Abstract
Production of gamma-aminobutyric acid (GABA) was carried out in Erlenmeyer flasks by Lactobacillus brevis NCL912. Traditional methods were first adopted to select the key factors that impact the GABA production to preliminarily determine the suitable concentration ranges of the key factors. It was found that glucose, soya peptone, Tween-80 and MnSO4·4H2O were the key factors affecting GABA production. Then, response surface methodology was applied to analyze the optimum contents of the four key factors in the medium, and the production of GABA was predicted as 349.69 mM under the optimized conditions with this model. Afterward, the experiment was performed under the optimized conditions, and the yield of GABA reached 345.83 mM, which was 130% higher than the initial medium. The results showed that experimental yield and predicted values of GABA yield were in good agreement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gamma-aminobutyric acid (GABA) is a non-protein amino acid that is widely distributed in nature (Manyam et al. 1981). It is the major inhibitory neurotransmitter in the mammalian brain. GABA has several well-known physiological functions, such as neurotransmission, induction of hypotensive, diuretic effects, and tranquilizer effects (Jakobs et al. 1993; Wong et al. 2003). GABA is also involved in the regulation of cardiovascular functions, such as blood pressure and heart rate, and plays a role in the sensations of pain and anxiety (Mody et al. 1994). A recent study showed that GABA is a strong secretagogue of insulin from the pancreas (Adeghate and Ponery 2002) and effectively prevents diabetic conditions (Hagiwara et al. 2004).
Gamma-aminobutyric acid can be produced by microorganisms including bacteria (Maras et al. 1992; Smith et al. 1992), fungi (Kono and Himeno 2000) and yeasts (Hao and Schmit 1993). In recent years, many studies have focused on the GABA-producing lactic acid bacteria (LAB) because LAB possess special physiological activities and commercial potential as starters in fermented foods, such as dairy products, bread, fermented vegetables, meats and fish, etc. Some fermented food such as yogurt and cheese containing GABA (Nomura et al. 1998; Park and Oh 2007; Skeie and Ardo 2000) have been developed with GABA-producing LAB as starters.
Several GABA-producing LAB species have been reported in recent decades, including Lactobacillus brevis (Huang et al. 2007b; Li et al. 2008; Park and Oh 2007; Siragusa et al. 2007; Ueno et al. 1997; Yokoyama et al. 2002), Lactococcus lactis (Nomura et al. 1998; Siragusa et al. 2007), Lactobacillus paracasei (Komatsuzaki et al. 2005; Siragusa et al. 2007), Lactobacillus delbrueckii subsp. bulgaricus (Siragusa et al. 2007), Lactobacillus buchneri (Park and Oh 2006; Cho et al. 2007) and Lactobacillus plantarum (Siragusa et al. 2007). Glutamate decarboxylase (GAD; EC 4.1.1.15) is the key enzyme for the bioconversion of GABA. GAD has been isolated from several LAB and its biochemical properties have been characterized (Huang et al. 2007a; Komatsuzaki et al. 2008; Nomura et al. 1998; Ueno et al. 1997).
Gamma-aminobutyric acid-producing ability of LAB widely varies among the strains (Huang et al. 2007b; Komatsuzaki et al. 2005; Park and Oh 2007; Yokoyama et al. 2002) and is affected significantly by culture conditions, such as medium. It is important to optimize the medium for enhancing GABA production during fermentation. Traditional optimizing methods involve changing one independent variable while fixing the others at given levels. This single-dimensional search technique is simple, but often fails to yield optimized conditions because it does not consider possible interactions among factors. Response surface methodology (RSM) is an efficient experimental strategy to seek optimal conditions for multivariable system. RSM has been successfully employed for optimizing the medium composition (Lee and Chen 1997). Based on the key factors influencing the GABA production opted with one independent variable, RSM was applied to optimize the factors of medium for enhancing the GABA production.
Materials and methods
Strain, media and culture conditions
A GABA-producing LAB strain Lb. brevis NCL912 was isolated from paocai, a Chinese traditional fermented vegetable (Li et al. 2008). The initial medium used for GABA production was composed of (g L−1): glucose, 25; yeast extract, 6.25; soya peptone, 6.25; MgSO4·7H2O, 0.2; MnSO4·4H2O, 0.05; sodium l-glutamate, 500 mM; and Tween 80, 2 mL L−1. The pH was adjusted to 5.0 before autoclaving. Nitrogen sources, sodium l-glutamate and the other compositions were autoclaved separately at 121°C for 20 min and mixed together prior to inoculation. Unless otherwise stated, the strain was kept in static cultivation at 34°C for 48 h in 250-mL flasks containing 100-mL medium.
GABA assay
The GABA concentrations in the culture broths were determined by pre-staining paper chromatography (Li et al. 2009).
Response surface methodology
Box–Behnken design using three coded levels was employed for determining the optimal conditions of the four screened factors in the single-factor experiments. The behavior of the system was explained by the following quadratic equation:
where Y is the predicted response, β0 the offset term, β i the linear offset, β ii the squared offset, β ij the interaction effect, and x i is the dimensionless coded value of X i . Various assemblies used in the design are shown in Table 1.
Results and discussion
Evaluation of the key factors
Effects of carbon sources on GABA production
In a previous work, we found that the carbohydrates that could be fermented by Lb. brevis NCL912 were l-arabinose, ribose, d-xylose, galactose, glucose, fructose, maltose, melibiose, α-methyl d-glucoside, N-acetyl d-glucosamine and gluconate (Li et al. 2008). From these carbohydrates, only glucose and maltose were tested in the present study because the others were expensive or unavailable.
Two concentrations (1.25 and 2.5%, w/v) were tested for glucose and maltose, respectively, while fixing the other composition levels of the basal medium. The results showed that glucose was a better carbon source than maltose for GABA production (Fig. 1). It was also an important factor affecting GABA production since a higher GABA yield was observed at a concentration of 2.5%. Therefore, 2.5% glucose was used in the following tests.
Effects of nitrogen sources on GABA production
The effects of nitrogen sources on the GABA production were investigated when 2.5% glucose was used as the carbon source. Figure 2 showed that yeast extract, soya peptone and beef extract were the best nitrogen sources with high GABA concentrations. A low production of GABA was observed when maize extract was used as the nitrogen source. On the other hand, yeast extract, soya peptone and beef extract are all important factors affecting GABA production, because significant GABA concentration differences were obtained between 1.25 and 2.5% concentrations of the three nitrogen sources, respectively. Since soya peptone is cheaper and more available than yeast extract and beef extract, a 2.5% concentration of soya peptone was used in the subsequent single-factor experiments.
Effects of Tween-80 on the production of GABA
Tween-80 is a growth-stimulating factor for most of LAB. The effect of Tween-80 on GABA production was tested in a media containing 2.5% glucose and 2.5% soya peptone. As much as 2.0 mL L−1 of Tween-80 was added to the medium, and medium without Tween-80 was the control. The results showed that NCL912 could hardly convert glutamate to GABA when Tween-80 was not added, while the GABA concentration reached 208.50 mM in the medium with Tween-80.
Effects of other factors on the production of GABA
The other substances, such as MnSO4·4H2O, CaCl2, MgSO4·7H2O, VB6, VC and nicotinic acid that may possibly affect the production of GABA were determined experimentally. The effects of these factors on the GABA production were studied in the media containing 2.5% soya peptone as the nitrogen source, while the contents of other components were the same as the initial medium described in “Materials and methods”. The medium containing 2.5% glucose, 2.5% soya peptone, 500 mM glutamate and 2.0 ml L−1 Tween-80 was the experimental control. As shown in Table 2, addition of CaCl2, MgSO4·7H2O, VB6, VC and nicotinic acid had no significant effect on GABA production. MnSO4·4H2O was found to be essential for GABA production. GABA concentration reached 206.83 mM in the medium containing 0.1 g L−1 MnSO4·4H2O, which was higher than that of non-MnSO4·4H2O by about 18%.
Determination of optimal concentration ranges of the key factors
In the above experiments, glucose, soya peptone, Tween-80 and MnSO4·4H2O were determined as the key factors affecting GABA production by NCL912. To know the suitable concentration ranges of the four factors, a series of concentrations of these factors were selected for single-factor experiments in the following sections.
The effects of glucose concentration on GABA production
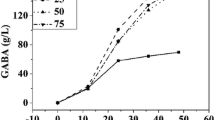
In this experiment, glucose concentrations were altered according to Table 3, and the other components in the medium were fixed as follows (g L−1): soya peptone, 25; MnSO4·4H2O, 0.05; sodium l-glutamate, 500 mM; and Tween 80, 2 ml L−1. As shown in Table 3, GABA concentration increased with increase in glucose concentration from 0.5 to 5.0%. When the concentration of glucose was above 5.0%, the GABA concentration was almost stable with only a slight increase.
The effects of soya peptone concentration on GABA production
In this experiment, soya peptone concentrations were altered according to Table 4, and the other components in the medium were fixed as follows (g L−1): glucose, 50; MnSO4·4H2O, 0.05; sodium l-glutamate, 500 mM; and Tween 80, 2 ml L−1. For soya peptone, GABA production significantly increased with concentration of soya peptone when the concentration of soya peptone was lower than 2.5%. GABA production was almost stable when the concentration of soya peptone was at or above 2.5%. Although a slight increase in GABA production was observed when soya peptone exceeded 2.5%, the higher the concentration of the soya peptone, the higher was the cost of the medium.
The effects of Tween-80 concentration on GABA production
The concentrations of Tween-80 used for testing are shown in Table 5. The other components in the medium were fixed as follows (g L−1): glucose, 50; soya peptone, 25; MnSO4·4H2O, 0.05; sodium l-glutamate, 500 mM. The results showed that almost no GABA could be detected in the absence of Tween-80, and the GABA concentration increased with increase in Tween-80 concentration from 0.05 to 2.0 mL L−1. The GABA concentration was almost stable, although a slight increase was observed when Tween-80 concentration exceeded 2.0 mL L−1.
The effects of MnSO4·4H2O concentration on GABA production
The media used in this experiment contained (g L−1): glucose, 50; soya peptone, 25; sodium l-glutamate, 500 mM; Tween-80, 2 mL L−1; and MnSO4·4H2O concentrations tested were 0, 0.005, 0.01, 0.02, 0.03 g L−1, respectively. GABA production increased with increase in MnSO4·4H2O concentration from 0 to 0.01 g L−1. In the absence of MnSO4·4H2O, there was a drastic reduction in the production of GABA (only 173.84 mM). GABA concentrations in 0.005, 0.01, 0.02 and 0.03 g L−1 MnSO4·4H2O-containing media reached 216.45, 323.83, 325.62 and 325.80 mM, respectively. The data indicated that, although essential, increasing MnSO4·4H2O concentrations above 0.01 g L−1 did not result in increased GABA production.
Further optimization of the key factors by RSM
Glucose, soya peptone, Tween-80 and MnSO4·4H2O were identified as the key factors that influenced GABA production from the above experiments, and their suitable concentration ranges were also preliminarily determined. In the following experiments, the four important components were optimized using RSM. Table 1 shows various combinations used and the corresponding GABA yield (experimental and predicted). Data were analyzed by linear multiple regression using the Design-Expert (Version 6.0.4.0; Stat-Ease, Inc.) and the following equation was obtained.
where X 1, X 2, X3 and X 4 are the symbols of concentration of glucose, soya peptone, Tween-80 and MnSO4·4H2O, respectively.
To validate the regression coefficient, analysis of variance (ANOVA) for GABA production was performed (Table 6). Value of “Model Prob > F” less than 0.0001 implies that the model is significant. In this case X 1, X 2, X 21 and X 22 are significant model terms. The lack of fit measures the failure of the model to represent data in the experimental domain at points that are not included in the regression (Rastogi and Rashmi 1999). The “Lack of Fit Prob > F value” of 0.0538 implies that the lack of fit is insignificant. Normally, a regression model having an R 2 value higher than 0.9 is considered as having a very high correlation. The R 2 value of this model is 0.9791, so it is reasonable to use the regression model to analyze the trends in the responses.
The graphical representation provides a method to visualize the relationship between the response and experimental levels of each variable and the type of interactions between test variables to deduce the optimum conditions. In this design, the response surface curves for the four variables are shown in Fig. 3. The response surface representing GABA yield is a function of concentrations of the two medium components with another nutrient being at an optimal level.
Three-dimensional surface plots showing the effect of different variables on GABA production. a Effect of glucose (X 1) and soya peptone (X 2) on GABA production. b Effect of glucose (X 1) and Tween-80 (X 3) on GABA production. c Effect of glucose (X 1) and MnSO4·4H2O (X 4) on taxol production. d Effect of soya peptone (X 2) and Tween-80 (X 3) on GABA production. e Effect of soya peptone (X 2) and MnSO4·4H2O (X 4) on GABA production. f Effect of Tween-80 (X 3) and MnSO4·4H2O (X 4) on GABA production
From the study of plots, the maximum production of GABA was obtained when the concentration of glucose, soya peptone, MnSO4·4H2O and Tween-80 were at 55.25, 30.25, 0.0061 g L−1 and 1.38 mL L−1, respectively. The maximum value of GABA production predicted from this model was 349.69 mM. To verify the predicted results, the experiment was performed under the optimized nutrients levels, and the experimental value was 345.83 mM, which is 130% higher than the basal media, suggesting that experimental and predicted values of GABA yield were in good agreement. Therefore, the strain has great potential for industry applications.
Conventional process for optimization of conditions is time consuming and expensive, and fails to consider the interactions between different factors. The statistic method of Box–Behnken design that avoids the above problems has been proved to be useful in this optimization.
References
Adeghate E, Ponery AS (2002) GABA in the endocrine pancreas: cellular localization and function in normal and diabetic rats. Tissue Cell 34:1–6. doi:10.1054/tice.2001.0217
Cho YR, Chang JY, Chang HC (2007) Production of gamma-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J Microbiol Biotechnol 17:104–109
Hagiwara H, Seki T, Ariga T (2004) The effect of pre-germinated brown rice intake on blood glucose and PAI-1 levels in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem 68:444–447
Hao RY, Schmit JC (1993) Cloning of the gene for glutamate decarboxylase and its expression during conidiation in Neurospora crassa. Biochem J 293:735–738
Huang J et al (2007a) Purification and characterization of glutamate decarboxylase of Lactobacillus brevis CGMCC 1306 isolated from fresh milk. Chin J Chem Eng 15:157–161
Huang J, Mei LH, Xia J (2007b) Application of artificial neural network coupling particle swarm optimization algorithm to biocatalytic production of GABA. Biotechnol Bioeng 96:924–931. doi:10.1002/bit.21162
Jakobs C, Jaeken J, Gibson KM (1993) Inherited disorders of GABA metabolism. J Inherit Metab Dis 16:704–715
Komatsuzaki N et al (2005) Production of gamma-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol 22:497–504. doi:10.1016/j.fm.2005.01.002
Komatsuzaki N et al (2008) Characterization of glutamate decarboxylase from a high gamma-aminobutyric acid (GABA)-producer, Lactobacillus paracasei. Biosci Biotechnol Biochem 72:278–285. doi:10.1271/bbb.70163
Kono I, Himeno K (2000) Changes in gamma-aminobutyric acid content during beni-koji making. Biosci Biotechnol Biochem 64:617–619
Lee SL, Chen WC (1997) Optimization of medium composition for the production of glucosyltransferase by Aspergillus niger with response surface methodology. Enzyme Microb Tech 21:436–440
Li H et al (2008) A high γ-aminobutyric acid-producing ability: Lactobacillus brevis isolated from Chinese traditional paocai. Ann Microbiol 58:649–653
Li H et al (2009) Pre-staining paper chromatography method for quantification of gamma-aminobutyric acid. J Chromatogr A 1216:5057–5060. doi:10.1016/j.chroma.2009.04.044
Manyam BV et al (1981) Isoniazid-induced elevation of CSF GABA levels and effects on chorea in Huntington’s disease. Ann Neurol 10:35–37
Maras B et al (1992) The amino acid sequence of glutamate decarboxylase from Escherichia coli—evolutionary relationship between mammalian and bacterial enzymes. Eur J Biochem 204:93–98
Mody I et al (1994) Bridging the cleft at gaba synapses in the brain. Trends Neurosci 17:517–525
Nomura M et al (1998) Production of gamma-aminobutyric acid by cheese starters during cheese ripening. J Dairy Sci 81:1486–1491
Park KB, Oh SH (2006) Isolation and characterization of Lactobacillus buchneri strains with high gamma-aminobutyric acid-producing capacity from naturally aged cheese. Food Sci Biotechnol 15:86–90
Park KB, Oh SH (2007) Production of yogurt with enhanced levels of gamma-aminobutyric acid and valuable nutrients using lactic acid bacteria and germinated soybean extract. Bioresour Technol 98:1675–1679. doi:10.1016/j.biortech.2006.06.006
Rastogi NK, Rashmi KR (1999) Optimisation of enzymatic liquefaction of mango pulp by response surface methodology. Eur Food Res Technol 209:57–62
Siragusa S et al (2007) Synthesis of γ-aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl Environ Microbiol 73:7283–7290. doi:10.1128/AEM.01064-07
Skeie S, Ardo Y (2000) Influence from raw milk flora on cheese ripening studied by different treatments of milk to model cheese. Lebensm-Wiss Technol 33:499–505
Smith DK et al (1992) Escherichia coli has two homologous glutamate decarboxylase genes that map to distinct loci. J Bacteriol 174:5820–5826
Ueno Y et al (1997) Purification and characterization of glutamate decarboxylase from Lactobacillus brevis IFO 12005. Biosci Biotechnol Biochem 61:1168–1171
Wong CG, Bottiglieri T, Snead OC 3rd (2003) GABA, gamma-hydroxybutyric acid, and neurological disease. Ann Neurol 54:S3–S12. doi:10.1002/ana.10696
Yokoyama S, Hiramatsu J, Hayakawa K (2002) Production of gamma-aminobutyric acid from alcohol distillery lees by Lactobacillus brevis IFO-12005. J Biosci Bioeng 93:95–97
Acknowledgments
We thank Dr. Yu Bo for his assistance in the response surface methodology analysis. This work was financially supported by the Education Department of the Jiangxi Province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Qiu, T., Gao, D. et al. Medium optimization for production of gamma-aminobutyric acid by Lactobacillus brevis NCL912. Amino Acids 38, 1439–1445 (2010). https://doi.org/10.1007/s00726-009-0355-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-009-0355-3