Abstract
Self-diffusion of surfactants and counterions in premicellar and micellar solutions of sodium, lithium and cesium dodecyl sulfates has been examined by nuclear magnetic resonance (NMR)-diffusometry. Self-diffusion of surfactants obeys as a whole the well-known two-site exchange model in which surfactants diffuse as micelles and non-micellized molecules in monomer and dimer forms. To explain self-diffusion of counterions, the model which takes into account their diffusive motion about the surface of micelles is proposed. It is shown that this phenomenon contributes considerably to charge transfer in micellar solutions. Estimation of surface diffusion of counterions about the micellar surface is made on the basis of experimental results on self-diffusion of surfactants and counterions, the data obtained on critical micelle concentration and the degree of counterion binding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ionic surfactants are the most widely used family of surfactants in both academic research and industrial practice, being utilized in practical applications ranging from household uses, including detergents, pharmaceuticals, and personal care products, to industrial applications involving lubricants and coatings.
Below the critical micelle concentration (cmc) in water solutions of ionic surfactants, which belong to 1-1 electrolyte family, the surfactant ions and counterions do not form ion pairs similar to inorganic electrolyte solutions. Under surfactant micellization the charged surface of micelles binds a portion of counterions as a result of electrostatic [1] and, possibly, specific interactions [2]. But the data on the degree of counterion binding (β) obtained by means of different experimental techniques is rather conflicting even for one and the same surfactant [1, 3–6].
The nuclear magnetic resonance (NMR)-diffusometry approach for probing translational diffusive mobility of surfactants and counterions is one of the most versatile techniques to characterize surfactant self-assembly, to study micellar size/shape and to analyze the degree of counterion binding [7–11]. The established procedure to analyze self-diffusion NMR data is to make use of a two-site exchange model [12, 13], in which the observed diffusion coefficient is expressed as a population-weighed average between “free” (monomer) and “bound” (micelles) surfactant. Below cmc, surfactant is considered to exist in the form of monomers. Under the increase of surfactant concentration, at cmc and over it, a part of surfactant turns into micelles, whose concentration increases with the growth of total surfactant concentration.
According to the two-site exchange model, the self-diffusion coefficients of surfactant molecules and counterions D ± follow the equation
where superscripts – and + correspond to surfactant and counterions, m ±1 and m ±2 (m ±1 + m ±2 = 1) are the portions of “free” and “bound” species, D ±1 and D ±2 are their on self-diffusion of coefficients. In our previous work on the self-diffusion of sodium dodecyl sulfate (SDS) in premicellar and micellar solutions [14], we made this approach more realistic when it was determined that below cmc in addition to “free” SDS molecules the surfactant dimers give remarkable contribution to the measured self-diffusion coefficient. By this one can see that the two-site exchange model is rather simplified to describe NMR data in surfactant solutions where additional species can present. However, to the present day it is the main model which is very helpful in analysis of NMR data for such systems as surfactant solutions where the rapid exchange of molecules between states exists. And we are using the term “two-site exchange model” since the introduction of the third participant into the process does not contradict the basic principles of this model.
The two-site exchange model can be also used for analysis of counterion diffusive motion on the basis of NMR self-diffusion experiment [9–11, 15]. In this case one has to take into account D +2 = D −2 . By definition the degree of counterion binding to micelles looks like
In the other form it can be written as
since on the background of the two-site exchange model:

At high values of the surfactant concentration when overwhelming majority of the surfactant presents in the micellar state and the obstruction effect for self-diffusion of micelles is taken into account, D − → D −2 and
Although the combination of NMR-diffusometry and the two-site exchange model constitutes a direct way to obtain the degree of counterion binding, practically its application is rather restricted. At the same time the independent data in this field may be exclusively useful for confirmation of assumptions about the surface mobility of bound counterions based on the results of electric conductivity [16] and dielectric relaxation [17–20] studies, the structure of electric double layer, the protolytic balance at the micelle surface, the solubilizing capacity and reactivity of micellar solutions of surfactants [3, 21–23].
In the present study we have chosen several representatives of dodecyl sulfates of alkali-earth elements for comprehensive analysis of diffusion of all species using the basic principles of the quasi-chemical theory of micelle forming [3]. One of them, SDS, and its micelle properties have been under different investigations over the years [24]. Moreover, SDS is the generally accepted model system to study micellization phenomena. That is why the SDS self-diffusion data could be found in many papers (e.g., Refs. [7, 25, 26]). We also selected SDS for our study as a reference system to compare self-diffusion behavior of three dodecyl sulfates, which are studied more poorly. Moreover, the self-diffusion data for inorganic counterions in surfactant solutions is a rare occurrence in scientific literature [26].
In this work we have (1) investigated self-diffusion of surfactant ions and counterions in premicellar and micellar solutions of sodium, lithium and cesium dodecyl sulfates by means of NMR-diffusometry, (2) developed and (3) tested the model of counterion diffusion taking into account their self-diffusion about the micelle surface for different alkaline-earth elements.
2 Experimental
2.1 Chemicals and Samples
The chemicals of reagent grade (99%) from Sigma (SDS), ACROS Organics (lithium dodecyl sulfate, LiDS), Fluka (LiCl), ECROS (NaCl, CsCl) were used as received. Cesium dodecyl sulfate (CsDS) was obtained from SDS by ion exchange in CsCl solution. Mixed solution of SDS (0.5 M) and CsCl (1.0 M) was prepared at 50°C. Then the solution was allowed to stand at 50°C for 2 h and at room temperature for next 12 h. The sediment was separated off solution using Büchner funnel and again dissolved in CsCl solution. After three consecutive recrystallizations the sediment was washed with acetone and dried over sulphuric acid till constant mass is obtained. The product yield was about 85%. The degree of ion exchange Na+ and Cs+ was determined by flame photometry (air–acetylene mixture) at 2,300°C and it was not lower 96%. Solutions for NMR measurements were prepared using Milli-Q water. The concentration of surfactants was varied from 0.003 to 0.1 M. To determine self-diffusion of free counterions the water solutions of LiCl, NaCl and CsCl in concentration of 0.007, 0.02 and 0.1 M were used. All measurements were fulfilled at 40°C, i.e., higher than the Kraft point for CsDS (28–32°C) [27].
2.2 Self-Diffusion Measurements
Bruker AVANCE 400 NMR spectrometer equipped with z-gradient probe head (BBO) with a gradient strength up to 53.5 G cm−1 was used. Stimulated-echo sequence [28] incorporating bipolar gradient pulses and a longitudinal eddy current delay (BPP-LED) was employed. For uniform warming-up of the sample volume and for minimization of convection effects, the air stream of 400 L/min was used. Samples were kept at 40°C for 20 min before measurement. Self-diffusion measurements for surfactants were made using 1H signal (400.13 MHz). For counterions the resonance signals of 23Na (105.84 MHz), 7Li (155.51 MHz), and 133Cs (52.48 MHz) were used. The gradient strength was incremented from 0 up to 50.0 G cm−1 under constant diffusion time (50–60 ms) and diffusion gradient pulse (3–6.5 ms). The proton self-diffusion measurements were made with the pulse sequence 3–9–19 for solvent signal suppression [29]. The 90°-pulse duration was 9.5 ms for nuclei of protons, 8.6 ms for sodium, 7.25 ms for lithium, and 4.87 ms for cesium. A recycle delay of 5 s was used to collect 16–128 transients in 32–64 k of data points. The deviation of adjustable curves on diffusive decays did not exceed 3–5 × 10−3.
2.3 Determination of cmc and Degree of Counterion Binding
For cmc determination a potentiometry method was used [30]. For LiDS and SDS solutions, we used a galvanic cell with ion transfer consisting of glass ion-selective electrodes with hard contact and a chlorosilver electrode, the last being a reference electrode. To determine the mean activity of CsDS ions the ion-selective electrode with plasticized membrane was used. This membrane represents a polyvinyl chloride film plasticized with dioctyl phthalate which also operates as the solvent for electrode-active substance, cesium tetra(p-chloride-phenyl)borate. The interelectrode potential was determined with the help of the ionometry converter I-500 (Russain Federation). The mean activity of ions in LiDS, SDS and CsDS was determined with the help of calibration curves for LiCl, NaCl and CsCl. All samples were stored for 1.5–2 h at 40°C. The values of cmc were defined as X-coordinate of the intersection of two rectilinear lines which approximate the concentration dependences of the ion activity in ions in premicellar and micellar domains [31]. The slope of lines for the micellar domain was equal to a degree of dissociation of counterions bound to micelles, i.e., (1 − β).
2.4 Modeling the Effective Self-Diffusion Coefficients of Surfactant and Counterions in Surfactant Solutions
Let us modify the two-site exchange model taking into account the processes of dimerization of surfactant molecules [15, 31] and surface diffusion of counterions. In this case Eq. (1) for the surfactant and the counterion can be rewritten, respectively, as
and
Here, m −2 = c −2 /c = α, α is the degree of micellization, c −2 is the surfactant concentration in the micellar form, c is the total surfactant concentration, D −11 and D −12 are the self-diffusion coefficients of monomer and dimer forms of surfactant ions, m −11 and m −12 are their portions and D +s is the surface diffusion coefficient of bound counterions. The terms in Eq. (3) determine diffusive contributions of surfactant monomers, dimers and micelles into the effective self-diffusion coefficient. The effective self-diffusion coefficient of counterions [Eq. (4)] is composed of four terms. First one reflects the contribution from free counterions which are present as a result of dissociation of the non-micellized surfactant. It is evident that m +1 = m −1 . The second term characterizes free counterions which appear under dissociation of the micelle-forming surfactant. The bound counterions take part in two independent diffusive motions: about the surface of micelles (third item) and translating with micelles (forth item). From Eq. (4) it follows that
Equation (5) characterizes the dependence of the effective self-diffusion coefficient on α, and consequently on the total surfactant concentration, the dependence on total surfactant concentration. In extreme case under α → 1 one has
and
Thus, at a high degree of micellization the effective self-diffusion coefficient of counterions tends to a certain limit which depends on their self-diffusion coefficients in free and bound states so as on the self-diffusion coefficients of the surfactant in the micellar state. From Eq. (7) it is seen that the surface self-diffusion coefficient of counterions is determined as
The right-hand side of Eq. (5) holds the parameters D +1 , D −2 , β, which are available from literature or experiment. To calculate D +(c), first, it is necessary to have a value of the unknown parameter D +s , and, second, to determine the functions α(c), m −11 (c) and m −12 (c). The last can be solved on the basis of the quasi-chemical theory of micelle forming [3] with the known data on dimerization constants for SDS [31].
There is a need to state that Eqs. (3)–(8) are correct only when the obstruction effect for diffusion of micelles can be neglected. This effect originates from overlapping of double electric layers in concentrated micellar solutions. In the present work we restricted the total surfactant concentration to 0.1 M. Taking into account the aggregation number of surfactant in micelles n 1 = 40.1 at 40°C [1] and an average radius of micelles a m = 2.2 × 10−9 m [14], one has the maximum volume fraction of micelles of 6.6%. The mean intermicellar distance r is equal to 8.7 × 10−9 m and we have the condition κr ≫ 1 (κ is the reciprocal of the Debye length which is no more than 3.2 × 108 m−1) when there is no noticeable overlapping of double electric layers. So the obstruction effect can be regarded as inessential in the concentration range studied.
2.5 The Composition of Premicellar and Micellar Solutions
We consider that surfactants in premicellar solutions and intermicelle medium present in monomer and dimer forms. To determine the concentrations of monomers, c m, and dimers, c d, we defined the dimerization degree α 0 = c d /c 11, where c 11 = c d + c m is the concentration of non-micellized surfactant molecules (in the premicellar range c 11 = c). It is evident that
The equilibrium constant of coexistence of monomers and dimers, K 1, is equal to
or, in the other form, to
The unknown parameter K 1 can be found [31] on the basis of the quasi-chemical theory of micelle forming as
With the known K 1 it is possible to determine the dimerization degree α 0 and then the concentrations of monomers and dimers. The dependence of the degree of micellization, α, on the total surfactant concentration for 1-1 ionic surfactants obeys the equation [3]
where \( \left( {K_{c} n_{1} } \right)^{{ - {\frac{1}{n - 1}}}} \,\approx\, c_{\text{cmc}} \) [28], K c is the micellization constant, n 1 is the aggregation number of surfactant ions in micelles, n = n 1(1 + β). When the function α(c) is known it is possible to determine the concentration of free surfactant in the intermicelle space:
the concentration of micellized surfactant:
the values of m −1 and m −2 :
3 Results and Discussion
Figure 1 depicts, as an example, the mean activity of ions, a, as a function of total concentration of SDS in solution. For solutions of LiDS and CsDS similar dependences were detected. Within the bounds of experimental errors for all studied systems these dependences were characterized by two rectilinear domains, which correspond to premicellar and micellar states of surfactant solutions. According to the current opinion the breaking points of this dependence correspond to the cmc value, which was calculated by joint regression procedure for both rectilinear domains. At c < cmc the slope of the curve characterizes the mean activity coefficient of ions, γ ±, in premicellar solutions, or the association (dimerization) of free surfactant ions. At c > cmc the slope corresponds to the degree of dissociation of the micellized surfactant, or to the value (1 − β). The results followed from the ions activity data are presented in Table 1. One can see that the nature of the counterion considerably influences cmc, premicellar association of surfactants and the degree of counterion binding by micelles. At that, the following pattern has to be marked: the higher the hydrated ion radius (Li > Na > Sc), the stronger the deviation of premicellar solutions from ideality (γ ± = 1). In the micellar solutions, one can see a noticeable increase of β under the passage from LiDS to CsDS.
Figures 2 and 3 depict experimental self-diffusion coefficients of counterions and surfactant molecules, respectively. The results are in good qualitative agreement with the proposed model [Eqs. (3) and (4)]. All curves show sharp inflections at definite values of the total surfactant concentrations which in the first approximation agree with cmc values. The radii of hydrated counterions strongly influence their self-diffusion coefficients which below cmc coincide with corresponding values determined for LiCl, NaCl and CsCl solutions (Fig. 2a–c). At the same time the nature of the counterion does not give significant effect on self-diffusion coefficients of surfactants (Fig. 3a–c). They do not differ one from another at c < cmc, showing slight decrease as the result of premicellar surfactant association [14, 31]. At the total surfactant concentrations when the contribution from non-micellized molecules is very low, the self-diffusion coefficients of all species [D +(c) and D −(c)] tend asymptotically to the values presented in Table 2. We used these values to estimate micelle radius a m and the surface self-diffusion coefficients of counterions D +s .
Concentration dependence of effective (measured) self-diffusion coefficients of counterions in LiDS (a), SDS (b) and CsDS (c) solutions. Curves 1 correspond to the results of modeling which includes the surface self-diffusion coefficients of counterions. Curves 2 correspond to the concentration dependences of self-diffusion coefficients of Li+ (a), Na+ (b) and Cs+ (c) in LiCl, NaCl and CsCl solutions
Concentration dependence of effective (measured) self-diffusion coefficients (,) of surfactants in LiDS (a), SDS (b) and CsDS (c) solutions. Curves correspond to the results of modeling [Eq. (3)] which takes into account the coexistence of surfactant monomers and dimers
From the Stokes–Einstein equation it follows that
where k is the Boltzmann constant, T is the absolute temperature and η is the viscosity of medium. Since viscosity of water at 40°C is equal to 0.653 mPa·s, for LiDS and SDS we have estimated the value of micelle radius a m = 2.3 × 10−9 m. For CsDS micelles, the radius is equal to 1.5 × 10−9 m. These values are in satisfactory agreement with many results on micelle radii of ionic surfactants obtained with the help of different methods [32–35].
There are two ways to estimate the surface self-diffusion coefficient of counterions from experimental data. One is the direct use of Eq. (8) with the limit values of self-diffusion coefficients for counterions and a surfactant. Using the values of \( D_{\alpha \to 1}^{ + } \), \( D_{2}^{ - } = D_{\alpha \to 1}^{ - } \) and \( D_{1}^{ + } = D^{ + }_{\alpha \to 0} \) from Table 2 together with the value of β from Table 1, one can obtain the surface self-diffusion coefficients D +s equal to 3.8 × 10−10, 4.6 × 10−10 and 5.9 × 10−10 m2s−1 for Li+, S+ and Cs+, respectively.
Another approach to determine the values of D +s is based on the analysis of self-diffusion data for both the surfactant and counterions using Eqs. (3) and (4) and taking into account the composition of premicellar and micellar solutions which are calculated as described in Sect. 2. The minimization procedure of mean-square deviations of experimental data for concentration dependences of the surfactant and counterions self-diffusion coefficients using Eqs. (3) and (4) gives values for the surface self-coefficients of counterions. The calculations were simplified since micelles practically do not contribute to measures self-diffusion coefficient below cmc whereas monomers and dimers of the surfactant do the same at high total surfactant concentrations. Fitting curves 1 in Figs. 2a–c and 3a–c correspond to D +s values of 2.8 × 10−10, 4.3 × 10−10 and 5.8 × 10−10 m2s−1 for Li+, Na+ and Cs+, respectively. As follows from comparison of the D +s values obtained by two different methods, both methods give very close results for Na+ and Cs+ and a slight difference for Li+.
It should be mentioned that both approaches for determination of surface self-diffusion coefficient of counterions bound to micelles need to use the known value of the degree of counterion binding to micelles, β. It is well known [3] that this parameter is strongly dependent on the method of its determination. As follows from our analysis, a range [D +s , β] exists for which D +s > 0. This range is characterized by the curve shown in Fig. 4. As follows from the results presented in Fig. 4, D +s turns to zero at β < 0.58. The obliteration of the counterions surface diffusion in the analysis of self-diffusion data which is based on the simple two-site exchange model results in systematic lowering of the β value. For example, the use of Eq. (2) gives β = 0.57 ± 0.03 for SDS, which is significantly lower in comparison with the results of other methods [1], the predictions of quasi-chemical theory for micelle formation [3], and our results on ion activity.
Range [D +s , β] satisfying to the solution of Eq. (6) for micellar SDS solution
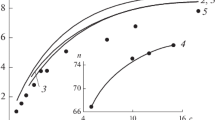
It is interesting to analyze the concentration alterations of different diffusive constituents of the effective self-diffusion coefficient of counterions [Eq. (4)]. Figure 5 depicts experimental data on counterion self-diffusion in SDS solutions (shown as solid circles) and contributions from four terms in the right-hand side of Eq. (4). At low concentration of micelles, the main contribution comes from counterions which appear as a result of dissociation of the non-micellized surfactant (curve 1 in Fig. 5). This contribution decreases with increasing surfactant concentration, giving only about 5% of the contribution to the effective self-diffusion coefficient for 0.1 M SDS. With increasing micellization degree, one can see the growth of contributions from the surface diffusion of counterions about micelle and from the diffusive motion of free counterions, which appear as a result of dissociation of the micellized surfactant (curves 2 and 3 in Fig. 5, respectively). At surfactant concentration of 0.1 M, the total portion of these two constituents in the effective self-diffusion coefficient exceeds 80%. The contribution from counterions which diffuse together with micelles (curve 4 in Fig. 5) is relatively weak due to low mobility of micelles in comparison with mobility of other species. And the total of all four constituents (curve 5 in Fig. 5) agrees rather well with experimental data. Consequently, the effective self-diffusion coefficient of counterions at c ≥ cmc depends on the contributions of all constituents which give different deposits in dependence on the total concentration of surfactant.
4 Conclusions
A model is proposed to explain the patterns of self-diffusion of counterions in micellar solutions of ionic surfactants which integrates additional diffusive processes in comparison with classic two-site exchange model. The estimation of surface diffusion of counterions about the micellar surface is made on the basis of experimental results on the self-diffusion of surfactants and counterions, obtained data on critical micelle concentration, and the degree of counterion binding. The proposed model may be rather useful in the analysis of the ion diffusion, in the studies of electric conductivity in micellar solutions and specific interactions in the dense part of double electric layer of micelles.
References
S.S. Shah, N.U. Jamroz, Q.M. Sharif, Colloids Surf. A: Physicochem. Eng. Asp. 178, 199 (2001)
M. Quesada-Pérez, R. Hidalgo-Álvarez, A. Martín-Molina, Colloid Polym. Sci. 288, 151 (2010)
A.I. Rusanov, Micelle Formation in Surfactant Solutions (Khimiya, St. Petersburg, 1992)
V. Srinivasan, D. Blankschtein, Langmuir 19, 9932 (2003)
E. Ruckenstein, J.A. Beunen, Langmuir 4, 77 (1988)
M.H. Ropers, G. Czichocki, G. Brezesinski, J. Phys. Chem. B 107, 5281 (2003)
O. Söderman, P. Stilbs, W.S. Price, Concepts Magn. Reson. 23A, 121 (2004)
P.C. Griffiths, A.Y.F. Cheung, J.A. Davies, A. Paul, C.N. Tipples, A.L. Winnington, Magn. Reson. Chem. 40, S40 (2002)
P. Stilbs, B. Lindman, J. Phys. Chem. 85, 2587 (1981)
M. Jansson, P. Stilbs, J. Phys. Chem. 89, 4868 (1985)
M. Jansson, P. Stilbs, J. Phys. Chem. 91, 113 (1987)
P. Stilbs, Prog. Nucl. Magn. Reson. Spectrosc. 19, 1 (1987)
O. Söderman, P. Stilbs, Prog. Nucl. Magn. Reson. Spectrosc. 26, 445 (1994)
Yu.F. Zuev, R.H. Kurbanov, B.Z. Idiyatullin, O.G. Us’yarov, Colloid J. 69, 482 (2007)
M. Jansson, B. Jönsson, J. Phys. Chem. 93, 1451 (1989)
A.S. Aleiner, O.G. Us’yarov, Colloid J. 72, 580 (2010)
T. Shikata, S. Imai, Langmuir 14, 6804 (1998)
C. Baar, R. Buchner, W. Kunz, J. Phys. Chem. B 105, 2914 (2001)
P. Fernandez, S. Schrödle, R. Buchner, W. Kunz, Chem. Phys. Chem. 4, 1065 (2003)
R. Buchner, C. Baar, P. Fernandez, S. Schrödle, W. Kunz, J. Mol. Liq. 118, 179 (2005)
O.G. Us’yarov, Colloid J. 69, 102 (2007)
N.O. Mchedlov-Petrosyan, Varied Forces of Organic Acids in True and Order Solutions (Kharkov National University, Kharkov, 2004)
L.Ya. Zakharova, F.G. Valeeva, L.A. Kudryavtseva, Yu.F. Zuev, Russ. J. Phys. Chem. 74, 2006 (2000)
C. Tanford, The Hydrophobic Effect, Formation of Micelles and Biological Membranes (Wiley, New York, 1980)
P. Stilbs, J. Colloid Interface Sci. 87, 385 (1982)
B. Lindman, M.C. Puyal, N. Kamenka, R. Rymden, P. Stilbs, J. Phys. Chem. 88, 5048 (1984)
B.L. Bales, J. Phys. Chem. B 106, 9033 (2002)
E.O. Steiscal, J.E. Tanner, J. Chem. Phys. 42, 288 (1965)
M. Piotto, V. Saudek, V. Sklenar, J. Biomol. NMR 2, 661 (1992)
E.S. Podchasskaya, O.G. Us’yarov, Colloid J. 67, 177 (2005)
O.G. Us’yarov, Colloid J. 66, 684 (2004)
J.B. Hayter, J. Penfold, Colloid Polymer Sci. 261, 1022 (1983)
S.S. Berr, M.J. Coleman, R.M. Jones, J.S. Johnson Jr., J. Phys. Chem. 90, 6492 (1986)
M. Avdeev, V. Garamus, L. Rosta, I. Smirnova, N. Smirnova, Physica B 276, 341 (2000)
N.A. Mazer, G.B. Benedek, M.C. Caray, J. Phys. Chem. 80, 1975 (1976)
Acknowledgments
We thank Prof. K.N. Mihelson for assistance with ion-selective electrodes. This work was supported by the Russian Foundation for Basic Research (grant 08-03-00491) and the grant of Leading Scientific Schools (NSh. 6267.2010.2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gnezdilov, O.I., Zuev, Y.F., Zueva, O.S. et al. Self-Diffusion of Ionic Surfactants and Counterions in Premicellar and Micellar Solutions of Sodium, Lithium and Cesium Dodecyl Sulfates as Studied by NMR-Diffusometry. Appl Magn Reson 40, 91–103 (2011). https://doi.org/10.1007/s00723-010-0185-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-010-0185-1