Abstract
Protein phosphorylation is a common signalling mechanism in both prokaryotic and eukaryotic organisms. Whilst the focus of protein phosphorylation research has primarily been on protein serine/threonine or tyrosine phosphorylation, there are other phosphoamino acids that are also biologically important. Two of the phosphoamino acids that are functionally involved in the biochemistry of protein phosphorylation and signalling pathways are phosphoaspartate and phosphoglutamate, and this review focuses on their chemistry and biochemistry. In particular, we cover the biological aspects of phosphoaspartate and phosphoglutamate in signalling pathways and as phosphoenzyme intermediates. In addition, we examine the synthesis of both of these phosphoamino acids and the chemistry of the acyl phosphate group. Although phosphoaspartate is a major component of prokaryotic two-component signalling pathways, this review casts its net wider to include reports of phosphoaspartate in eukaryotic cells. Reports of phosphoglutamate, although limited, appear to be more common as free phosphoglutamate than those found in phosphoprotein form.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphoamino acids, when referred to in the context of phosphoproteins in biochemical textbooks, are usually only mentioned in terms of the phosphoesters, phosphoserine (1) threonine (2) and tyrosine (3) (Fig. 1). This is certainly true with respect to eukaryotic biochemistry. However, there are many other less common but equally important phosphoamino acids, but the nature of their phosphoryl linkage makes them difficult to study. One example of these other phosphoamino acids is the phosphoramidates (containing P–N bonds), phosphohistidine (4, 5), phosphoarginine (6) and phospholysine (7) (Fig. 1). The N-linked phosphoryl group of phosphoramidate phosphoamino acids makes them labile under acidic conditions, a subject that has been extensively reviewed elsewhere (Attwood et al. 2007; Besant et al. 2009).
What this review focuses on, to some, might be termed the ‘lesser’ known phosphoamino acids, these being the phosphoanhydrides or acyl phosphates, phosphoaspartate (β-aspartyl phosphate) (8) and phosphoglutamate (γ-glutamyl phosphate) (9) (Fig. 2).
One of the best-known occurrences of nature’s use of phosphoanhydrides of amino acids as reactive intermediates is in the biosynthesis of aminoacyl-tRNAs. Here, the carboxyl group of an amino acid attacks the α-phosphorous of ATP to form the aminoacyl-adenylate containing the mixed anhydride. This primes the carboxyl carbon for attack by the ribosyl hydroxyl oxygen of tRNA. Here, however, we want to concentrate on the phosphorylation of the side chain carboxyl groups of aspartate and glutamate, although the formation and chemistry of the mixed anhydride is central to the functional roles of these phosphoamino acids.
The phosphoanhydride groups of phosphoaspartate and phosphoglutamate are unstable under neutral, acidic and alkaline conditions, as described in more detail below (“Chemistry of mixed anhydrides”). This instability has meant that studying the biochemistry of protein aspartate/glutamate phosphorylation and the associated protein kinases has been challenging. This review covers the chemistry of phosphoaspartate and phosphoglutamate, how they can be synthesised, purified, identified and their kinetic and thermodynamic stabilities. We examine how phosphoaspartate and phosphoglutamate have been identified as enzymatic intermediates in numerous biochemical reactions and how both play a functional role in biosynthetic pathways. Furthermore, we highlight how this chemistry relates to the roles of phosphoaspartate and phosphoglutamate in other biological functions.
Chemistry of mixed anhydrides
Phosphoaspartate and phosphoglutamate are mixed carboxylic–phosphoric anhydrides, which can be thought of conceptually as being derived from dehydrative combination of hydrogen phosphate and the side chain carboxyl group of the amino acid. This modification kinetically and thermodynamically activates both the carboxyl and phosphoryl groups towards nucleophilic substitution, and nature takes advantage of this mode of activation to achieve step-wise chemical transformations that are impossible to achieve from a direct reaction of the carboxylate (or phosphate) with nucleophiles. There are very close analogies in the synthetic chemistry lab, where acyl and phosphoryl chlorides, anhydrides and related activated species are used to make carboxylic and phosphoric acid derivatives (such as amides/phosphoramides and carboxylic/phosphoric esters), reactions that can normally only otherwise be achieved at very high temperatures or low pH.
Kinetic activation and stability
With respect to the carboxyl group of acyl phosphates, kinetic activation towards nucleophilic substitution is imparted in several ways. At physiological pH, carboxyl groups are primarily ionised and the negative charge on the carboxylate oxygen increases the electron density on the carbonyl carbon and repels the approach of electron-rich nucleophiles. In contrast, in a mixed carboxylic–phosphoric anhydride 10 (Scheme 1), the carboxyl oxygens have no formal charge. Indeed, the phosphoryl group, perhaps best represented in structure 10b (Denehy et al. 2007), is electron withdrawing, and inductively pulls electron density away from the carbonyl carbon. This also makes delocalisation of the lone pair of electrons on the bridging oxygen onto the carbonyl carbon less effective. Both of these effects increase the electrophilicity of the carbonyl carbon, enhancing the rate of the first step in the substitution mechanism—nucleophilic attack on the sp2-hybridised carbon. The second step of the mechanism, elimination of the leaving group from the tetrahedral intermediate 11, is also accelerated as the phosphate group is better leaving group than hydroxide, because the phosphoryl group is better (than a proton) at stabilising the developing negative charge on the bridging oxygen in the transition state 12.
The mechanism of acyl substitution of acyl phosphates. Although phosphoryl groups are usually represented as an octet-violating structure having a π-like double bond, the π-bonding is weak at best and an octet-conforming structure with a formal positive charge on phosphorus is probably a more accurate depiction (Denehy et al. 2007). Nu nucleophile
Similar arguments can be posited for the kinetic activation of the phosphoryl group, although in this case the mechanism of substitution is quite different, and involves a single, concerted, SN2-like step (Jencks 1992) (Scheme 2); the electron-withdrawing acyl group increases the electrophilicity of the phosphorus atom in 10a and lowers the energy of the transition-state 13 by stabilising the incipient negative charge on the carboxylate oxygen in the leaving group.
Clearly, activation of the carboxyl/phosphoryl groups in acyl phosphates also reduces their stability towards hydrolysis and nucleophilic attack by other adventitious nucleophiles. At neutral pH, the free phosphoamino acids phosphoaspartate and phosphoglutamate hydrolyse readily, polymerise, and react with organic bases such as hydroxylamine, which can also catalyse polymerisation (Katchalsky and Paecht 1954). At 30°C in the pH range 4–10, phosphoaspartate is approximately 30% hydrolysed after 30 min (Black and Wright 1953), but is reported to be “relatively stable” for several hours under similar conditions at 15°C. Presumably, hydrolytic stability could be enhanced by the immediate chemical environment of phosphoaspartate/glutamate residues in proteins (Wolfenden and Liang 1989).
Importantly, in the prototype acetyl phosphate (10a, R = Me, Schemes 1, 2), it has been demonstrated that hydrolysis occurs predominantly by cleavage of the C–O bond at high or low pH (Koshland 1952; Phillips and Fife 1968), but by P–O cleavage at near-neutral pH (Koshland 1952). At high pH, attack of hydroxide at the phosphorus atom is no doubt repelled by the negatively charged phosphoryl group (Westheimer 1987). Thus, enzyme-catalysed reactions involving phosphoaspartate/glutamate as substrates could take advantage of acid or base catalysis to effect acyl substitution rather than the phosphoryl transfer that occurs when the corresponding phosphorylated amino acid residues in proteins are intermediates. However, in both cases, it is more likely that substrate alignment and/or steric hindrance (Koshland 1952) are the key factors in directing the attack of nucleophiles specifically to one of the electrophilic sites.
The rate of hydrolysis of acetyl phosphate is fairly constant over the pH range 5–10, but accelerates rapidly at higher pH (Fig. 3) (Koshland 1952). The profile in the pH region 1–5 resembles a titration curve with significant increases in reaction rate between pH 5 and 4, and a slower rate acceleration over the pH range 4–1, corresponding to protonations to give the monoanion and neutral species, respectively. Below pH 1, the rate of hydrolysis increases dramatically, and is proportional to hydrogen ion concentration (Koshland 1952). Phosphoaspartate and phosphoglutamate are likely to display a similar pH-dependent hydrolysis rate profile.
The effect of pH on rate of hydrolysis of acetyl phosphate (L mol−1 min−1) (reprinted with permission from Koshland 1952). Copyright 1952, American Chemical Society
Magnesium ion also dramatically accelerates the hydrolysis of acetyl phosphate at near-neutral pH where the dianion predominates, but not at lower pHs where the monoanion or neutral species are more abundant (Koshland 1952). Other studies on the effects of various metal ions and other cations have been reported (Kurz and Gutsche 1960; Oestreich and Jones 1966; Briggs et al. 1970), including isotope labelling experiments that show that both P–O and C–O bond cleavage occurs, but P–O bond cleavage predominates with Mg2+, whereas C–O bond cleavage is the major pathway in the presence of Ca2+ (Klinman and Samuel 1971). Bivalent metal ions, Mn2+ and/or Mg2+ in particular, are essential to the role of many enzymes involving acyl phosphates as substrates, products or intermediates.
Thermodynamic activation and stability
Acyl phosphates are high-energy biochemical species used as intermediates to drive thermodynamically unfavourable reactions. Thermodynamic stability and, therefore, relative phosphoryl transfer capacity is most commonly compared with other energy-rich species via the free energy of hydrolysis. The free energy of hydrolysis of phosphoaspartate and phosphoglutamate appears not to have been determined, but the calorimetrically measured heats of hydrolysis were reported to be of the order of 20 kcal mol−1 (Katchalsky and Paecht 1954), although the authors noted difficulties in determining these values accurately. Indeed, the heat of hydrolysis of acetyl phosphate at pH 5.6 and 33°C is only 7.2 kcal mol−1 (Meyerhof and Shatas 1952), so ~20 k cal mol−1 does seem to be a significant overestimate.
Most thermodynamic studies have been undertaken on acetyl phosphate, which serves as a prototype for all acyl phosphates, including phosphoaspartate and phosphoglutamate. The standard free energy of hydrolysis of acetyl phosphate is approximately −10 kcal mol−1 (Carpenter 1960; Wurmser 1967). Although it is understood that the free energies of hydrolysis of phosphorylated compounds under standard conditions are different from those in biological systems (Ruben et al. 2008), this value does indicate that acetyl phosphate and (therefore) other acyl phosphates have a substantially greater thermodynamic propensity to transfer their phosphoryl group than does ATP [\( \Updelta G^{\circ\prime}_{\text{hydrolysis}} \approx - 7.3\,{\text{kcal}}\,{\text{mol}}^{ - 1} \) (Ruben et al. 2008); \( \Updelta G^{\circ\prime}_{\text{hydrolysis}} \) (MgATP) is even lower ≈6–7Footnote 1 kcal mol−1 (Guynn and Veech 1973)].
The origin of the large negative free energy of hydrolysis and phosphoryl transfer capacity of acyl phosphates and related energy-rich compounds has been the subject of much interest and conjecture (Hayes et al. 1978). With respect to the high free energy of hydrolysis of acyl phosphates, two factors appear to be most important: decreased electron delocalisation (resonance) and solvation, relative to their hydrolysis products.
A once-popular explanation for the large negative free energy of hydrolysis of acyl phosphates was opposing resonance. This would predicate that the lone pair of electrons of the bridging oxygen in 10a is delocalised onto both carbonyl (10c) and phosphoryl oxygens (10d) (Scheme 3). As both groups ‘compete’ for the lone pair of electrons, delocalisation in either direction is less effective and, as delocalisation of electrons is usually a stabilising phenomenon, the molecule is higher in energy relative to its hydrolysed counterparts in which there is no competition.
Given that it has been demonstrated that the phosphoryl group has little π-character (Denehy et al. 2007), and therefore limited ability to partake in resonance, perhaps a better explanation is illustrated in Scheme 4. Assuming octet-rule conformity, when acetyl phosphate is represented by its more accurate Lewis structure 10b, delocalisation of the bridging oxygen’s lone pair of electrons onto the phosphoryl group is not possible. However, the positively charged phosphorus atom would draw electron density away from the bridging oxygen inductively (through the σ-bond), lowering the energy of the lone pair of electrons and making delocalisation onto the carbonyl oxygen (10e) less effective. This rationalisation is supported by ab initio calculations (Uchimaru et al. 2003) on the free energy of hydrolysis of trifluoromethyl acetate 14 (Scheme 4). The trifluoromethyl group can only withdraw electron density from the bridging ester oxygen inductively and yet the free energy of hydrolysis of trifluoromethyl acetate (~−7 kcal mol−1) is predicted to be significantly more negative than that of methyl acetate (~+4 kcal mol−1) (Uchimaru et al. 2003).
Whichever model one chooses to accept, calculations have suggested that reduction of delocalisation of the bridging oxygen’s lone pair of electrons is at least partly responsible for the large negative free energy of hydrolysis of acyl phosphates (Hayes et al. 1978).
Most recently, it has been proposed that an anomeric effect—a bonding interaction of the phosphoryl oxygen lone pair electrons with the σ* orbital of the P–O bond—is primarily responsible for the destabilisation of the scissile P–O bond and that, as the strength of this interaction correlates with free energy of hydrolysis, there is likely to be a causal connection (Ruben et al. 2008). This seems difficult to reconcile, however, as the same authors state that the anomeric effect lowers the ground state energy of acetyl phosphate, so even if it does destabilise the P–O bond, it stabilises the molecule, and cannot increase (i.e. make more negative) the free energy of hydrolysis.
As with ATP and the phosphagens, enhanced solvation of the products of hydrolysis relative to the starting materials is a major (if not the most important) factor in determining the large negative free energy of hydrolysis of acyl phosphates (Hayes et al. 1978; Wolfenden and Liang 1989). What seems to have been largely overlooked is the energy released from the neutralisation of the acid produced upon hydrolysis (Scheme 5) (Carpenter 1960).
Chemical synthesis of phosphoaspartate and phosphoglutamate
The first chemical synthesis of phosphoaspartate began with the protected aspartic acid derivative 15 (Scheme 6) (Black and Wright 1953, 1955). Reaction of this acid chloride with silver phosphate gave the acyl phosphate 16 in about 80% yield. Deprotection by hydrogenolysis in cold aqueous potassium bicarbonate gave the free phosphoamino acid 8a in solution in 28% yield, as determined by its reaction with hydroxamic acid (described below). Thus prepared, the solution of phosphoaspartate at approximately pH 6.5 and −20°C deteriorated slowly, but remained useful for several weeks (Black and Wright 1955).
Due to the lability of the phosphoaspartate, attempts to purify the substance as a silver, lithium, potassium or barium salt, or using ion-exchange columns, were unsuccessful. However, it was possible to remove most of the contaminating inorganic phosphate by treatment with 1 M silver nitrate, whilst maintaining the pH at 6–7 with small additions of potassium hydroxide (Black and Wright 1955).
The other literature synthesis of phosphoaspartate, which was also applied to the only reported chemical synthesis of phosphoglutamate (9b), involves the initial reaction of the silver carboxylate of the protected amino acid derivatives 17 with dibenzyl chlorophosphate, providing fully protected phosphoamino acids 18 in excellent yields (Scheme 7) (Katchalsky and Paecht 1954). Protonolysis with anhydrous hydrogen bromide gave the zwitterionic free amino acids 8b/9b, which were shown by conversion to the corresponding hydroxamic acids and subsequent colourimetric assay, to be 92% pure, the major impurity being benzyl bromide. Attempts to remove the benzyl bromide led to extensive decomposition.
Detection of acyl phosphates: phosphoaspartate and phosphoglutamate
Colourimetric assays on the hydroxamic acid derivatives
As indicated above, the original assay developed for detection of the unstable phosphoaspartate and phosphoglutamate involved treatment with hydroxylamine, and colourimetric determination of the resultant hydroxamic acids, i.e. N-hydroxy-asparagine and -glutamine (19) (Black and Wright 1953; Katchalsky and Paecht 1954) (Scheme 8). The hydroxamic acids are stable and can be used for chromatographic comparison (Black and Wright 1955). They also form strongly red-coloured complexes with iron(III) (Shuaib et al. 2002), which can be used for qualitative and quantitative measurements (Katchalsky and Paecht 1954; Black and Wright 1955).
Simple hydroxamates form bidentate octahedral coordination complexes with iron(III). The chelates formed with N-hydroxy-asparagine and -glutamine are tridentate and more complex, involving co-coordination by the carboxylate residue (Farkas and Buglyo 1990; Farkas et al. 1993; Shuaib et al. 2002).
The detection of acyl phosphates via their hydroxamic acid derivatives now seems to have been largely supplanted by reductive cleavage.
Sodium borohydride reductive cleavage
The most commonly used method of detection of free or protein-bound phosphoaspartate and phosphoglutamate involves the reductive cleavage of these phosphoamino acids with NaBH4, in a method originally described by Degani and Boyer (1973) in a study of the sarcoplasmic reticulum Ca2+-ATPase (EC 3.6.3.8), and later more generally reviewed by Purich 2002. The phosphoaspartate and phosphoglutamate react with sodium borohydride to give homoserine (20) and 4-hydroxy-1-aminovaleric acid (21), respectively (Scheme 9), whereas aspartate and glutamate are resistant to reduction under these conditions.
Often, [3H]NaBH3 is used so as to produce the tritiated derivatives. If the phosphoamino acid residue is part of a protein polypeptide chain, the reduced products are then released as the free amino acids by acid hydrolysis of the protein. The free homoserine (20) or δ-hydroxy-α-aminovalerate (21) is then separated by paper electrophoresis or thin layer chromatography (Purich 2002) and their positions usually detected by the radioactivity from the incorporated tritium. Collet et al. (1999) have used the NaBH4 reductive cleavage method coupled with tandem mass spectrometry to identify the phosphorylated aspartate in the active site of phosphoserine phosphatase (EC 3.1.3.3).
Direct detection of phosphoaspartate in phosphoenzyme intermediates and phosphopeptides
Fourier transform infrared spectroscopy has been used to detect phosphoaspartate in the active site of the Ca2+-ATPase and the effects of enzymic conformational transitions on the phosphorylated residue (Barth and Mäntele 1998; Andersson and Barth 2006). In these studies, the vibrations of both the phosphoaspartyl C=O bond and the PO3 2− bonds were studied amongst a background of 50,000 protein vibrations, and led to the conclusion that hydrolysis is catalysed by distortion of the acyl phosphate moiety towards the dissociative transition state, in which the PO3 2− moiety is trigonal and a partial negative charge develops on the leaving bridging oxygen (Barth and Bezlyepkina 2004) (see structure 13, Scheme 2—a dissociative transition state is one in which P–O bond cleavage is significantly in advance of P–+OH2 bond formation, although the process is still concerted). Interactions with a coordinated Mg2+ and active-site lysine residue are thought to be particularly important in lengthening the bridging P–O bond, and therefore weakening it by about 20%, compared to that in the model compound acetyl phosphate. In addition, the O–P–O bond angles are larger and there is more electron density on the bridging acyl oxygen.
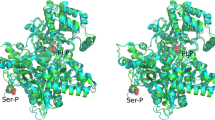
In one instance, phosphoaspartate has been directly observed in an X-ray crystallographic structure of the phosphoenzyme intermediate of β-phosphoglucomutase (EC 5.4.2.6) (Lahiri et al. 2002). The acyl phosphate moiety is stabilised by ionic/hydrogen-bonding interactions with serine and lysine residues and an octahedrally coordinated Mg2+ ion (Fig. 4).
Important active-site interactions of the phosphoaspartate residue (shown in bold) in β-phosphoglucomutase, as determined by X-ray crystallography. Adapted from Lahiri et al. (2002)
A model peptide, GlyGly(Asp-P)Ala, which was generated by in situ phosphorylation of the aspartate residue, has been characterised by 31P NMR spectroscopy. The chemical shift varied between −6.45 and −1.27 ppm at low pH (1.5) and high pH (8.0), respectively, and a pK a2 value of 4.6 was determined for the acyl phosphate group (Schlemmer et al. 1988).
Phosphoaspartate in biological systems
Free phosphoaspartate
As discussed above, phosphorylation of the side chain carboxyl group (β-carboxyl) of free aspartate (22) to form the mixed anhydride activates it, rendering the carboxyl carbon susceptible to nucleophilic attack. Phosphoaspartate (8) is formed in a reaction with ATP catalysed by aspartate kinase (aspartokinase; EC 2.7.2.4) and is itself a substrate for the enzyme aspartate-semialdehyde dehydrogenase (ASADH) (β-aspartyl-semialdehyde dehydrogenase; EC 1.2.1.11) (Scheme 10). Aspartate-semialdehyde (23) is a key intermediate in the biosynthesis of the essential amino acids threonine (26) and methionine (27), via homoserine (20) (Scheme 10), and lysine (25), via meso-diaminopimelic acid (24), which is also a cross-linking agent in the cell walls of bacteria. Accordingly, ASADH is an attractive target for antibiotics.
Phosphoaspartate in proteins
Enzyme-catalysed reactions in which an active-site aspartate is phosphorylated as an intermediate in the reaction
Phosphoaspartate also occurs as an enzymic intermediate in a number of enzyme-catalysed reactions; most prominent amongst these enzymes are members of the haloacid dehalogenase (HAD) superfamily (Ridder and Dijkstra 1999; Allen and Dunaway-Mariano 2004). These include the P-type ATPases, which are ion pumps that include Ca2+-ATPase, H+-ATPase (EC 3.6.3.6), and Na+/K+-ATPase (EC 3.6.3.9) (Scarborough 1999; Jorgensen et al. 2001; Wiedemuller and Hauser 2009). In addition, there are phosphatases such as phosphoserine phosphatase (Collet et al. 1999), phosphomutases such as β-phosphoglucomutase (Dai et al. 2006) and phosphonoacetaldehyde hydrolase (3.11.1.1) (Morais et al. 2004; Allen and Dunaway-Mariano 2004; Szefczyk 2008). All of these enzymes contain a conserved active-site sequence motif: DXXX(T/V), where the first aspartate residue is phosphorylated (Ridder and Dijkstra 1999; Collet et al. 1999).
The general mechanism of the ATPases, phosphatases, β-phosphoglucomutase and phosphonoacetaldehyde hydrolase enzymes involves nucleophilic attack of an oxygen nucleophile on the phosphorus in the key phosphoaspartate residue (29) (Scheme 11).
In contrast, catalytic hydrolysis of α-haloacids by the HAD enzymes involves attack on the ester carbonyl carbon of intermediate 31 (Scheme 12), regenerating the catalytic aspartate residue (28).
The enzyme phosphonoacetaldehyde hydrolase uses nucleophilic catalysis by an aspartate residue to catalyse a chemically difficult C–P bond cleavage (Scheme 13). The free energy released upon hydrolysis of the phosphoaspartate intermediate in the subsequent step thermodynamically drives the overall transformation. The substrate is first activated by formation of the imine with Lys53, which is then attacked by the aspartate (Asp12) and, subsequently, hydrolysis of the phosphoaspartate is facilitated by general base catalysis by the resultant enamine (Morais et al. 2004). This mechanism of substrate activation by formation of the imine has recently been questioned by Szefczyk (2008), based on a quantum mechanics/molecular mechanics (QM/MM) study, which suggested that phosphoryl transfer to the aspartate might occur directly from the substrate, promoted by substrate protonation by Lys53.
The proposed mechanism of C–P bond hydrolysis catalysed by phosphonoacetaldehyde hydrolase. Adapted from Allen and Dunaway-Mariano (2004)
Aspartate 8 in β-phosphoglucomutase also acts as a nucleophilic catalyst for the transfer of phosphoryl groups in the interconversion of glucose-1- and 6-phosphate. The enzyme is ‘primed’ by initial phosphorylation of the active-site aspartate by either β-glucose-1-phosphate or β-glucose-1,6-bisphosphate. Consequently, the aspartate starts and ends each catalytic cycle in the phosphorylated state (Scheme 14). This suggests that the difference in thermodynamic stability of the acyl phosphate versus the phosphate ester is minimal inside the active site of the enzyme, and again indicates the importance of solvation in determining the large difference in free energy of hydrolysis between these two species.
The mechanism of phosphoryl transfer in β-phosphoglucomutase. Adapted from Allen and Dunaway-Mariano (2004)
The general base (Asp10) (DXDX(T/V)) in the conserved motif is only positioned correctly to assist phosphotransfer from the phosphoaspartate 8 when glucose-1-phosphate is bound and not when water is bound. Thus, hydrolysis of the phosphoaspartate intermediate is minimised. Asp10 also acts as a general acid in phosphotransfer from glucose-1,6-bisphosphate to Asp8 (Allen and Dunaway-Mariano 2004).
There has been much conjecture about whether the β-phosphoglucomutase-catalysed reaction involves a concerted transition state or a stabilised high energy intermediate. A paper by Lahiri et al. (2003) reported an X-ray crystal structure with a captured high-energy pentavalent phosphorus intermediate (a phosphorane) in the active site (Fig. 5). However, this interpretation was immediately challenged and it was proposed that the phosphorane had been confused with a pentavalent magnesium trifluoride with two additional coordinating oxygens, which acts as a transition-state analogue (Blackburn et al. 2003). Although the phosphorane-containing structure was defended (Allen and Dunaway-Mariano 2003; Tremblay et al. 2005), subsequent QM/MM calculations indicated that the phosphorane is an energy maximum—a transition state—and could therefore not be observed crystallographically (Webster 2004). The magnesium fluoride complex, on the other hand, is an energy minimum, and therefore seems more probable. Most recently, a reanalysis of the original X-ray data along with 19F NMR characterisation appears to have confirmed that the active site contains the magnesium transition-state analogue structure (Baxter et al 2010).
A representation of the active site in a β-phosphoglucomutase–transition-state analogue complex (adapted from Webster 2004). The crystal structure was originally purported to contain a stabilised high-energy phosphorane (Y = P, X = O); however, it now seems more likely that a pentavalent transition-state analogue (Y = Mg, X = F) occupies the active site
In the P-type ATPases, although hydrolysis of the phosphoaspartate intermediate is part of the catalytic cycle, it is relatively slow, at about 5 s−1 (Sorensen et al. 2000; Clausen et al. 2001). This reduced rate of hydrolysis is achieved by substitution of aspartate in the conserved motif for the phosphatases by Thr (DXTXT/V) (Ridder and Dijkstra 1999), which forms a hydrogen-bonding interaction with the attacking water, rather than acting as a general base catalyst in the way that the aspartate does in the hydrolases (Clausen et al. 2001; Allen and Dunaway-Mariano 2004). Allen and Dunaway-Mariano (2004) suggested that the reduced rate of hydrolysis of the phosphoaspartate might provide a ‘pause’ to allow the conformational transition that results in the release of the translocated ions that the pump has carried across the membrane. However, Toyoshima (2009) proposes that in the sarcoplasmic reticulum ATPase, hydrolysis of phosphoaspartate is triggered by the positioning of Glu183 to act as a general base, in a conformational change that follows the release of its transported Ca2+ ions (see below).
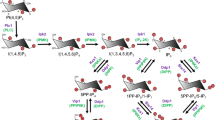
The structure and function of the sarcoplasmic Ca2+-ATPase has been intensively studied (for reviews, see Toyoshima et al. 2003; Møller et al. 2005; Toyoshima 2009). Scheme 15 shows how phosphotransfer events are coupled to structural conformational changes that result in the binding and release of Ca2+ ions (Toyoshima 2009).
Architecture of Ca2+-ATPase and its ion pumping mechanism. A cartoon illustrating the structural changes of the Ca2+-ATPase during the reaction cycle, based on the crystal structures in seven different states (see text for full description). The cytoplasmic domains shown are labelled A, N and P, α-helices in the transmembrane domain are labelled 1, 2, 4L, 4C and 5. Ca2+ ions are solid circles (e.g. between transmembrane domains 4L and 5 in E1·2Ca2+) whilst open circles are protons. The region in the N domain that contains F487 is the adenosine-binding site (see E1·ATP/E1P structure). The region labelled TGE is a loop in the A domain that contains the sequence 181TGES. The aspartate (D351) that is phosphorylated is in the P domain. Reproduced from Toyoshima (2009) with permission from Elsevier
In Scheme 15, as protons are released from acidic residues in the Ca2+-binding pocket, E2 is converted to E1 and allows the binding of two Ca2+ ions from the cytoplasm to form E1.2Ca2+. At the same time, the transmembrane helices 1 and 5 are straightened, causing movement of the N and A domains away from Asp351 in the P domain. This allows the binding of MgATP to form E1·ATP in which the P domain is bent and transmembrane helix 1 is pulled up and bent, closing the cytoplasmic gate of the Ca2+ ion-binding site sequestering the bound Ca2+. The movement of the N domain correctly positions the ATP to phosphorylate Asp351. The phosphorylation of Asp351 initiates the large rotation of the A domain, placing the TGE loop between the N and P domains, across the top of the phosphoaspartate, which protects it from bulk water in the E2P conformation. The large A domain rotation, in turn, causes drastic rearrangements in the transmembrane helices, which result in the destruction of the Ca2+-binding sites and opening of the lumenal gate of the binding site to allow the Ca2+ to exit into the sarcoplasmic reticulum lumen. The empty Ca2+-binding sites are then occupied by water and protons from the lumen. Further rotation of the A domain results in the introduction of a single water molecule and moves Glu183 into position to act as a general base to catalyse the attack of the water on the phosphoaspartate. Pi is released and the ATPase returns to the E2 state, ready for the next cycle of Ca2+ transport and ATP hydrolysis. Thus, both the phosphorylation of the active-site aspartate and the hydrolysis of the phosphoaspartate are coupled to large protein conformational changes that result in the transfer of Ca2+ ions from the cytoplasm into the lumen of the sarcoplasmic reticulum. It is in this way that the free energy of hydrolysis of ATP is used to drive the movement of the Ca2+ against the concentration gradient that exists across the sarcoplasmic reticulum membrane. It is anticipated that the other P-type ATPases function in similar ways to the Ca2+-ATPase.
The role of phosphoaspartate in two-component histidine kinase signalling systems
Two-component signalling systems, although most familiar in microorganisms, also function in plants to regulate processes such as ripening and circadian rhythms (Mizuno 2005). There are numerous excellent reviews on two-component (and multi-component) signalling systems (Wolanin et al. 2002; West and Stock 2001), which adequately describe the biological pathways they control and regulate. Hence, this section focuses only on the role of aspartate phosphorylation of the response regulator in two-component systems.
In general, the role of phosphoaspartate in two-component signalling systems is to relay the signal from the sensor histidine kinase, via the response regulator, to the DNA and, along with transcription factors, initiate transcription of appropriate response genes. However, there are exceptions like the bacterial chemotaxis-related protein CheY and the yeast osmosensing pathway that activates a MAP kinase cascade. The phosphorylated form of CheY has an increased affinity for the flagellar switch protein FliM that directly regulates flagellar rotation, turning it from counter-clockwise to clockwise. CheY, although not a transcription factor-like response regulator, has been extensively studied and there are several crystal structures of this protein from various different bacteria (Lam et al. 2010; Lee et al. 2001a, b).
Phosphoaspartate in the two-component signalling system is found on the response regulator protein after the transfer of the phosphoryl group from a conserved histidine residue of the cognate histidine kinase (for a review on phosphohistidine, see Attwood et al. 2007). In general, most response regulator proteins can be divided into an N-terminal regulatory domain containing the phospho-target aspartate, and a C-terminal effector domain that interacts with DNA as part of the response regulator’s transcriptional role. The general mechanism for phosphorylation of the canonical response regulator aspartate involves nucleophilic attack on the phosphorus atom. In the structure of the response regulator, the covalently bound phosphate is hydrogen bonded to conserved threonine and lysine residues. The phospho-transfer also requires a divalent cation, (Mg2+ or Mn2+), which is coordinated by two well-conserved aspartate residues and an asparagine, with the phosphoryl oxygen and two water molecules located in the active-site pocket (for a detailed structure, see Lee et al. 2001a, b).
Activation of the response regulator as a result of the aspartate being phosphorylated has been hotly debated and there are several models that describe how this occurs. One model, based on the crystal structure of CheY-P (or BeF3 − activated CheY), suggests that the phosphate moiety causes structural displacement of two (β4/α4 and β5/α5) domains (Zhu et al. 1997; Lee et al. 2001a, b). There are numerous amino acid conformational changes associated with either aspartate phosphorylation or chemical activation and it is these structural changes that are thought to act as the “on” switch for response regulator activation. However, Stock and Da Re (2000) present several compelling arguments that challenge the idea of this conformational change being the defining component of the switching on mechanism. They question how the structural α/β domain is able to convert metabolic energy into regulatory outputs. The main point of conjecture lies in the thermodynamic principles behind aspartate phosphorylation (see “Chemistry of mixed anhydrides”). They argue that although response regulator phosphorylation does cause some perturbation to the structure, as is evident in the crystal structure models (Zhu et al. 1997; Lee et al. 2001a, b), these conformational changes are not directly relevant to response regulator activation. This is explained by a comparison between the structures of phosphorylated or mutationally activated response regulators and unphosphorylated proteins where there appears to be little difference in conformational change. Instead, Stock and Da Re suggest that the energy released upon cleavage of the aspartyl-phosphoanhydride of the phosphorylated response regulator protein is used in binding to the target molecule and this is the “on” switch in the activation pathway. Hence, only when there is an interaction with the target, will the high-energy phosphoanhydride be used to generate a structural rearrangement that leads to a response.
In its transcriptional role, the phosphorylation state of the regulatory domain of the response regulator can act on the effector domain eliciting either a positive or negative transcriptional response. The regulatory role of aspartate phosphorylation in this system appears to be counter-balanced by autophosphatase activity of the response regulator protein or by additional phosphatase proteins in the system.
The ease of hydrolysis of the mixed anhydride bond of phosphoaspartate (see “Chemistry of mixed anhydrides”) enhances the non-enzymatic rate of hydrolysis, adding to the existing autophosphatase activity of the response regulator. The capacity for both phosphorylation and dephosphorylation by the response regulator allows rapid fine-tuning of the signalling pathway according to the needs of the organism.
In some instances, response regulators such as CheY, involved in chemotaxis, utilise the phosphatase activity of a separate protein (i.e. CheZ) to enhance the dephosphorylation of CheY-P. CheY does possess some intrinsic phosphatase activity but the dephosphorylation rate of CheY-P is increased through interaction with CheZ (Wolanin et al. 2003). CheZ is thought to accelerate the hydrolysis of the phosphoaspartyl group from CheY-P by structurally orienting a water molecule for nucleophilic attack (Zhao et al. 2002). However, it has also been suggested that phosphoaspartate hydrolysis may occur via a succinimide intermediate and that this may play a role in the on/off activation mechanism of response regulator proteins such as CheY (Napper et al. 2003).
Stable analogues of phosphoaspartate
Given the critical role of aspartate-semialdehyde dehydrogenase (ASADH) in bacterial amino acid and cell wall biosynthesis (see “Free phosphoaspartate”), and its absence from mammalian biochemistry, analogues of phosphoaspartate have been investigated as potential antibiotics. The first such compounds reported (Cox et al. 2001, 2002) have isosteric replacements of the bridging mixed anhydride oxygen atom, as shown in Fig. 6. The α,α-difluoro-β-ketophosphonate 32 and phosphoramidate, γ-N-phospho-l-asparagine (34), are indeed modest reversible inhibitors of ASADH, with K i values of the order of 0.09 mM. The β-ketophosphonate 31 also inhibited the enzyme, but with much less potency, whilst the β-hydroxyanalogues 35 (mixtures of epimers at C4), which were designed to mimic tetrahedral intermediate formed when an active cysteine residue attacks the carbonyl carbon of phosphoaspartate, were completely inactive.
A subsequent paper showed the α-fluorophosphonate 33 to irreversibly inhibit ASADH, but only with a K i of 1.2 mM (Cox et al. 2005). The simple analogue with a hydrocarbon linker between the phosphate and amino acid groups 36 was inactive at the concentrations studied, as were the E alkenes 37, which were designed as potential Michael acceptors to irreversibly inhibit the enzyme by the formation of a covalent bond with the active-site serine residue. The Z alkene 38 was marginally active at 20 mM and the acetylenic derivative 39 had a K i of 1.3 mM, but neither of these compounds were irreversible inhibitors, indicating that the postulated conjugate addition of the cysteine thiol had not occurred.
Protected analogues incorporating a cyclopropane moiety have also been synthesised (Adams et al. 2004), with the aim of rigidifying the side chain and therefore enhancing binding affinity (by minimising entropic losses, for example), however, deprotection of these compounds resulted in decomposition and, consequently, the free phosphoamino acids have not been evaluated as inhibitors of ASADH.
A stable phosphoaspartate analogue residue has also been incorporated into a protein, the bacterial response regulator methyl esterase CheB (Saxl et al. 2001). CheB is activated by phosphorylation of Asp56; Saxl et al. prepared a mutant in which the two native cysteine residues were replaced by serine, and Asp56 was substituted by a cysteine residue (D56C/C207S/C309S CheB). The Cys56 (40) was then chemically modified by treatment with Ellman’s reagent (41), followed by a second disulfide exchange of the intermediate (42) with thiophosphate to give the phosphoprotein (43) (Scheme 16), which had a half-life of 28 days and displayed activity equivalent the native phosphorylated CheB (44).
Phosphoglutamate in biological systems
Free phosphoglutamate
The best-known form of phosphoglutamate is the free amino acid, which is phosphorylated on its side chain carboxyl group and is better know as γ-glutamyl phosphate. As with phosphoaspartate, the formation of this mixed anhydride renders the carboxyl carbon more reactive and makes γ-glutamyl phosphate an important intermediate in the biosynthesis of two amino acids. The first such reaction involving phosphoglutamate is the amination of glutamate to form glutamine, catalysed by glutamate-ammonia ligase (glutamine synthetase; EC 6.3.1.2). The reaction involves the formation of phosphoglutamate (9) by nucleophilic attack of glutamate (45) on the γ-phosphorous of ATP (Scheme 17). Ammonia then attacks the carbonyl carbon of 9, displacing phosphate and forming glutamine (46) (see, e.g., Liaw and Eisenberg 1994). Glutamine, thus formed, is a precursor for the synthesis of a number of biologically important molecules, e.g. histidine, tryptophan, carbamoyl phosphate, AMP and CTP.
The second biosynthetic transformation involving phosphoglutamate begins with the phosphorylation of glutamate catalysed by glutamate 5-kinase (γ-glutamyl kinase; EC 2.7.2.11). The product is the substrate for glutamate-5-semialdehyde dehydrogenase (γ-glutamyl phosphate reductase; EC 1.2.1.41) leading to the formation of glutamate-5-semialdehyde (47), which is a precursor for the synthesis of proline (48) (Scheme 17). In plants and animals, both reactions are catalysed by a bifunctional enzyme, δ-1-pyrroline-5-carboxylate synthetase (Hu et al. 1992; Aral et al. 1996).
The intermediate, phosphoglutamate (9), is also formed in the reaction catalysed by glutamate-cysteine ligase (γ-glutamylcysteine synthetase; EC 6.3.2.2). The product, γ-glutamylcysteine (49), is subsequently converted into to glutathione (see Griffith and Mulcahy 1999).
Phosphoglutamate in proteins
Unlike aspartate phosphorylation, we have found few references to phosphorylation of glutamate residues in proteins; most of these refer to prothymosin α (Trumbore et al. 1997; Wang et al. 1997; Tao et al. 1999). This nuclear protein contains a very high proportion of acidic amino acids (50%) and Berger and co-workers found that human and monkey prothymosin α in cultured cells is phosphorylated on several glutamate residues. These workers postulated that phosphorylated prothymosin α acts as an energy reserve for nuclear processes (Trumbore et al. 1997) and later obtained evidence of its role in the production, processing or export of RNA (Tao et al. 1999). Apart from in prothymosin α, phosphoglutamate has been reported to occur in α2 chains of collagen (Cohen-Solal et al. 1979).
Stable analogues of phosphoglutamate
The first analogues of phosphoglutamate (9) were synthesised and evaluated as inhibitors of glutamine synthetase (GS) more than 30 years ago (Wedler and Horn 1976; Wedler et al. 1980), at a time when the intermediacy of phosphoglutamate was only postulated. The first reported, 3-(phosphonoacetylamido)-l-alanine (50) (Fig. 7), was found to be a weak competitive inhibitor of E. coli. GS, with a K i (3 mM) similar to the substrate, glutamate (2.5 mM) (Wedler and Horn 1976). In contrast, initial binding of 50 to pea-seed GS was rapid and tenfold tighter than with glutamate, and was followed by a slower but still reversible, ‘very tight’ binding. In addition, the affinity of 50 for pea-seed GS was markedly enhanced (K i = 18 μM) by preincubation of the enzyme with MgADP, whereupon inhibition became non-competitive.
The β-ketophosphonate 51 was subsequently investigated and displayed a very similar interaction with pea-seed GS. Again, inhibition of the E. coli enzyme was competitive and the K i was enhanced in the presence of MgADP (4.8–1.6 mM). In contrast, inhibition of GS from ovine brain by 51 was non-competitive and with about ten times the affinity of glutamate (i.e. tenfold lower K i).
More recently, the α,α-difluoro-β-ketophosphonate 52 (Scheme 18) was synthesised and evaluated as an inhibitor of E. coli GS and γ-glutamylcysteine synthetase (γ-GCS) (Hiratake et al. 2002). The incorporation of electron-withdrawing fluorine atoms at the α-position is a common ploy to enhance the electrophilicity of the carbonyl group in non-hydrolysable analogues of carboxylic acid derivative enzyme substrates. In this case, the fluorine atoms also lower the pK a of the phosphonate, bringing it closer to the phosphoglutamate, and therefore better matching the protonation state of the phosphono group in the analogue with the phosphate group in phosphoglutamate at physiological pH (Hiratake et al. 2002).
At pH 5.5 and 6, the difluorophosphonate analogue is indeed a significantly more potent inhibitor of GS (IC50 = 0.3 mM at pH 6) than 51, if the concentration of the keto form 52 is taken into consideration. However, even at this slightly acidic pH, the cyclic iminium form 52b predominates, with minor amounts of the hydrate 52a also present, and so the ‘real’ IC50 is much higher. At physiological pH (7.5), the phosphoglutamate analogue exists almost exclusively as the cyclic iminium 52b, and there is no inhibition up to 6.9 mM. The epimeric mixture of alcohols 53 is also inactive, suggesting that it is the keto form 52 that is the active inhibitor, rather than the hydrate 52a (Hiratake et al. 2002).
Inhibitors of glutamine synthetase have recently been reviewed (Berlicki 2008).
Conclusion
Acyl phosphates are nature’s equivalent to the synthetic chemist’s acyl chlorides and anhydrides. The side chain carboxyl groups of free glutamate and aspartate are activated by phosphorylation, facilitating carbonyl substitution and reduction in a number of essential metabolic pathways. Phosphorylation of active-site aspartate residues also provides reactive intermediates in several enzyme-catalysed reactions. It is clear that in some cases both the phosphorylation and subsequent dephosphorylation of aspartate residues are also involved in the induction of major conformational changes that correspond to different functional states of the protein. In these instances, the thermodynamics associated with changes in the phosphorylation state are used as an integral part of the machinery of protein function. Why nature has evolved to favour aspartate over glutamate for this purpose has not been explained. Perhaps, it is simply that the reactive acyl phosphate must not be too solvent-accessible to avoid adventitious hydrolysis, or that the shorter aspartate side chain constrains the orientation of the acyl phosphate moiety to maximise interactions with proximal amino acid residues and the enzyme substrate(s). Alternatively, it may be that many instances of protein glutamate phosphorylation are yet to be identified.
The inherently instability of phosphoaspartate and phosphoglutamate free amino acids and residues under neutral, acidic and alkaline conditions has presented challenges for their study in biological systems. Robust techniques now exist to detect transient aspartate or glutamate phosphorylation in proteins. What is more demanding is the study of how such phosphorylation affects protein conformation and function. In the future, hydrolytically stable analogues may play a part in bringing into focus the roles of phosphoaspartate and phosphoglutamate residues in proteins, through their incorporation into proteins using techniques such as chemical modification, native chemical ligation and outright protein synthesis.
Finally, as a complement to mutation studies, chemical biology could contribute to a better understanding of protein aspartate phosphorylation. The reactivity of acyl phosphates may provide opportunities to intercept the phosphorylated proteins with appropriate nucleophilic small molecules. The inactivation of proteins by the formation of covalently bound adducts should help elucidate the associated signalling pathways and their role in normal and abnormal systems.
Notes
Guynn and Veech (1973) detail a range of values for the \( \Updelta G^{\circ\prime}_{\text{hydrolysis}} \) of MgATP in tabular form sourced from other research cited in the literature.
References
Adams LA, Charmant JPH et al (2004) Efficient synthesis of protected cyclopropyl beta-aspartylphosphates. Org Biomol Chem 2:542–553
Allen KN, Dunaway-Mariano D (2003) Technical comment: response to comment on “The pentacovalent phosphorus intermediate of a phosphoryl transfer reaction”. Science 301:1184
Allen KN, Dunaway-Mariano D (2004) Phosphoryl group transfer: evolution of a catalytic scaffold. Trends Biochem Sci 29:495–503
Andersson J, Barth A (2006) FTIR studies on the bond properties of the aspartyl phosphate moiety of the Ca2+-ATPase. Biopolymers 82:353–357
Aral B, Schlenzig JS, Liu G, Kamoun P (1996) Database cloning human delta 1-pyrroline-5-carboxylate synthetase (P5CS) cDNA: a bifunctional enzyme catalyzing the first 2 steps in proline biosynthesis. C R Acad Sci III 319:171–178
Attwood PV, Piggott MJ, Besant PG (2007) Focus on phosphohistidine. Amino Acids 32:145–155
Barth A, Bezlyepkina N (2004) P–O bond destabilization accelerates phosphoenzyme hydrolysis of sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem 279:51888–51896
Barth A, Mäntele W (1998) ATP-induced phosphorylation of the sarcoplasmic reticulum Ca2+-ATPase: molecular interpretation of the infrared difference spectra. Biophys J 75:538–544
Baxter NJ, Bowler MW et al (2010) Atomic details of near-transition state conformers for enzyme phosphoryl transfer revealed by MgF3− rather than by phosphoranes. Proc Natl Acad Sci USA 107:4555–4560, S4555/1–S4555/6
Berlicki L (2008) Inhibitors of glutamine synthetase and their potential application in medicine. Mini Rev Med Chem 8:869–878
Besant PG, Attwood PV, Piggott MJ (2009) Focus on phosphoarginine and phospholysine. Curr Protein Pept Sci 10:536–550
Black S, Wright NG (1953) Enzymatic reduction of beta-aspartyl phosphate to homoserine. J Am Chem Soc 75:5766
Black S, Wright NG (1955) Beta-aspartokinase and beta-aspartyl phosphate. J Biol Chem 213:27–38
Blackburn GM, Williams N et al (2003) Comment on “The pentacovalent phosphorus intermediate of a phosphoryl transfer reaction”. Science 301:1184
Briggs PJ, Satchell DPN et al (1970) Acylation. Part XXX. Metal ion catalyzed hydrolysis of acetyl phosphate. J Chem Soc Sect B Phys Org 5:1008–1012
Carpenter FH (1960) Free-energy change in hydrolytic reactions: nonionized-compound convention. J Am Chem Soc 82:1111–1122
Clausen JD, McIntosh DB, Woolley DG, Andersen JP (2001) Importance of Thr-353 of the conserved phosphorylation loop of the sarcoplasmic reticulum Ca2+-ATPase in MgATP binding and catalytic activity. J Biol Chem 276:35741–35750
Cohen-Solal L, Chen-Solal M, Glimcher MJ (1979) Identification of gamma-glutamyl phosphate in the alpha 2 chains of chicken bone collagen. Proc Natl Acad Sci USA 76:4327–4330
Collet JF, Stroobant V, Van Schaftingen E (1999) Mechanistic studies of phosphoserine phosphatase, an enzyme related to P-type ATPases. J Biol Chem 274:33985–33990
Cox RJ, Hadfield AT et al (2001) Difluoromethylene analogues of aspartyl phosphate: the first synthetic inhibitors of aspartate semi-aldehyde dehydrogenase. Chem Commun 18:1710–1711
Cox RJ, Gibson JS et al (2002) Aspartyl phosphonates and phosphoramidates: the first synthetic inhibitors of bacterial aspartate-semialdehyde dehydrogenase. Chembiochem 3:874–886
Cox RJ, Gibson JS et al (2005) Design, synthesis and analysis of inhibitors of bacterial aspartate semialdehyde dehydrogenase. Chembiochem 6:2255–2260
Dai J, Wang L, Allen KN, Radstrom P, Dunaway-Mariano D (2006) Conformational cycling in beta-phosphoglucomutase catalysis: reorientation of the beta-d-glucose 1,6-(bis)phosphate intermediate. Biochemistry 45:7818–7824
Degani C, Boyer PD (1973) A borohydride reduction method for characterization of the acyl phosphate linkage in proteins and its application to sarcoplasmic reticulum adenosine triphosphatase. J Biol Chem 248:8222–8226
Denehy E, White JM et al (2007) Electronic structure of the sulfonyl and phosphonyl groups: a computational and crystallographic study. Inorg Chem 46:8871–8886
Farkas E, Brown DA et al (1993) Metal complexes of glutamic acid-gamma-hydroxamic acid (Glu-gamma-ha) (N-hydroxyglutamine) in aqueous solution. J Chem Soc Dalton Trans: Inorg Chem (1972–1999)18:2803–2807
Farkas E, Buglyo P (1990) Complex formation between transition metals and dl-aspartic acid-beta-hydroxamic acid (N-hydroxyasparagine) J Chem Soc Dalton Trans: Inorg Chem (1972–1999)5:1549–1551
Griffith OW, Mulcahy RT (1999) The enzymes of glutathione synthesis: gamma-glutamylcysteine synthetase. Adv Enzymol Relat Areas Mol Biol 73:209–267
Guynn RW, Veech RL (1973) Equilibrium constants of the adenosine triphosphate hydrolysis and the adenosine triphosphate-citrate lyase reactions. J Biol Chem 248:6966–6972
Hayes DM, Kenyon GL et al (1978) Theoretical calculations of the hydrolysis energies of some “high-energy” molecules. 2. A survey of some biologically important hydrolytic reactions. J Am Chem Soc 100:4331–4340
Hiratake J, Inoue M et al (2002) Alpha-glutamyltranspeptidase and alpha-glutamyl peptide ligases: fluorophosphonate and phosphonodifluoromethyl ketone analogs as probes of tetrahedral transition state and alpha-glutamyl-phosphate intermediate. Methods Enzymol 354:272–295
Hu CA, Delauney AJ, Verma DP (1992) A bifunctional enzyme (delta 1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc Natl Acad Sci USA 89:9354–9358
Jencks WP (1992) Mechanism of reactions of monosubstituted phosphates in water. Appearance and reality. ACS Symp Ser 486:102–114
Jorgensen PL, Jorgensen JR, Pedersen PA (2001) Role of conserved TGDGVND-loop in Mg2+ binding, phosphorylation, and energy transfer in Na,K-ATPase. J Bioenerg Biomemb 33:367–377
Katchalsky A, Paecht M (1954) Phosphate anhydrides of amino acids. J Am Chem Soc 76:6042–6044
Klinman JP, Samuel D (1971) Oxygen-18 studies to determine the position of bond cleavage of acetyl phosphate in the presence of divalent metal ions. Biochemistry 10:2126–2131
Koshland DE Jr (1952) Effect of catalysts on the hydrolysis of acetyl phosphate. Nucleophilic displacement mechanisms in enzymic reactions. J Am Chem Soc 74:2286–2296
Kurz JL, Gutsche CD (1960) Association phenomena. I. Specific cation effects on the hydrolysis and glycinolysis of acetyl phosphate. J Am Chem Soc 82:2175–2181
Lahiri SD, Zhang G, Dunaway-Mariano D, Allen KN (2002) Caught in the act: the structure of phosphorylated β-phosphoglucomutase from Lactococcus lactis. Biochemistry 41:8351–8359
Lahiri SD, Zhang G et al (2003) The pentacovalent phosphorus intermediate of a phosphoryl transfer reaction. Science 299:2067–2071
Lam KH, Ling TKW, Au SWN (2010) Crystal structure of activated CheY1 from Helicobacter pylori. J Bacteriol 192:2324–2334
Lee SY, Cho HS, Pelton JG, Yan D, Henderson RK, King DS, Huang L, Kustu S, Berry EA, Wemmer DE (2001a) Crystal structure of an activated response regulator bound to its target. Nat Struct Biol 8:52–56
Lee SY, Cho HS, Pelton JG, Yan D, Berry EA, Wemmer DE (2001b) Crystal structure of activated CheY. Comparison with other activated receiver domains. J Biol Chem 276:16425–16431
Liaw SH, Eisenberg D (1994) Structural model for the reaction mechanism of glutamine synthetase, based on five crystal structures of enzyme–substrate complexes. Biochemistry 33:675–681
Meyerhof O, Shatas R (1952) Heat of hydrolysis of acetyl phosphate. Arch Biochem Biophys 40:253–262
Mizuno M (2005) Two-component phosphorelay signal transduction systems in plants: from hormone responses to circadian rhythms. Biosci Biotechnol Biochem 69:2263–2276
Møller JV, Nissen P, Sørensen TL-M, le Maire M (2005) Transport mechanism of the sarcoplasmic reticulum Ca2+-ATPase pump. Curr Opin Struct Biol 15:387–393
Morais MC, Zhang G, Zhang W, Olsen DB, Dunaway-Mariano D, Allen KN (2004) X-ray crystallographic and site-directed mutagenesis analysis of the mechanism of Schiff-base formation in phosphonoacetaldehyde hydrolase catalysis. J Biol Chem 279:9353–9361
Napper S, Wolanin PM, Webre DJ, Kindrachuk J, Waygood B, Stock JB (2003) Intramolecular rearrangements as a consequence of the dephosphorylation of phosphoaspartate residues in proteins. FEBS Lett 538:77–80
Oestreich CH, Jones MM (1966) The effect of metal ions on labile phosphates. I. The hydrolysis of acetyl phosphate dianion. Biochemistry 5:2926–2931
Phillips DR, Fife TH (1968) Acid-catalyzed hydrolysis of acyl phosphates. J Am Chem Soc 90:6803–6809
Purich DL (2002) Use of sodium borohydride to detect acyl-phosphate linkages in enzyme reactions. Methods Enzymol 354:168–177
Ridder IS, Dijkstra BW (1999) Identification of the Mg2+-binding site in the P-type ATPase and phosphatase members of the HAD (haloacid dehalogenase) superfamily by structural similarity to the response regulator protein CheY. Biochem J 339:223–226
Ruben EA, Plumley JA et al (2008) Anomeric effect in “high energy” phosphate bonds. Selective destabilization of the scissile bond and modulation of the exothermicity of hydrolysis. J Am Chem Soc 130:3349–3358
Saxl RL, Anand GS et al (2001) Synthesis and biochemical characterization of a phosphorylated analogue of the response regulator CheB. Biochemistry 40:12896–12903
Scarborough GA (1999) Structure and function of the P-type ATPases. Curr Opin Cell Biol 11:517–522
Schlemmer H, Sontheimer GM et al (1988) Phosphorus-31 nuclear magnetic resonance spectroscopy of the phosphorylated tetrapeptide Gly-Gly-Asp-Ala. Magn Reson Chem 26:260–263
Shuaib NM, Marafie HM et al (2002) The binding affinity of Fe(III) to aspartic acid and glutamic acid monohydroxamates. J Coord Chem 55:933–949
Sorensen TL, Dupont Y, Vilsen B, Andersen JP (2000) Fast kinetic analysis of conformational changes in mutants of the Ca(2+)-ATPase of sarcoplasmic reticulum. J Biol Chem 275:5400–5408
Stock J, Da Re S (2000) Signal transduction: response regulators on and off. Curr Biol 10:R420–R424
Szefczyk B (2008) Towards understanding phosphonoacetaldehyde hydrolase: an alternative mechanism involving proton transfer that triggers P–C bond cleavage. Chem Commun 35:4162–4164
Tao L, Wang RH, Enkemann SA, Trumbore MW, Berger SL (1999) Metabolic regulation of protein-bound glutamyl phosphates: insights into the function of prothymosin alpha. J Cell Physiol 178:154–163
Toyoshima C (2009) How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim Biophys Acta 1793:941–946
Toyoshima C, Nomuri H, Sugita Y (2003) Structural basis of ion pumping by Ca2+-ATPase of sarcoplasmic reticulum. FEBS Lett 555:106–110
Tremblay LW, Zhang G et al (2005) Chemical confirmation of a pentavalent phosphorane in complex with beta-phosphoglucomutase. J Am Chem Soc 127:5298–5299
Trumbore MW, Wang RH, Enkemann SA, Berger SL (1997) Prothymosin α in vivo contains phosphorylated glutamic acid residues. J Biol Chem 272:26394–26404
Uchimaru T, Kutsuna S et al (2003) Effect of fluorine substitution on the rate for ester hydrolysis: estimation of the hydrolysis rate of perfluoroalkyl esters. Theochem 635:83–89
Wang RH, Tao L, Trumbore MW, Berger SL (1997) Turnover of the acyl phosphates of human and murine prothymosin alpha in vivo. J Biol Chem 272:26405–26412
Webster CE (2004) High-energy intermediate or stable transition state analogue: theoretical perspective of the active site and mechanism of beta-phosphoglucomutase. J Am Chem Soc 126:6840–6841
Wedler FC, Horn BR (1976) Catalytic mechanisms of glutamine synthetase enzymes. Studies with analogs of possible intermediates and transition states. J Biol Chem 251:7530–7538
Wedler FC, Horn BR et al (1980) Interaction of a new gamma-glutamyl phosphate analog, 4-(phosphonoacetyl)-l-alpha-aminobutyrate, with glutamine synthetase enzymes from Escherichia coli, plant, and mammalian sources. Arch Biochem Biophys 202:482–490
West AH, Stock AM (2001) Histidine kinases and response regulator proteins in two-component signalling systems. Trends Biochem Sci 26:369–376
Westheimer FH (1987) Why nature chose phosphates. Science 235:1174–1178
Wiedemuller C, Hauser K (2009) Ion transport and energy transduction of P-type ATPases: implications from electrostatic calculations. Biochim Biophys Acta 1787:721–729
Wolanin PM, Thomason PA, Stock JB (2002) Histidine protein kinases: key signal transducers outside the animal kingdom. Genome Biol 3:3013.1–3013.8
Wolanin PM, Webre DJ, Stock JB (2003) Mechanism of phosphatase activity in the chemotaxis response regulator CheY. Biochemistry 42:14075–14082
Wolfenden R, Liang YL (1989) Contributions of solvent water to biological group-transfer potentials: mixed anhydrides of phosphoric and carboxylic acids. Bioorg Chem 17:486–489
Wurmser R (1967) Biochemical energetics. II. Experimental determination of free energies. 3:28–35
Zhao R, Collins EJ, Bourret RB, Silversmith RE (2002) Structure and catalytic mechanism of the E. coli chemotaxis phosphatase CheZ. Nat Struct Biol 9:570–575
Zhu X, Rebello J, Matsumura P, Volz K (1997) Crystal structures of CheY mutants Y106W and T871/Y106W. CheY activation correlates with movement of residue 106. J Biol Chem 272:5000–5006
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Attwood, P.V., Besant, P.G. & Piggott, M.J. Focus on phosphoaspartate and phosphoglutamate. Amino Acids 40, 1035–1051 (2011). https://doi.org/10.1007/s00726-010-0738-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0738-5