart with 500 ml of sterile normal saline (containing 1% heparin). The cerebrum was withdrawn, weighed and homogenized under a bacteria-free environment. The brain capillary was depleted on sterile filter paper. The homogenate was mixed together with rapid-growth E. coli (ER2738 host strain) for amplification. The following rounds of biopanning were conducted by intravenously injecting with newly amplified phage (1 × 1011 pfu in 100 μl TBS). Other procedures were repeated as described above.
Titering of phage
The phage titer was determined after each round of biopanning and amplification. Phages were diluted in TBS serially. 10 μl of phage dilution was incubated with 200 μl of late log phase E. coli ER 2738 bacteria for 10 min at room temperature to allow infection. Then the infected bacteria were spread on LB agar plate containing IPTG and Xgal. Plates were incubated overnight in an incubator (37°C). The number of transducing units was calculated by counting the blue plaques on the plates after 24 h of incubation.
where a is the number of blue plaques on the LB plates after incubating overnight and b is the times of dilution.
DNA extraction and sequencing
After isolation of selected clones, 12 bacteriophage clones from the last round were randomly picked up and subjected to DNA sequencing (ABI3730). The peptide-encoding nucleotide sequences were determined with -96 gIII primer (5′-HOCCC TCA TAG TTA GCG TAA CG-3′) included in Ph.D.-C7C Phage Display Library. The phage-displayed peptide sequences were translated and aligned with the Laseregene program (v7.1).
Immunohistochemistry
Selected phage clones and native M13 phage were given to two groups of ICR mice (n = 3) via tail vein, respectively (1011pfu in 100 μl TBS). One hour after administration, mice were anesthetized using 5% chloral hydrate (0.4 g/kg) and perfused through the heart with 100 ml normal sodium followed by 50 ml of 4% paraformaldehyde. Cerebrum samples were fixed by 4% paraformaldehyde overnight and immersed in 15% sucrose at 4°C for 24 h and 30% sucrose at 4°C till deposition. Samples were frozen quickly in O.C.T. compound. Frozen samples were subject to immunohistochemistry, performed on 10 μm freezing sections blocked with goat serum for 1 h and incubated with mouse anti-M13 monoclonal antibody (1:100) at 4°C overnight followed by a secondary antibody (Cy3-labeled goat anti-mouse IgG, 1 h, 37°C). Finally, distribution of phages in cerebrum was observed by fluorescence microscope (OLYMPUS IX-70 microscope).
Peptide synthesis
PepC7 (CTSTSAPYC), Pep7 (ATSTSAPYA), scrambled peptide PepSC7 (CSPATSYTC), Cy5.5 labeled peptides and other peptides were synthesized by Shanghai Sangon Biological Engineering Technology & Services Co. Ltd, using standard solid-phase FMOC method and purified to >95% by high-performance liquid chromatography (HPLC). All peptides were verified using a mass spectrometer (lcms-2010a, Shimadzu, Japan).
Peptide competition
The peptide-competitive inhibition assay was performed to determine whether the synthetic peptide and phage clone competed for the same binding site. Competitive inhibitions of brain homing phage clone were detected by the addition of increasing concentrations of synthetic PepC7, Pep7 and PepSC7 (0–200 pg). ICR mice (n = 3) were injected with 1011 pfu of phage Clone 7-1 intravenously (i.v.) in 100 μl TBS (50 mM Tris–HCl, 150 mM NaCl, pH 7.5). Phages were allowed to circulate for 1 h in vivo followed by brain harvest. The inhibitory effect of the added peptide was quantified by evaluating phage titers recovered from mice brain. The detailed method of phage titering is in “Titering of phage”.
In vivo imaging analysis
Nude mice were injected with 1 μM (in 200 μl PBS) Cy5.5 labeled PepC7 (Cy5.5-PepC7) via tail vein . Nude mice intravenously administrated with PBS, Cy5.5 labeled Pep7 (Cy5.5-Pep7) and a scrambled cyclic peptide (CSPATSYTC) which was also labeled by Cy5.5 (denoted as Cy5.5-PepSC7) were set as control. 30 min and 2 h after injection, Images were taken by the Maestro in vivo imaging system (Cambridge Research & Instrumentation, MA; excitation = 675 nm, emission = 695 nm). During the injection and image acquiring process, the mice were anesthetized with 5% chloral hydrate.
Statistical analysis
The statistical analysis of the samples was undertaken using a Student’s t test when one group was compared with the control group. p < 0.05 were considered statistically significant. All statistical analyses were performed using Stata version 8.0. All data reported are means ± standard deviations, unless otherwise noted.
Results and discussion
In vivo phage display
Since the in vivo phage display was first introduced by Pasqualini in 1996 (Pasqualini and Ruoslahti 1996), this technique has been expanded and has been proven to be very effective in selecting phages with high organ specificity upon systemic injection (Smith and Petrenko 1997). Therefore, we decided to select in vivo for brain targeting peptides. The brain capillary endothelial cells are specialized in homing of blood cells and can serve as addresses for the in vivo selection.
Previous researches on phage display biopanning for brain homing peptide have been performed during a very short circulation time in vivo, usually 5–15 min being allowed before phages recovery from brain tissues (Pasqualini and Ruoslahti 1996; Kolonin et al. 2006). However intravenous administration of phages usually can lead to high backgrounds of phages in the circulation thus affecting the screening results. Phages recovered from the brain could be easily “polluted” by phages from the circulation especially the first a few hours after intravenous injection.
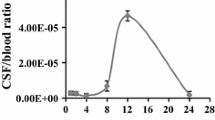
Zou (2004) studied the biodistribution of filamentous phage peptide libraries in mice. He reported that the accumulation of intact infectious phage particles in tissues (calculated by dividing the titer (in TU/g tissue) in a given organ at a given time by the titer in blood (in TU/ml) at that time differed. We found that the brain/blood ratios of phage particles from the library we employed in this article peaked at 24 h (Fig. 1). Thus, we chose 24 h as the optimal time point to recover phages from the brain after tail-vein administration.
We conducted four rounds of consecutive biopanning. The efficiency of phage to reach the brain increased after each round of panning and the amount of recovered phage increased about 13 times compared to the first round (Fig. 2). A total of 12 phage clones were chosen randomly from the final round of biopanning and subjected to DNA sequencing. The sequences of the displayed peptides are shown in Table 1.
A multiple sequence-alignment analysis using Lasergene (version 7.1.0) program revealed that 42% of the peptides shared a consensus sequence. Consensus amino acid motifs appearing with high frequencies were commonly observed in phage display experiments and considered as an important evidence of the phage display selection with the progressive enrichment in phage titers along with the succeeding rounds of biopanning. The consensus motifs among phages selected via biopanning on living cells or upon injection in vivo, often appear to be three or four amino acid sequences (Rajotte et al. 1998; Nicklin et al. 2000). We named this sequence PepC7 for short and the phage clone encoding PepC7 was named as Clone 7-1. The sequence of PepC7 was analyzed by BLAST (http:#www.ncbi.nlm.nih.gov) search database. There were no putative conserved domains detected.
In vivo specificity of Clone 7-1 phage
To further evaluate the targeting potential of PepC7, PepC7-encoding phages were systemically injected into ICR mice through tail vein. M13 library phages were set as the control. 1011 pfu of each of the two phages was injected. Phages were then recovered from the brain. As shown in Fig. 3, Clone 7-1 recovered from mice brain gave an enrichment of approximately 41 fold when compared with library phage recovered from the mice brain.
High efficiency of Clone 7-1 phage homing to the brain. Library phage, phages from the fourth round and phage Clone 7-1 were injected intravenously. into three groups of ICR mice (1011pfu in 100 μl TBS) respectively. Random phage clones from the peptide library was set as control (mean ± SD, n = 3, ***p < 0.005)
Immunohistochemistry
Fluorescence microscopy examination revealed that the phage Clone 7-1 encoding PepC7 showed a significant superiority on transport efficiency into the brain compared with native M13 phage (Fig. 4). Especially, they distributed extensively in the cortex and the third ventricle (Fig. 4c and f, respectively). In contrast, there were very weak signal in those regions mentioned above for native M13 phages under the same background level (Fig. 4i and l, respectively). The PepC7 played a critical role in the transport into the brain. From a morphological point of view, filamentous phages such as M13 are approximately 6.5 nm in diameter and 930 nm in length, which are comparable with nanoparticles (Webster et al. 2001). Therefore, the identified PepC7 could be used as a leading ligand to be modified on the drug delivery system such as nanoparticles or liposome and could facilitate efficient transport of drugs into the brain.
Peptide competition assay
The efficiency of Clone 7-1 homing to brain was detected with the addition of synthetic PepC7, Pep7 and PepSC7. This assay was performed to determine whether the synthetic peptides and Clone 7-1 competed for the same binding site. The Clone 7-1 phage homing to the brain was competitively inhibited by PepC7 in a dose-dependent manner while there was not significant competitive effect of Pep7 or PepSC7 (Fig. 5). For Clone 7-1, the presence of PepC7 in the concentration from 0.2 pg to 200 pg resulted in an inhibition of the phage homing from 10% to 84%. The presence of Pep7 or PepSC7 in the concentration from 0.2 pg to 200 pg resulted in an inhibition of the phage Clone 7-1 homing from 9% to 18% and 4% to 10%, respectively. The competitive-inhibition effect might be due to the binding sites on the BBB which Clone 7-1 interacted with were saturated by the free PepC7. Less binding sites remained for Clone 7-1 will lead to decrease of Clone 7-1 transport into the brain.
Compared with PepC7, PepSC7 showed rarely competitive efficiency which is due to the incorrect structure of the peptides. It illustrated that the correct structure of PepC7 is responsible for the competitive inhibition capacity. With the comparison between PepC7 and Pep7, the importance of the disulfide bond in the sequences is indicated by the fact that the competitive activity of linear peptide Pep7 was greatly diminished after reduction of the disulfide bond formed by the cysteines. Peptides containing two cysteines, when fused to the NH2-terminus of phage protein III are capable of forming the expected disulfide bridge and that the presence of such a bond can greatly enhance the brain-homing activity of the peptides. Conformationally constrained peptides are known to have an improved affinity for targets like integrins (Ruoslahti and Pierschbacher 1987; Cardarelli et al. 1992). Koivunen et al. (1994) found that the RGD and NGR sequences had higher activity when presented in cyclic peptides. The peptide displayed in a cyclic peptide library can meet the conformational requirement of the receptors, thus providing better chance for the identification of a specific ligand.
In vivo Optical images
Optical in vivo images were taken 30 min and 2 h after injection. An obvious accumulation of Cy5.5 signal was detected in the brain of nude mice administered with Cy5.5-PepC7 (Fig. 6aII) compared with that in those treated with Cy5.5-Pep7 or Cy5.5-PepSC7 (Fig. 6aIII, IV). 2 h after administration, main organs of nude mice were harvested. Cy5.5-PepC7 showed significantly higher brain accumulation efficiency than Cy5.5-PepSC7. It indicated that the scrambled control sequence had little affinity to BBB. It is the correct structure of PepC7 that is responsible for the brain targeting ability. Compared with nude mice which was injected with Cy5.5-Pep7, higher brain accumulation was observed in that injected intravenously with Cy5.5-PepC7 while there was lower accumulation in liver (Fig. 6b). The optical images demonstrated again that the conformation structure formed by the disulfide bridge played an important role in brain-homing activity. It is notable that all of the peptides had certain distribution in the kidney which might be due to the soluble character of the peptides. It is promising that when conjugated with long-circulating drug delivery systems PepC7 could mediate efficient brain-homing drug delivery while avoiding non-specific capture by liver and kidney.
In vivo distribution of PepC7. a Optical in vivo imaging of nude mice vein administrated II Cy5.5-PepC7; III Cy5.5-Pep7; IV Cy5.5-PepSC7 respectively. I nude mouse administrated with PBS was set as blank control. Images were taken 30 min and 2 h after injection. b Ex vivo imaging of Cy5.5 labeled peptides in main organs. Nude mice were tail intravenously injected with II Cy5.5-PepC7; III Cy5.5-Pep7; IV Cy5.5-PepSC7, respectively. I nude mouse administrated with PBS was set as blank control. Organs were harvested 2 h after administration
By optical imaging techniques, we have demonstrated the brain-specific targeting capability of PC7. Despite the encouraging results, some questions are still open, such as which receptor or molecules PepC7 interact with on vascular endothelial cells, and how the PepC7 overcome the BBB. Further studies are underway to answer these questions. Identification of those receptors/molecules will be a great help in understanding the mechanism of PepC7 and will accelerate the development of applications for PepC7 in targeted drug delivery to the brain.
Conclusions
In conclusion, we have identified a novel peptide sequence of CTSTSAPYC associated with high affinity of brain. Phage Clone 7-1 displaying PepC7 revealed a significant superiority on brain transport efficiency compared with native M13 phage. The cyclic conformation of PepC7 formed by disulfide bond and the correct structure itself play the critical role in maintaining the selectivity and affinity for the brain. To the best of our knowledge, PepC7 is a brain-target motif never been reported before and could be applied to the design of drug delivery system homing to the brain. Further studies are right under way to identify the molecules or sites to which the PepC7 interact with on the BBB.
References
Abbott NJ (2005) Physiology of the blood-brain barrier and its consequences for drug transport to the brain. Int Congr 1277:3–18
Abbott NJ, Patabendige AAK, Dolman DEM et al (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25
Arap W, Pasqualini R, Ruoslahti E (1988) Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 279:377–380
Arap W, Haedicke W, Bernasconi M et al (2002) Targeting the prostate for destruction through a vascular address. Proc Natl Acad Sci 99:1527–1531
Barbas CF, Burton DR, Scott JK, Silverman GJ (2001) Phage Display: A laboratory manual. Cold Spring Harbor Laboratory Press, New York, pp 1.1–1.37
Barry MA, Dower WJ, Johnston SA (1996) Toward cell-targeting gene therapy vectors: selection of cell-binding peptides from random peptide-presenting phage libraries. Nat Med 2:299–305
Béduneau A, Saulnier P, Benoit JP (2007) Active targeting of brain tumors using nanocarriers. Biomaterials 28:4947–4967
Begley DJ (2004) Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther 104:29–45
Cabilly S (1999) The basic structure of filamentous phage and its use in the display of combinatorial peptide libraries. Mol Biotechnol 12:143–148
Cardarelli PM, Yamagata S, Taguchi I et al (1992) The collagen receptor-alpha-2-beta-1, from MG-63 and HT1080-cells, interacts with a cyclic RGD peptide. J Biol Chem 267:23159–23164
Costantino L, Tosi G, Ruozi B et al (2009) Colloidal systems for CNS drug delivery. Prog Brain Res 180:35–69
Demeule M, Regina A, Che C et al (2008) Identification and design of peptides as a new drug delivery system for the brain. Pharmacol Exp Ther 324:1064–1072
Gabathuler R (2010) Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol Dis 37:48–57
Hu KL, Li JW, Shen YH et al (2009) Lactoferrin-conjugated PEG–PLA nanoparticles with improved brain delivery: In vitro and in vivo evaluations. J Control Rel 134:55–61
Huwyler J, Wu D, Pardridge WM (1996) Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci 93:14164–14169
Joyce JA, Laakkonen P, Bernasconi M et al (2003) Stage-specific vascular markers revealed by phage display in a mouse model of pancreatic islet tumorigenesis. Cancer Cell 4:393–401
Kehoe JW, Kay BK (2005) Filamentous phage display in the new millennium. Chem Rev 105:4056–4072
Koivunen E, Wang BC, Ruoslahti E (1994) Isolation of a highly specific ligand for the α 5 β 1 integrin from a phage display library. J Cell Biol 124:373–380
Kolonin MG, Sun J, Do KA et al (2006) Synchronous selection of homing peptides for multiple tissues by in vivo phage display. FASEB J 20:E99–E107
Lee JH, Engler JA, Collawn JF, Moore BA (2001) Receptor mediated uptake of peptides that bind the human transferrin receptor. Eur J Biochem 268:2004–2012
Lee TY, Wu HC, Tseng YL, Lin CT (2004) A novel peptide specifically binding to nasopharyngeal carcinoma for targeted drug delivery. Cancer Res 64:8002–8008
Liu X, Chen C (2005) Strategies to optimize brain penetration in drug discovery. Curr Opin Drug Disc 8:505–512
Murai KK, Nguyen LN, Koolpe M et al (2003) Targeting the EphA4 receptor in the nervous system with biologically active peptides. Mol Cell Neurosci 24:1000–1011
Nicklin SA, White SJ, Watkins SJ et al (2000) Selective targeting of gene transfer to vascular endothelial cells by use of peptides isolated by phage display. Circulation 102:231–237
Pande J, Szewczyk MM, Grover AK (2010) Phage display: concept, innovations and future. Biotechnol Adv 28:849–858
Pardridge WM (2005) Molecular biology of the blood-brain barrier. Mol Biotechnol 30:57–70
Pasqualini R, Ruoslahti E (1996) Organ targeting in vivo using phage display peptide libraries. Nature 380:364–366
Pasqualini R, Koivunen E, Ruoslahti E (1997) Integrins as receptors for tumor targeting by circulating ligands. Nat Biotechnol 15:542–546
Pasqualini R, Koivunen E, Kain R et al (2000) Aminopeptidease N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res 60:722–727
Praveen B, Alex B, Maiken N (2004) The blood-brain barrier: an overview: structure, regulation and clinical implications. Neurobiol Dis 16:1–13
Rajotte D, Arap W, Hagedorn M et al (1998) Molecular heterogeneity of the endothelium revealed by in vivo phage display. J Clin Invest 102:430–437
Re F, Cambianica I, Zona C et al (2011) Functionalization of liposomes with ApoE-derived peptides at different density affects cellular uptake and drug transport across a blood-brain barrier model. Nanomed Nanotech biol Med (in Press)
Régina A, Demeule M, Ché C et al (2008) Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Brit J Pharmacol 155:185–197
Roney C, Kulkarni P, Arora V et al (2005) Targeted nanoparticles for drug delivery through the blood-brain barrier for Alzheimer’s disease. J Control Rel 108:193–214
Rooy IV, Tascioglu SC, Couraud PO et al (2010) Identification of peptide ligands for targeting to the blood-brain barrier. Pharm Res 27:673–682
Ruoslahti E, Pierschbacher MD (1987) New perspectives in cell-adhesion-RGD and integrins. Science 238:491–497
Sidhu SS (2001) Engineering M13 for phage display. Biomol Eng 18:57–63
Smith GP (1985) Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 228:1315–1317
Smith GP, Petrenko VA (1997) Phage Display. Chem Rev 97:391–410
Ulbrich K, Hekmatara T, Herbert E, Kreutre J (2009) Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB). Eur J Parm Biopharm 71:251–256
Wan XM, Chen YP, Xu WR et al (2009) Identification of nose-to-brain homing peptide through phage display. Peptides 30:343–350
Zhang Y, Pardridge WM (2005) Delivery of β-galactosidase to mouse brain via the blood–brain barrier transferrin receptor. J Pharmaco Exp Ther 313:1075–1108
Zou J, Dickerson MT, Owen NK et al (2004) Biodistribution of filamentous phage peptide libraries in mice. Mol Biol Rep 31:121–129
Acknowledgments
This work was supported in part by grants from the National Basic Research Program of China (973 Program) (2007CB935800), National Science and Technology Major Project 2009ZX09310-006 and Doctorial Innovation Fund of Fudan University. We thank Prof. L.P. Wen and Dr. X.M. Wang (University of Science and Technology of China, School of Life Sciences) for technological support in phage display screening.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Zhang, Q., Pang, Z. et al. Identification of peptide sequences that target to the brain using in vivo phage display. Amino Acids 42, 2373–2381 (2012). https://doi.org/10.1007/s00726-011-0979-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-0979-y