Abstract
Endothelial cell-specific molecule-1 (ESM-1) is a secretory proteoglycan comprising a mature polypeptide of 165 amino acids and a single dermatan sulfate. The aim of this study was to evaluate endothelial cell-specific molecule-1 (ESM-1) as a hepatocellular carcinoma (HCC) marker and to analyze the effect of ESM-1 gene silencing in hepatocellular carcinoma cells. RT-PCR and Western Blot analysis revealed overexpression of ESM-1 in human HCC liver tissue and in serum from patients with HCC. Sandwich ELISA assay was used for quantitative analysis of ESM-1 in serum. Levels of ESM-1 were significantly elevated in the serum of patients with HCC (n = 40) as compared to serum from patients with hepatitis (AH, n = 40; CH, n = 39) or liver cirrhosis (n = 40) or from healthy subjects (n = 40). The accuracy of ESM-1 for HCC was higher than that of α-fetoprotein (AFP) according to ROC curve analysis. Expression of ESM-1 siRNA decreased cell survival through the inhibition of NF-κB pathway and induced cell cycle arrest by PTEN induction resulting in the inhibition of cyclin D1 in SK-Hep1 cells. Furthermore, ESM-1 silencing inhibited cell migration and invasion of SK-Hep1 cells. This study demonstrates that ESM-1 as a potential tumor marker is overexpressed in most tissues and serum in the presence of HCC and is involved with cell survival, cell cycle progression, migration, and invasion of hepatocellular carcinoma cells. Based on our results, we suggest that ESM-1 or a combination of ESM-1 and AFP is useful markers for diagnosis of HCC and ESM-1 may be useful therapeutic target of hepatocellular carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary cancer of the liver, and it has become the fifth most common malignancy worldwide and the third leading cause of cancer-related death (Gomaa et al. 2008; Zhou et al. 2006). Surgical resection is the most effective therapy for HCC, but many patients are not good candidates for surgery because their cancers involve intrahepatic or distant metastases. Furthermore, the long-term survival of postoperative HCC patients is unsatisfactory. Therefore, the optimal means of curing HCC is early diagnosis and monitoring using biomarkers that can characterize tumor formation and progression.
Endothelial cell-specific molecule-1 (ESM-1), also known as endocan, is a 50 kDa soluble proteoglycan that comprises a mature polypeptide of 165 amino acids and a single dermatan sulfate chain covalently linked to the serine residue at position 137 (Sarrazin et al. 2006). ESM-1 is a key player in the regulation of cell adhesion, inflammatory disorders, and tumor progression. ESM-1 specifically associates with insulin-like growth factor to promote cell growth and proliferation, and its expression is induced by TNF-alpha and IL-1 beta (Lassalle et al. 1996; Sarrazin et al. 2006). ESM-1 is expressed by the vascular endothelium and freely circulates in the bloodstream and has only one glycosaminoglycan chain (Sarrazin et al. 2006). ESM-1 is known to be a diagnostic marker for sepsis (Filep 2006; Reinhart et al. 2006; Scherpereel et al. 2006). Recently, it was reported that ESM-1 is expressed in tumor endothelium and induced tumor formation (Aitkenhead et al. 2002; Scherpereel et al. 2003). Overexpression of ESM-1 has been found in renal and lung cancer patients (Grigoriu et al. 2006; Perey et al. 2001; Rennel et al. 2007). In contrast, it has also been shown that ESM-1 is down-regulated in colorectal cancer and is positively correlated with tissue differentiation (Zuo et al. 2008). Survival analysis of HCC patients showed that overall survival was inversely associated with endocan microdensity as assessed by immunohistochemical detection (Yuen and Lai 2005). Typically, ESM-1 expression levels in human tissues have been analyzed using molecular biological methods and immunostaining, while circulating levels (protein levels) have been measured by Western blotting or ELISA (Bechard et al. 2000).
In this study, we evaluated ESM-1 as a potential tumor marker in human hepatocellular carcinoma. We examined ESM-1 expression in liver tissue from patients with HCC using RT-PCR and Western blot analysis. We also measured ESM-1 levels in patients’ serum using a sandwich ELISA assay kit. To analyze the role of ESM-1 in hepatocellular carcinoma, we performed the ESM-1 siRNA experiments. We examined cell survival, cell cycle analysis, cell migration, and invasion assay after the expression of ESM-1 siRNA in SK-Hep1 cells.
Materials and methods
Tissues
Primary HCC tissue was obtained from Kangnam St. Mary’s Hospital (Seoul, Korea) and Chonbuk National University Hospital (Chunju, Korea). Normal liver tissue was obtained from patients who had a disease other than HCC and was provided by Kangnam St. Mary’s Hospital (Seoul, Korea). Tissue specimens were frozen in liquid nitrogen and stored until use. Total RNA was extracted using an RNeasy midi-kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
Serum
At Kangnam St. Mary’s Hospital (Seoul, Korea), serum specimens were collected from patients who had not received any treatment against HCC. Serum specimens obtained from Kangnam St. Mary’s Hospital (Seoul, Korea) and Dankook University School of Medicine (Cheonan, Korea) were stored in a deep freezer until use. Consent was obtained from each patient for research use of the serum specimen, and all protocols were carried out in accordance with the guidelines approved by the Catholic University, St. Mary’s Hospital, and Dankook University School of Medicine.
RT-PCR
Oligonucleotide sequences corresponding to the ESM-1 gene were designed using Primer3 software (http://frodo.wi.mit.edu). The first strand cDNA mixture contained 5 μg of total RNA as a template for RT-PCR or real-time RT-PCR analysis and SYBR® premix Ex TagTM (TaKaRa Bio, Otsu, Japan). The primer sequences for β-actin were 5′-AAGGCCAACCGCGAGAAGAT-3′ (sense) and 5′-TGATGACCTGGCCGTCAGG-3′ (antisense). The primer sequences for ESM-1 were 5′-CTTGCTACCGCACAGTCTCA-3′ (sense) and 5′-GCGTGGATTTAACCATTTCC-3′ (antisense). Optimized PCR was carried out as follows: 1 cycle of 94°C for 5 min; 35 cycles of 94°C for 40 s, 56°C for 40 s, and 72°C for 30 s; and final extension at 72°C for 7 min. The primers used for ESM-1 real-time RT-PCR were 5′-TTGCTACCGCACAGTCTCAG-3′ (sense) and 5′-AGGGGAATTTCAGGCATTTT-3′ (antisense). Optimized PCR was carried out as follows: 1 cycle of 95°C for 10 s; 40 cycles of 95°C for 5 s, 60°C for 30 s and 95°C for 15 s; and final extension at 60°C for 15 s. Relative intensity was calculated that ESM-1 expression was normalized to β-actin expression and then intensity of HCC was compared with that of normal subjects.
Western blot analysis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was conducted using a Phast PAGE System (Pharmacia, Piscataway, NJ, USA) and a 12% gel, according to the manufacturer’s instructions. Proteins were then transferred to a PVDF membrane and probed with an anti-ESM-1 antibody (R&D, Minneapolis, MN, USA) followed by an HRP-conjugated secondary antibody (Sigma-Aldrich, St. Louis, MO, USA). Immunolabeled proteins were detected by incubation with enhanced chemiluminescence (ECL) substrate followed by exposure of the membrane to autoradiographic film. Density analysis was performed by Gel Doc system (Bio-Rad).
Confocal microscope analysis
Normal and hepatocellular carcinoma tissue was fixed in the 4% paraformaldehyde in 0.1 M PB (pH 7.4) and then replaced with 30% sucrose in 0.1 M PB at 4°C overnight. Transverse frozen sections (free-floating, 30 μm) were cut in a cryostat and processed for immunofluorescence. All the sections were blocked with 5% goat serum in 0.2% Triton X-100 for 1 h at room temperature (RT) and then incubated overnight at 4°C with mouse monoclonal anti-ESM-1 (1:1000, Abnova). The sections were then incubated with FITC-conjugated mouse IgG antibody (1:400, Jackson ImmunoResearch) for 2 h at RT. Nuclei were counterstained by DAPI (Invitrogen). An irrelevant mouse IgG of the same isotype served as a negative control. The samples were mounted in ProLong Gold antifade reagent (Invitrogen), and the stained sections were observed under a LSM510 confocal microscope (Carl Zeiss).
Detection of ESM-1 in human serum using sandwich ELISA
ESM-1 EIA kits were obtained from Atila Biosystems, Inc. (Palo Alto, CA, USA) and serum ESM-1 was detected according to the manufacturer’s instructions. All samples were coded using a numbering system and analyzed by technicians in our laboratory who were not aware of the disease state of the patients. A microwell plate was coated with 100 μl of capture antibody (1 µg/ml) against ESM-1, incubated overnight at 4°C, and then coated again with 1% BSA. Then 100 μl of serially diluted ESM-1 standard solution in the ESM-1 ELISA kit (Atila Biosystems) and samples diluted fivefold in dilution buffer (2% BSA in PBST) were applied to wells in duplicate and incubated with the 0.25 μg/ml detection antibody for 1 h at room temperature. After washing the wells with PBST solution, 100 μl of diluted HRP-conjugated streptavidin was applied to each well for 30 min. Subsequently, tetramethylbenzidine (TMB) solution was added to the wells, and then the reaction was stopped with 1 N H2SO4 solution. The absorbance was then measured at 450 nm on a spectrophotometer (Molecular Devices, Sunnyvale, CA, USA).
Measurement of AFP
Serum AFP levels were measured using a bead-based immunoassay developed by Luminex Corp. Carboxy-coated microspheres (Luminex Corp., Austin, TX) were coupled with 10 μg/ml capture antibody (mAb 11C3, Boditech Med, Korea). Detection antibody (mAb 14C2, Boditech Med, Korea) was biotinylated using the EZ-Link-sulfo-NHS-biotinylation kit (Pierce, Rockford, IL) according to the manufacturer’s instructions and dialyzed against PBS.
Statistical analysis
Samples were grouped into the following categories: normal, acute hepatitis, chronic hepatitis, liver cirrhosis, and HCC. Statistical analysis was conducted using SPSS version 10.0 (SPSS Inc, Chicago, IL). Serum ESM-1 levels are shown as means ± standard deviations (means ± SD). Means among groups were compared using an unpaired Student’s t test; p < 0.05 was considered statistically significant. Receiver operating characteristics (ROC) were calculated to assess the diagnostic value of ESM-1 or combination of ESM-1 and AFP.
siRNA knockdown of ESM-1 gene expression
ESM-1 specific siRNA and control siRNA were purchased from Samchully Pharm. Co. (Seoul, Korea). The targeted sequences of ESM-1 were sense siRNA: 5′-CUC UCA CGG AGC AUG ACA UTT -3′; antisense siRNA: 5′-AUG UCA UGA UCC GUG AGA GTT -3′. ESM-1 siRNA transfection was performed in opti-MEM with the transfection reagent LipofectAMINE Plus (Invitogen Life Technologies) following the manufacturer’s instructions.
WST-1 assay
WST-1 assay (Boehringer Mannheim, Mannheim, Germany) was performed following the manufacturer’s instructions to measure the cell survival rate of SK-Hep1 after treatment with 200 nM of ESM-1 siRNA and control siRNA, respectively.
Cell cycle analysis
Cell cycle was analyzed by flow cytometry. Cells were washed twice with cold-PBS, and permeabilized in a cold-saponin buffer (0.1% saponin in PBS). Subsequently, cells were treated with a propidium iodide (PI) buffer (10 μg/ml PI, 0.1 mM EDTA, 0.1% Triton X-100 in PBS, pH 7.4) containing 1 mg/ml RNase A for 30 min as previously described (Zou et al. 1989), and analyzed with the FACSCalibur system (Becton Dickinson, Mountain View, CA). The distribution of the cells in different phases of the cell cycle was calculated using the Cell-FIT software (Becton Dickinson Instruments).
Cell migration assays
The migration assay was conducted by the transwell assay using BD FalconTM Cell Culture Inserts. The SK-Hep1 cells were expressed in the ESM-1 siRNA for 24 h before the assay. The top chambers of the transwells were added with 0.2 ml of cells (4 × 105 cells/ml) in serum-free medium, and the bottom chambers were added with 0.6 ml of DMEM medium containing 10% FBS. The cells were incubated in the transwells at 37°C in 5% CO2 for 24 h. Migrated cells were stained with crystal violet. The migrated cells in each well were counted in three different fields per experiment under the microscope. The mean values were obtained from three replicate experiments and were subjected to the t test.
Cell invasion assays
The SK-Hep1 cells were cultured for 24 h after the expression of ESM-1 siRNA in DMEM medium containing 10% FBS before the assay. The cells were harvested by trypsinization and washed in DMEM medium with soybean trypsin inhibitor and without serum. The cells were suspended in serum-free medium at 4 × 105 cells/ml. Prior to preparing the cell suspension, the dried layer of matrigel matrix was rehydrated with serum-free DMEM medium for 2 h at 37°C. The rehydration solution was carefully removed, 0.1 ml serum-free medium was added to top chambers, and 0.1 ml (4 × 104 cells) of cell suspension was added to each top chamber. The bottom chambers were added with 0.6 ml of DMEM medium containing 10% FBS. The plates were incubated for 48 h at 37°C. The invaded cells on the bottom surface of the membrane were stained with crystal violet. The cells were enumerated by taking photomicrographs at 100× magnification. The cells in three different fields of each well were counted with two wells per treatment. The mean values were obtained from three replicate experiments and were subjected to the t test.
Results
ESM-1 expression in tissue from patients with HCC
We examined gene expression in paired HCC and adjacent non-tumor tissue from 10 patients using RT-PCR. Expression of ESM-1 mRNA was significantly higher in most HCC tissues than in adjacent non-tumor tissues (Fig. 1a). To quantify mRNA in HCC tissue, we conducted real time RT-PCR on 20 independent paired tumor and non-tumor tissue samples. The relative expression level of ESM-1 was calculated as the ratio of ESM-1 to β-actin expression. ESM-1 levels in tumor samples were expressed relative to ESM-1/β-actin (value = 1.0) of matched normal controls. ESM-1 RNA levels were much higher (21.09 ± 29.49) in most HCC tissues than in the corresponding non-tumor tissues or in normal liver tissue (Fig. 1b) and were dramatically higher in Edmond grade 2 HCC tissues compared to other grades of HCC tissue (Fig. 1c, Table 1). ESM-1 RNA levels were much higher (33.13 ± 41.2) in metastatic tumor samples than in non-metastatic liver tumor samples (Table 1). There was no relationship between tumor size (R 2 = 0.25), length of survival in months (R 2 = 0.05) (Table 1) and serum AFP (R 2 = 0.16) (Table 2).
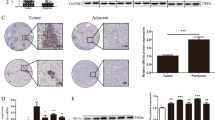
Higher expression of ESM-1 in HCC tissues. a ESM-1 gene expression analyzed by RT-PCR. RT-PCR analysis for ESM-1 was performed on 10 pairs of non-tumor liver (NT) and HCC (T) samples. cDNA was synthesized from mRNA from paired HCC tissues and adjacent non-tumor tissues, which was used as a template for reverse transcription. β-actin was used as the loading control. b Expression of ESM-1 mRNA analyzed by real time RT-PCR. The relative expression of ESM-1 was calculated as the ratio of ESM-1 to β-actin. Tumor-originated ESM-1 levels are expressed relative to ESM-1/β-actin (value = 1.0) from matched normal controls (n = 20). c ESM-1 mRNA was grouped and analyzed by Tumor grade (Ed grade). d Expression of ESM-1 protein. Western blot analysis for ESM-1 was performed on 4 pairs of non-tumor liver (NT) and HCC (T) tissues
We also examined the levels of ESM-1 protein in tissue from patients with HCC by Western blot analysis using an ESM-1-specific monoclonal antibody. Tissues were extracted from four patients with liver cancer who underwent surgical treatment. The extracted cells were then lysed and analyzed by Western blotting. As shown in Fig. 1d, ESM-1 levels in the tumor tissues were clearly higher than in the non-tumor tissues (in three of four cases). However, ESM-1 was lower in one tumor sample.
Expression of ESM-1 in the cytoplasm and periphery of tumor cells
We performed the immunofluorescent staining for ESM-1 protein in tissues of hepatocellular carcinoma with a monoclonal antibody against human ESM-1. Immunofluorescent staining showed strong expressions of ESM-1 in the cytoplasm of tumor cells in hepatocellular carcinoma tissues when compared to normal tissues. ESM-1 is also expressed at the periphery of the tumor cells in hepatocellular carcinoma tissue (Fig. 2).
Localization of ESM-1 expression in hepatocellular carcinoma tissues by confocal microscope. Subcellular localization of ESM-1 in normal and hepatocellular carcinoma tissues was detected with FITC-conjugated secondary antibodies to capture ESM-1. The mature ESM-1 protein was located in the cytoplasm and periphery of tumor cells in hepatocellular carcinoma tissues
ESM-1 levels in HCC serum samples
We compared serum ESM-1 in normal subjects and in patients with HCC. ESM-1 levels were significantly higher in HCC patients than in normal subjects (Fig. 3). The ESM-1 concentration in serum was then measured by ELISA. A dose-response curve for the ESM-1 assay was constructed using serially diluted ESM-1 samples in ESM-1 EIA kit (Atila Biosystems). Figure 4a shows the relationship between colorimetric intensity and ESM-1 concentration in the range 0–25.0 pg/ml. The minimum ESM-1 concentration that could be detected by sandwich ELISA was <0.3 pg/ml. In order to determine the optimum dilution of serum for use in the assay, serum samples were diluted 2- to 25-fold with dilution buffer and assayed for ESM-1 using the previously described protocol. The optimum dilution was found to be fivefold.
Serum concentration of ESM-1. a Serum ESM-1 in normal subjects (N) and in patients with acute hepatitis (AH), chronic hepatitis (CH), liver cirrhosis (LC), or hepatocellular carcinoma (HCC). b Serum ESM-1 concentrations in liver cirrhosis (LC), and in HCC stage 1 (HCCS1), stage 2 (HCCS2), stage 3 (HCCS3), and stage 4 (HCCS4)
Serum specimens from 40 patients with acute hepatitis (AH), 39 patients with chronic hepatitis (CH), 40 patients with liver cirrhosis (LC), 36 patients with HCC and 40 healthy subjects were evaluated in this study. We measured serum ESM-1 using an ESM-1 ELISA kit according to the manufacturer’s instructions. Samples were run in duplicate after fivefold dilution. The analyzed, summarized results are shown in Table 2 and Fig. 4. Serum ESM-1 concentrations were significantly elevated in patients with HCC (87.30 ± 25.69 pg/ml) as when compared to patients with AH (54.29 ± 20.02 pg/ml), CH (57.04 ± 19.21 pg/ml), LC (59.76 ± 23.55 pg/ml), and normal subjects (26.82 ± 10.80 pg/ml). Serum ESM-1 was particularly elevated in patients with stage 2 HCC (99.98 ± 10.06 pg/ml) as compared with patients with stage 1 HCC (68.03 ± 0.66 pg/ml). These results suggest that ESM-1 may be elevated in patients with early liver cancer and can therefore be used as a biomarker of HCC.
Cutoff values for serum ESM-1 and AFP and combined sensitivity in HCC diagnosis
We next analyzed the receiver operator characteristics (ROC) of the samples. The ROC curve shows the tradeoff between sensitivity and specificity and the area under the curve (AUC) is representative of diagnostic accuracy. For an AUC value of 0.9–1.0, the accuracy of the test is considered excellent, 0.8–0.9 is good, 0.70–0.80 is fair, 0.6–0.7 is poor, and 0.5–0.6 represents a failed test. For ESM-1, the areas under the curve were 0.880 (normal subjects vs. HCC), and 0.780 (LC vs. HCC) (Fig. 5). These AUCs were better than those for AFP (0.758 and 0.721). Serum ESM-1 concentration did not show a significant correlation (R 2 = 0.129) with AFP. We analyzed the specificity and sensitivity of ESM-1 in HCC patients and compared it with those of AFP (Fig. 5). In order to distinguish between HCC and normal samples, we used an ESM-1 cutoff value of 67.5 pg/ml. The specificity of ESM-1 was 80.8% and sensitivity was 83.8% at the cutoff value. For AFP, the specificity was 90.8% and sensitivity was 56.8% (cutoff value 20.0 ng/ml). In order to distinguish between LC and HCC, the cutoff value for ESM-1 was 87.2 pg/ml, and the specificity and sensitivity were 92.5 and 54.1%, respectively. For AFP, the specificity was 85.0% and sensitivity was 56.8% (cutoff value 20.0 ng/ml). When ESM-1 and AFP were combined as an HCC diagnostic marker, their accuracy and sensitivity were greater than those of the individual markers. The AUC was increased to 0.932 (normal subjects vs. HCC) and 0.893 (LC vs. HCC) (Fig. 5). The specificity and sensitivity comparing normal subjects versus HCC patients were increased to 89.2 and 86.5%, respectively (Fig. 5). The specificity and sensitivity comparing LC versus HCC were 85.0 and 83.8%, respectively. These results indicate that serum ESM-1 may be an independent parameter capable of identifying certain clinical conditions and that ESM-1 or a combination of ESM-1 and AFP may be useful markers for diagnosing HCC in patients with a high level of sensitivity and specificity.
ROC curves of HCC patients versus normal subjects (a) and in HCC patients versus liver cirrhosis patients (b). In an ROC curve the true positive rate (sensitivity) is plotted as a function of the false positive rate (1-specificity) for different cutoff points. Each point on the ROC plot represents a sensitivity/specificity pair corresponding to a particular decision threshold. A test with perfect discrimination has an ROC plot that passes through the upper left corner (1-sensitivity, 1-specificity). Therefore, the closer the ROC plot is to the upper left corner, the higher the overall accuracy of the test. N normal, LC liver cirrhosis, HCC hepatocellular carcinoma
Gene silencing of ESM-1 decreased cell survival and growth by PTEN-induced cell cycle arrest in hepatocellular carcinoma cells
SK-Hep1 cells were used to monitor the cell survival and growth based on cell cycle after treatment with ESM-1 siRNA and control siRNA, respectively. The cell viability was evaluated with WST-1 reagent. The viability of ESM-1 siRNA-expressed cells was decreased in comparison to the control siRNA-expressed cells (Fig. 6a). At 24 h, after ESM-1 siRNA expression, cell viabilities were reduced up to 11% (Fig. 6a). Using immunoblotting, we then evaluated the expression levels of IκB and NF-κB p65, both members of the NF-κB subfamily. Increased phospho-IκB and down-regulated NF-κB p65 expression levels (Fig. 6b) suggested that NF-κB would be suppressed in ESM-1 siRNA-expressed SK-Hep1 cells. These results indicated that ESM-1 was able to increase SK-Hep1 cell survival rate via the IκB-dependent NF-κB pathway. To study cell proliferation in further detail, we then performed cell cycle analysis and examined cell cycle regulatory proteins involved in cell cycle control. The changes in DNA content during the progression of cell cycle were evaluated by a flow cytometric assay. In the DNA content histogram (Fig. 6c), the cell cycle was arrested at the G1/S phase by EMS-1 gene silencing in the same cell lines, as compared to that of control siRNA-expressed cells (Fig. 6c). Cell cycle regulated proteins were analyzed by immunoblotting assay. We found that levels of cyclin D1 and CDK4 were inhibited by ESM-1 silencing, and that the level of PTEN, which has tumor suppressor function in most tumor cells by inducing G1-phase cell cycle arrest (Radu et al. 2003), was induced by ESM-1 siRNA when compared to control siRNA (Fig. 6d). These results suggested that ESM-1 gene silencing regulated the cell cycle arrest by PTEN induction and PTEN-induced growth suppression in the SK-Hep1 cells.
Analysis of cell survival and cell cycle regulators following ESM-1 siRNA expression in SK-Hep1 cells. a Cell viability was evaluated using a cell proliferation reagent, WST-1, at 24 h after expression of ESM-1 siRNA, as described in the “Methods” section. *p = 0.0023. c Profiling of DNA content using a FACS Calibur. M1 indicates the percentage of G1 peak of DNA content. M2 and M3 indicate the percentage of S and G2/M peak of DNA content respectively. b and d Western blot analysis of phospho-IκB, IκB, NFκB p65, PTEN, cyclin D1, and Cdk4. The cell lysates were subjected to 10, 12, and 15% SDS-PAGE and electro-transferred onto PVDF membrane, respectively. They were then incubated with specific antibodies to phospho-IκB, IκB, NFκB p65, PTEN, cyclin D1, Cdk4 and β-actin
Expression of ESM-1 siRNA inhibited hepatoceluular carcinoma cell migration and invasion
Cell migration is a key cellular feature of tumor progression because it regulates metastasis. Both tumor cell migration and invasion through the basement membranes are crucial steps in the multistage process that leads to metastasis formation (Jimenez et al. 2000). To determine the function of the ESM-1 in hepatocellular carcinoma cell migration, we used SK-Hep1 cells that highly express ESM-1. We showed that ESM-1 mRNA level and protein level were decreased in ESM-1 siRNA-expressed cells when compared to ESM-1 mRNA level and protein level in control siRNA-expressed cells (Fig. 6a). We analyzed cell migration using transwell assays to test the migration ability of cells expressing ESM-1 siRNA. Our results showed that ESM-1 siRNA-expressed cells had a 1.6-fold decrease in cell migration rate in 24 h when compared to the control siRNA-expressed cells (Fig. 7a, b).
Inhibition of cell migration and invasion in ESM-1 siRNA-expressed SK-Hep1 cells. a The migration assay was conducted by transwell assay. Migrated cells were stained with crystal violet. The representative fields were photographed. c The invasion assay was carried out according to the invasion assay method described in “Material and methods”. The invaded cells were enumerated by taking photomicrographs at ×100 magnification. The representative fields were photographed. b and d The migrated and invaded cells in each well were counted in three different fields under the microscope, respectively. The mean values and the standard error were obtained from three individual experiments. *p = 0.0028, **p = 0.0007
Cancer cells need to cross the basement membranes when they initially invade the lymphatic or vascular beds during dissemination. The invasion process is comprised of distinct events which include the attachment of cells to the basement membrane, and the migration of cells into the target tissue in response to specific chemotactic stimuli (Lauffenburger and Horwitz 1996). To study whether ESM-1 plays an important role in cell invasion, we used the invasion chamber to measure the number of invasive cells migrating through matrigel, a solid gel of in vitro basement membrane. The matrigel layer contains the membrane and pores, blocking noninvasive cells from migrating through. We analyzed the number of invasive cells expressing ESM-1 siRNA or control siRNA alone. The results demonstrate that ESM-1 siRNA-expressing cells had a cell invasion rate of 2.2-fold less than those expressing control siRNA alone (Fig. 7c, d). These data showed that ESM-1 inhibition by its siRNA expression in hepatocellular carcinoma cells inhibited cell invasion.
Discussion
HCC, the most common primary cancer of the liver, has a particularly high incidence in Asia (Gomaa et al. 2008). Among the methods used to diagnose HCC, the detection of tumor markers in patients’ serum is most effective, because it is convenient, inexpensive and accurate. Therefore, it is important to further develop effective serological markers for HCC to enable early diagnosis, as well as to monitor tumor aggressiveness, treatment responsiveness, tumor recurrence, and survival.
Diagnosis of HCC is typically based on observation of a liver mass by radiologic imaging and serum AFP levels are still widely used as a serum marker. However, AFP levels have low sensitivity (only 60–70% sensitive) and specificity, particularly in the early stages of cancer. In addition, AFP levels can be elevated in patients with liver cirrhosis or chronic hepatitis and hepatitis reactivation. Several biomarkers, including des-γ-carboxyprothrombin/prothrombin induced by vitamin K absence-II (PIVKA II), lens cularis agglutinin-reactive (AFP-L3), and glypican-3, have been assessed for their ability to detect early HCC (Lopez 2005; Zhou et al. 2006); however, these markers have poor sensitivity and specificity.
ESM-1 is preferentially expressed in the lung and kidney. Recently, ESM-1 was recognized as being one of the most significant molecular signatures of several types of cancer. ESM-1 levels were increased in VEGF-A-treated endothelial cells and human renal clear cell carcinoma (Rennel et al. 2007). ESM-1 mRNA is overexpressed in non–small cell lung cancers compared with control lung tissue. Immunohistochemistry has shown that ESM-1 is expressed only in the tumor endothelium, and is expressed particularly in the periphery of the tumors. ESM-1 and vascular endothelial growth factor mRNA expression were found to be positively correlated in lung tumors (Grigoriu et al. 2006). We compared the expression of ESM-1 among the lung cancer (4 cases), breast cancer (4 cases), gastric cancer (4 cases), pancreatic cancer (4 cases) and hepatocellular carcinoma (10 cases) by RT-PCR. ESM-1 expression of hepatocellular carcinoma was higher than that of other cancers (data not shown).
In contrast, ESM-1 expression is down-regulated in colorectal cancer. Expression of ESM-1 is higher in normal colon and rectal tissue samples than in cancerous tissue samples and is lower in colorectal cancer tissue samples. However, the expression of ESM-1 is positively correlated with tissue differentiation in colorectal cancer (Zuo et al. 2008). Recently, it was reported that microvessel density (MVD), as determined by ESM-1 in HCC, was correlated with microscopic venous invasion and VEGF expression. Overall survival of patients was inversely associated with endocan-MVD (Huang et al. 2009; Yuen and Lai 2005).
In this study, we evaluated the clinical significance of ESM-1 or a combination of ESM-1 and AFP as a biomarker for detection of HCC. We analyzed gene expression in paired HCC and adjacent non-tumor samples from 20 patients using RT-PCR and real time PCR. ESM-1 RNA expression was significantly higher in HCC samples than in control liver samples. In addition, ESM-1 expression levels were higher in Edmond grade 2 cancers than in other HCC grades. Furthermore, no relationship was observed between AFP values and ESM-1 mRNA expression. These findings indicate that ESM-1 may be useful for the detection of HCC and that there is no relationship with AFP, which is one of the established clinical parameters.
ESMs have been shown to be critical regulators of chemokines and growth factors that influence inflammatory events and tumor behavior (Sarrazin et al. 2006). We have measured ESM-1 expression in human serum by both Western blot analysis and ELISA. When expression levels of ESM-1 were measured by Western blot in human serum, ESM-1 was more highly expressed in serum from patients with HCC than in control serum. Serum specimens from 40 patients with AH, 39 patients with CH, 40 patients with LC, 36 patients with HCC and 40 healthy subjects were evaluated in this study. The serum ESM-1 concentrations of the patients with HCC were significantly higher than those of normal individuals, or of patients with AH, CH, or LC. The accuracy, sensitivity and specificity as assessed by ROC analysis were better than those for AFP in detection of HCC. Furthermore, the accuracy, sensitivity, and specificity were increased when combined screening for AFP and ESM-1 was used. Serum ESM-1 levels exhibited no correlation with serum AFP. These results indicate that serum ESM-1 may be an independent parameter capable of identifying certain clinical conditions and that it may be useful as a biomarker for HCC.
We performed the Western blot of several proteins about other survival-related pathways. Using immunoblotting, we then evaluated the expression levels of phospho-JNK, -ERK1/2, -p38 MAPK, JNK, ERK1/2, and p38 MAPK. All of protein levels without phospho-ERK, which was not detected, were not altered in ESM-1 siRNA-expressed cells when compared to control siRNA (data not shown). It was suggested that JNK, ERK, and p38 pathway were not related to the suppression of survival in ESM-1 siRNA-expressed SK-Hep1 cells. These results indicated that ESM-1 may affect SK-Hep1 cell survival via the IκB-dependent NF-κB pathway than other survival-related pathways.
Cell cycle arrest in the G1 phase is the mechanism for the proliferation defect induced by PTEN in most glioblastoma and prostate cancer cells (Furnari et al. 1998; Li and Sun 1998). While information supporting the effect of PTEN on p27Kip1 and its role in cell cycle arrest accumulates, the influence of PTEN on cyclin D1 was marginally addressed and is subject to contradictory results. Some researchers have reported decreased cyclin D1 levels after PTEN overexpression (Paramio et al. 1999; Persad et al. 2001; Weng et al. 2001), while others have found no modification of cyclin D1 levels (Li and Sun 1998; Sun et al. 1999). Interestingly, one report described decreased levels of cyclin D3 but not of cyclin D1 after PTEN expression in PTEN-deficient endometrial cells (Zhu et al. 2001). We have shown that induction of PTEN by ESM-1 silencing decreased the levels of cyclin D1, and we demonstrated a role for cyclin D1 in the growth arrest induced by PTEN. The availability of nuclear cyclin D1/CDK4 complexes may promote the G1-to-S transition by phosphorylating Rb but also by sequestering p27Kip1 from cyclin E/CDK2 complexes (Sherr and Roberts 1999). The decreases in cyclin D1 expression and nuclear localization by PTEN may thus inhibit the cyclin E/CDK2 complexes by preventing the distribution of p27Kip1 from cyclin E/CDK2 to cyclin D1/CDK4 complexes (Radu et al. 2003). Our data showed that ESM-1 silencing decreased the levels of CDK4. In these results, we suggest that ESM-1 may modulate the cell cycle progression via the regulation of cyclin D1/CDK4 complexes by PTEN in hepatocellular carcinoma cells.
In conclusion, our data show that ESM-1 as a potential tumor marker is overexpressed in most tissues and serum in the presence of HCC and is involved with cell cycle progression, cell survival, migration, and invasion of hepatocellular carcinoma cells. The results of this study suggest that ESM-1 may be a useful biomarker for the diagnosis and therapy of HCC.
References
Aitkenhead M, Wang SJ, Nakatsu MN, Mestas J, Heard C, Hughes CC (2002) Identification of endothelial cell genes expressed in an in vitro model of angiogenesis: induction of ESM-1, (beta)ig-h3, and NrCAM. Microvasc Res 63:159–171
Bechard D, Meignin V, Scherpereel A, Oudin S, Kervoaze G, Bertheau P, Janin A, Tonnel A, Lassalle P (2000) Characterization of the secreted form of endothelial-cell-specific molecule 1 by specific monoclonal antibodies. J Vasc Res 37:417–425
Filep JG (2006) Endocan or endothelial cell-specific molecule-1: a novel prognostic marker of sepsis? Crit Care Med 34:574–575
Furnari FB, Huang HJ, Cavenee WK (1998) The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res 58:5002–5008
Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD (2008) Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol 14:4300–4308
Grigoriu BD, Depontieu F, Scherpereel A, Gourcerol D, Devos P, Ouatas T, Lafitte JJ, Copin MC, Tonnel AB, Lassalle P (2006) Endocan expression and relationship with survival in human non-small cell lung cancer. Clin Cancer Res 12:4575–4582
Huang GW, Tao YM, Ding X (2009) Endocan expression correlated with poor survival in human hepatocellular carcinoma. Dig Dis Sci 54:389–394
Jimenez C, Portela RA, Mellado M, Rodriguez-Frade JM, Collard J, Serrano A, Martinez AC, Avila J, Carrera AC (2000) Role of the PI3 K regulatory subunit in the control of actin organization and cell migration. J Cell Biol 151:249–262
Lassalle P, Molet S, Janin A, Heyden JV, Tavernier J, Fiers W, Devos R, Tonnel AB (1996) ESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokines. J Biol Chem 271:20458–20464
Lauffenburger DA, Horwitz AF (1996) Cell migration: a physically integrated molecular process. Cell 84:359–369
Li DM, Sun H (1998) PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc Natl Acad Sci USA 95:15406–15411
Lopez JB (2005) Recent developments in the first detection of hepatocellular carcinoma. Clin Biochem Rev 26:65–79
Paramio JM, Navarro M, Segrelles C, Gomez-Casero E, Jorcano JL (1999) PTEN tumour suppressor is linked to the cell cycle control through the retinoblastoma protein. Oncogene 18:7462–7468
Perey L, Benhattar J, Peters R, Jaunin P, Leyvraz S (2001) High tumour contamination of leukaphereses in patients with small cell carcinoma of the lung: a comparison of immunocytochemistry and RT-PCR. Br J Cancer 85:1713–1721
Persad S, Troussard AA, McPhee TR, Mulholland DJ, Dedhar S (2001) Tumor suppressor PTEN inhibits nuclear accumulation of beta-catenin and T cell/lymphoid enhancer factor 1-mediated transcriptional activation. J Cell Biol 153:1161–1174
Radu A, Neubauer V, Akagi T, Hanafusa H, Georgescu MM (2003) PTEN induces cell cycle arrest by decreasing the level and nuclear localization of cyclin D1. Mol Cell Biol 23:6139–6149
Reinhart K, Meisner M, Brunkhorst FM (2006) Markers for sepsis diagnosis: what is useful? Crit Care Clin 22:503–519 ix-x
Rennel E, Mellberg S, Dimberg A, Petersson L, Botling J, Ameur A, Westholm JO, Komorowski J, Lassalle P, Cross MJ, Gerwins P (2007) Endocan is a VEGF-A and PI3 K regulated gene with increased expression in human renal cancer. Exp Cell Res 313:1285–1294
Sarrazin S, Adam E, Lyon M, Depontieu F, Motte V, Landolfi C, Lortat-Jacob H, Bechard D, Lassalle P, Delehedde M (2006) Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy. Biochim Biophys Acta 1765:25–37
Scherpereel A, Gentina T, Grigoriu B, Senechal S, Janin A, Tsicopoulos A, Plenat F, Bechard D, Tonnel AB, Lassalle P (2003) Overexpression of endocan induces tumor formation. Cancer Res 63:6084–6089
Scherpereel A, Depontieu F, Grigoriu B, Cavestri B, Tsicopoulos A, Gentina T, Jourdain M, Pugin J, Tonnel AB, Lassalle P (2006) Endocan, a new endothelial marker in human sepsis. Crit Care Med 34:532–537
Sherr CJ, Roberts JM (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13:1501–1512
Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H (1999) PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci USA 96:6199–6204
Weng LP, Brown JL, Eng C (2001) PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum Mol Genet 10:599–604
Yuen MF, Lai CL (2005) Serological markers of liver cancer. Best Pract Res Clin Gastroenterol 19:91–99
Zhou L, Liu J, Luo F (2006) Serum tumor markers for detection of hepatocellular carcinoma. World J Gastroenterol 12:1175–1181
Zhu X, Kwon CH, Schlosshauer PW, Ellenson LH, Baker SJ (2001) PTEN induces G(1) cell cycle arrest and decreases cyclin D3 levels in endometrial carcinoma cells. Cancer Res 61:4569–4575
Zou AP, Cheng G, Pi YQ, Wang WH, Yu SB, Wang DX (1989) The role of lipoxygenase and cyclooxygenase metabolites in acute hypoxic pulmonary vasoconstriction in piglets. J Tongji Med Univ 9:239–243
Zuo L, Zhang SM, Hu RL, Zhu HQ, Zhou Q, Gui SY, Wu Q, Wang Y (2008) Correlation between expression and differentiation of endocan in colorectal cancer. World J Gastroenterol 14:4562–4568
Acknowledgments
We thank the staff of the Kangnam St. Mary’s Hospital (Seoul, Republic of Korea) for supplying serum samples and BioInfra Inc. (Seoul, Korea) for analysis of AFP. This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF, 20100011583) and a KRIBB Research Initiative fund from the Ministry of Education, Science and Technology (MEST) provided by the Korean Government. No potential conflicts of interest were disclosed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y.H. Kang and N.Y. Ji contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kang, Y.H., Ji, N.Y., Lee, C.I. et al. ESM-1 silencing decreased cell survival, migration, and invasion and modulated cell cycle progression in hepatocellular carcinoma. Amino Acids 40, 1003–1013 (2011). https://doi.org/10.1007/s00726-010-0729-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0729-6