Abstract
An isochemical kelyphite (orthopyroxene+spinel+plagioclase) that has nearly the same bulk chemical composition as the precursor garnet was found within a matrix of ordinary kelyphites (orthopyroxene+clinopyroxene+spinel±amphibole) in garnet peridotites from the Czech part of the Moldanubian Zone. It was shown that the kelyphitization of garnet took place in three stages: (1) the garnet-olivine reaction, accompanied by a long-range material transfer across the reaction zone, and (2) the isochemical breakdown of garnet, essentially in a chemically-closed system, and finally, (3) an open-system hydration reaction producing a thin hydrous zone (amphibole+spinel+plagioclase), which is located between the isochemical kelyphite and relict garnet. The presence of relict garnet suggests that this breakdown reaction of the second stage did not proceed to a completion probably being hindered by the formation of the hydrous zone at the reaction front. It was found by electron back-scattered diffraction method that orthopyroxene and spinel do not show any topotaxic relationship in the first type of kelyphite; whereas they show locally topotaxic relationship in the isochemical kelyphite. The transition from the first type to the second type of kelyphite is discussed on the basis of the detailed observations in the transition zone between the two kelyphites. More widespread occurrence of isochemical kelyphite is expected to occur in orogenic peridotites as well as from xenoliths brought by volcanics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been widely observed that pyropic garnet is partly or completely decomposed into fine-grained symplectitic mineral intergrowths called ‘kelyphite’ when deep-sheeted rocks are decompressed either by a tectonic emplacement of rock masses accompanied by exhumation or by a more rapid transport of rock fragments to the Earth’s surface by volcanic eruptions (i.e., xenoliths). Many kelyphites are rarely isochemical with the precursor garnets and have been typically and significantly modified from their original garnet compositions, implying that kelyphite is not a simple breakdown product of garnet but a reaction product between garnet and its surroundings such as olivine (e.g., Obata 2011). ‘Isochemical kelyphite’ on the other hand have been reported to occur mostly from xenolithic garnet pyroxenites or mafic granulites, such as those in nephelinitic breccia, Delegate, Australia (Keankeo et al. 2000), in basanitic breccia, Sicily (Sapienza et al. 2001) and those in alkali basalt and basaltic tuffs, Central Pannonian Basin, Hungary (Degi et al. 2010). We report in this paper a first clear occurrence of isochemical kelyphite, surrounded by an ordinary non-isochemical kelyphite from orogenic garnet peridotites that occur in the Czech part of the Moldanubian Zone. We demonstrate that the isochemical breakdown of garnet took place after the formation of a non-isochemical kelyphite. The detailed study of the kelyphitization processes, together with the analysis of their physical conditions of formation will put important constraints on the exhumation history of the host peridotites and the reaction kinetics.
Geological background
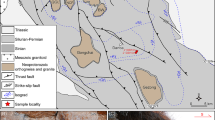
The Moldanubian Zone is a southern part of the European Variscan orogen which was active at around 340 Ma. Its metamorphic core complex is well exposed in the Bohemian Massif (Fig. 1). The Moldanubian Zone in the Bohemian Massif consists of three metamorphic units: the Monotonous Unit, the Varied Unit and the Gföhl Unit, in the structurally ascending order, where metamorphic grade increases in this order. The main rock types of the Gföhl Unit are garnet-kyanite-bearing orthogneisse of peraluminous granitic composition, subordinate amounts of pyroxene-bearing mafic granulite, garnet amphibolite, and calc-silicate rocks. Numerous small masses of garnet peridotite occur sporadically both in the granulite and migmatitic gneisses at many localities of the Gföhl Unit and they have been classified into three types by Medaris et al. (2005): Type I, transformed from high-temperature spinel peridotite to garnet peridotite, which is considered to be derived from “asthenospheric” mantle; Type II with Fe-Ti- rich compositions and therefore considered to represent disrupted fragments of a mafic-ultramafic cumulate complex; and Type III, equilibrated in a medium P/T regime and considered to represent fragments of a deep mantle wedge.
a Geotectonic subdivision of the Variscan orogen in central Europe and b simplified geologic map of the studied area showing the sample locality. P, Prahatice massif; K, Kristanov massif; BL, Blansky les massif; L, Lisov massif. The studied peridotite is from the Plešovice quarry (indicated by a star) within Blansky les massif. Modified from Naemura et al. (2009)
The studied sample is a garnet-spinel-peridotite taken from a boudinage mass (5 m × 10 m in size) of peridotite enclosed in a well-foliated felsic granulite in the Plešovice quarry, which is located at the eastern edge of the Blansky les granulite massif (of the Gföhl Unit)(Fig. 1). It is considered to belong to Type I of Medaris on the basis of a ubiquitous presence of spinel inclusions in garnet (Naemura et al. 2009). It was shown that the garnet peridotite was once equilibrated at 2.3–3.5 GPa and 850–1030 °C in the upper mantle and was then exhumed to the lower crustal level and got partially re-equilibrated in the spinel-lherzolites facies (Naemura et al. 2009). The host granulite records a peak metamorphism at around 750–850 °C and 1.6–1.8 GPa (Stipska and Powell 2005).
Observational methods and analytical techniques
Microstructural observation of kelyphites was made using a field-emission electron gun scanning microscope (FE-SEM; JEOL 7000F) at the University of Tokyo. X-ray compositional mapping and electron microprobe analysis were made using a wave-length dispersive electron microprobe (WDS) both at the Univ. Tokyo and Kyoto University. Quantitative analyses of minerals were made at Kyoto Univ. using WDS microprobe (EPMA). Acceleration voltage of 15KV and beam current of 20 nA were used throughout. For mineral analysis a focused electron beam (of ca. 3 μm size) was used and for bulk analysis of kelyphites, a defocused electron beam (5 to 10 μm size) was used and many analyses (typically 20 spot analyses) were averaged. A combination of both synthetic and natural minerals was used as standards and ZAF correction was utilized. Crystallographic orientation of minerals in the kelyphites was determined using an electron backscattered diffraction system (EBSD) attached to the FE-SEM (HKL Nordlys detector with Channel 5) at the Univ. Tokyo. The transmission electron microscopic work (TEM) was made both at Kyoto University and Kobe University. The thin foil preparation for the TEM observation was made using a focused ion beam (FIB: FEI Quanta 200 3DS) at Kyoto University and was observed with TEMs: H-8000 k (Hitachi) at Kyoto University and JEM2010 (JEOL co.) with NORAN System SIX (Thermo Fisher Scientifc Inc.) at Kobe University.
Petrographic details of kelyphites
The studied sample is a garnet-spinel peridotite of harzburgitic composition that is comprised of olivine, orthopyroxene (Opx), clinopyroxene (Cpx), and garnet (Grt) and spinel (Sp). Garnet typically forms large grains and is always, partially or completely, kelyphitized. Olivine is moderately serpentinized but the kelyphite is, for most part, free from secondary alteration except along rare fractures and/or veins. The majority part of the kelyphite is a fine-grained, radial and fibrous intergrowth that consists of Opx, Cpx, and spinel with subordinate amounts of Ca-amphibole (Amp). This type of kelyphite is referred to as ‘kelyphite I’ hereafter (Fig. 2a). As is typical of this type, the microstructure of kelyphite I is characterized by the occurrence of small elongated patches of Cpx enclosed in much larger crystals of Opx, both of which include many regularly-spaced thin vermicular lamellae of spinel defining a local lineament (Obata 2011; Fig. 4a, b, d). Spinel lamella are scarce or absent in amphibole crystals in kelyphite I. It is noted that the scale of kelyphite I (i.e., the size of Cpx patches and the spacing of the spinel lamella) rapidly decreases, to that of kelyphite II, in the vicinity of garnet and kelyphite II (Fig. 4b). In a more macroscopic scale, kelyphite I is surrounded or enveloped by a narrow rim or ‘moat’ (0.2–0.5 mm wide) that consists of polycrystalline coarse-grained Opx (grain size ca. 0.2 mm; abbreviated as ‘COR’, standing for ‘Coarse Opx Rim’) separating the fine-grained kelyphite inside from the olivine-dominant matrix outside, which is also typical of this type of kelyphite (Fig. 2a; Obata and Ozawa 2011; Obata 2011).
a Optical photomicrograph (plane polarized light) of a garnet peridotite, sample PL4, that contains three kinds of kelyphites, I, II and III (abbreviated as Kely I, II and III, respectively). Frame (α) is the area of Fig. 3 and that of (β) is for Fig. 4a. b Optical photomicrograph with crossed polarized light and a gypsum plate to emphasize the domain structure of kelyphites, which is recognized with the lineament structure and the interference color. Central part of the area of Fig. 2a. Points (b), (c) and (d) in kelyphite II indicate positions where crystallographic orientation data plotted in Fig. 5 (b, c, d) were obtained
Within a mass of kelyphite I, there occasionally occurs a region of much finer-grained and darker-colored (in transmitted light) than kelyphite I (Fig. 2a, b). It was found by X-ray mapping with an electron microprobe analyzer that this finer-grained part has nearly the same bulk composition as the adjacent garnet (Fig. 3); and this new, extremely fine-grained, isochemical kelyphite is referred to as ‘kelyphite II’. The kelyphite II part has relatively low birefringence (under cross-polarized light) and has typically a domain structure as recognized by the slight differences in interference colors and different extinction angles in cross-polarized light (Fig. 2b) and distinct lineament structures as described below. The boundary between kelyphite I and II is not necessarily sharp and a narrow transition zone may be defined according to the texture and mineralogy, which will be described in more details below. Very rarely relict garnets occur in association with kelyphite II as well as kelyphite I. A peculiar textural feature of kelyphite II is that it is developed only on one side of garnet (Fig. 2a), so that, unlike kelyphite I, kelyphite II does not form a corona structure surrounding the garnet. Examination of many thin sections from this locality revealed that such asymmetric (i.e., non-concentric) configuration is universal wherever kelyphite II and relict garnet occur together and we refer to such textural feature as the ‘asymmetric nature’ of kelyphite II.
X-ray compositional maps (Al, Ca, Mg, Fe) of the kelyphites and their surroundings. Area (α) in Fig. 2a. Warmer colors represent higher contents of respective elements. Kelyphite II is nearly isochemical with garnet except along fracture veins (“f” in Fig. 4a), where slight depletion in Ca and enrichment in Mg and Na (not shown here) are noted. Note that kelyphite III is not isochemical
The kelyphite II has a different mineral assemblage than the kelyphite I, which is Opx+Sp+plagioclase (Pl) (An99). (It is so fine-grained that phase identification was made with the aid of combinations of EBSD and EDS attached to a FE-SEM.) Although kelyphite II is mostly anhydrous, small amounts of amphibole occur in addition to Opx, Sp and plagioclase at some margins of kelyphite II. Each domain of kelyphite II is characterized by a very straight lineament, which is more straight than those in kelyphite I (Figs. 2b and 4b). The lineament consists of fine intergrowths of laths of Opx and plagioclase, where spinel occurs as discontinuous thin lamellae (less than 1 μm thick) exclusively within the Opx laths (Fig. 4c). The Opx-Pl spacing is only a few microns, and accurate microprobe analysis of these phases was not possible in kelyphite II. Sporadically, however, there are spots of large granular grains of spinel and Opx, where microprobe analysis was possible (Fig. 4c).
a Back scattered electron images (BSE) of the kelyphites and their surroundings (area β in Fig. 2a). Abbreviations are the same as in Fig. 2. ‘Trans’ is a transition zone between kelyphites I and II; ‘f’, fracture veins that are either void or filled with unidentified hydrous minerals. Frames (b), (c), (d) and (e) indicate the areas of the following photographs: b Kelyphites I, II and III. Domains in kelyphite II are labeled 1, 2 and 3 and the domain boundaries and transition zone are indicated by white lines. Black areas are either voids or unidentified hydrous phases of probable secondary origin. c coarse grained Opx and spinel in kelyphite II as indicated by an arrow; d A close up of the transition zone, between kelyphite I (Opx+Cpx+Sp±Amp: Zone A) and kelyphite II (Opx+Sp+Pl: Zone D). The transition zone may be subdivided into two subzones, B (Opx+Sp+Amp) and C (Opx+Sp+Pl+Amp). The domain boundary in kelyphite II is indicated by white dashed lines. The domains are labeled 1, 2, 3 as in (b). Frame (c) is the area of photograph 4(c). The area where gentle curvatures occur in the lineament is indicated with a white dotted circle near the outer margin of kelyphite II (domain 3) (see text for more details). e Transition subzones B and C, and kelyphites I, II and III around garnet. In the upper half of the photograph, a transition subzone B is directly juxtaposed to kelyphite II (Zone D) without subzone C, where internal structure of Zone B is sharply truncated by kelyphite II. In the lower-half, the same zone B is directly juxtaposed to the garnet, without Zone C in between. Note a narrow zone of kelyphite III developed between kelyphite II and garnet. Kelyphite III is a vermicular intergrowth of amphibole (gray), thin numerous spinel lamellae (lighter colored) and subordinate amount of plagioclase (the darkest) nearly perpendicular to the garnet surface. ‘f’ is fracture vein (See (a) for explanation). A white circle marked ‘s’ in an Opx–spinel symplectite in Zone B indicates the area where bulk chemical analysis (Table 2, No. 11) was obtained
A transition zone of up to 100 μm width may be defined between the kelyphite I and kelyphite II (Fig. 4a) and this zone may be further subdivided into two subzones, according to their characteristic mineral assemblages, B and C (Fig. 4d). The outer zone of the transition zone, B, consists of Opx, Sp and amphibole and is composed of two structural components in alternation: (a) fine symplectitic intergrowth of Opx and Sp, and (b) coarser spinel-lamella-sparse amphibole (pargasite; of a few micron width, Fig. 4d). Texturally, the Opx-spinel intergrowths appear to be continuous from those in the adjacent kelyphite I (Zone A) to Zone B and even to Zone C (Fig. 4d). Moving from kelyphite I toward kelyphite II, the extinction of Cpx defines the beginning of transition zone B. The appearance of plagioclase defines the beginning of subzone C in the transition zone, where Opx, spinel, plagioclase and amphibole coexist. The microstructure in this zone is more erratic without any clear lineament in contrast to the adjacent kelyphite II (Zone D). Plagioclase, a characteristic phase in Zone C, forms discrete round ‘pools’ of up to 10 μm size, and thus microprobe analyses of plagioclase, and amphibole too, were barely possible in this zone. The transition to kelyphite II is defined by the emergence of a clear lineament of the symplectitic structure and the grain size reduction. A small amount of amphibole remains in the margins (up to tens of microns width) of kelyphite II. The proportion of Zone B to C varies greatly in the transition zone. On a left-side boundary (Fig. 4d) Zone B is thinner than Zone C and is even locally missing, where kelyphite I is in direct contact with Zone C; whereas at a right-side corner adjacent to garnet (Fig. 4e), Zone B is widely developed and is in direct contact with kelyphite II or relict garnet without Zone C, where the lineament in Zone B is sharply truncated by that of kelyphite II. It is noted that Zone B, although narrow, partly envelopes the garnet, where kelyphite II is not developed (Fig. 4a, e), implying that Zone B may be regarded as a genetic extension of kelyphite I. It is also noted that the lamella spacing of Zone-B kelyphite becomes rapidly reduced, as in kelyphite I, approaching the garnet (Fig. 4e).
Facing to the garnet, kelyphite II is separated from the relict garnet by a narrow zone of another kind of symplectite (10 to 20 μm width) that consists of amphibole, spinel and subordinate amounts of plagioclase; this boundary zone will be referred to as ‘kelyphite III’ in this paper (Figs. 1 and 4a, b, e). (The phase identification in kelyphite III was confirmed with TEM in addition to the EBSD and EDS analyses.) Although there occurs locally a slight kink in direction in the spinel lamellae across the kelyphite II and III boundary (Fig. 4e), the lineament is on average nearly normal to the garnet grain boundary as the kelyphite I against garnet. Chemically, kelyphite III is not isochemical to garnet and contains more Ca and Na (and therefore, less Mg and Fe) than the garnet (and thus kelyphite II) as described below (Fig. 3; Table 1).
Crystallographic orientation relationships
The EBSD analysis of the kelyphites revealed that the kelyphite I has the same features as observed for the lower-temperature group of kelyphite as described in Obata and Ozawa (2011). That is, kelyphite I consists of multiple domains of single crystal of Opx and, within each Opx domain, Cpx patches have all the same crystallographic orientation and show a common systematic crystallographic relationship with their host Opx by sharing their (100) and (010) and [001]. Amphibole, wherever it occurs, also show the same crystallographic relationship with Cpx and Opx by sharing their (100) and (010) and [001]. (Such systematic crystallographic relationship is referred to as ‘topotaxy’ according to the suggestion of IMA: Bailey et al. 1977). Spinel, on the other hand, does not show any systematic relationship with Opx or Cpx, just similar to the garnet peridotite from Norwegian Caledonide, whose kelyphitization temperature was estimated to be 700~760 °C (Obata and Ozawa 2011)
The topotaxic relationship is, however, more complicated and variable in kelyphite II in the same sample. It was revealed that, within each domain of kelyphite II, crystallographic axes of the Opx is nearly constant in direction (except the coarse granular Opx grains, Fig. 4c) with some scatter, up to 15º in the Euler angle, which indicates that the Opx crystal laths are probably all crystallographically connected to each other in the three dimensions within the same domain forming a large (and spongy) single crystal of Opx. It is revealed, therefore, that the interference color observed optically (Fig. 2b) represents essentially that of Opx. It is also noted that the elongation of each Opx crystal lath or lamella is not related to any simple crystallographic direction or crystallographic axis of Opx. This morphological independency from crystallographic axes is more evident where curvature occurs in the Opx laths assembly within the same domain, where crystallographic orientation of Opx (as examined by EBSD) is remarkably constant regardless of the curvature, indicating that the curvature is primary in origin and not due to secondary deformation. Across domain boundaries, however, the lineament changes abruptly and discontinuously and so does the crystallographic orientation of the Opx, thereby defining the domain boundary (Fig. 4b).
The crystallographic orientation of the spinel is more erratic and variable even within the same domain of kelyphite II and so is the crystallographic relationship between the spinel and the host Opx. There is a clear tendency that, well inside an Opx domain (such as around point (c) in Fig. 2b), spinel tends to have the same topotaxic relationship with the Opx as is observed in the ‘higher-temperature’ kelyphites (i.e., Czech Mohelno peridotite: Obata and Ozawa 2011) or pyroxene-spinel symplectites from the Horoman peridotite (Odashima et al. 2008); that is, one of four equivalent {111} of spinel coincides with pyroxene (100) and one of six equivalent {110} of spinel coincides with pyroxene (010) (Fig. 5 (c)). Near the outer margin of kelyphite II and also close to the garnet (i.e., kelyphite III) within the same domain of Opx, however, such topotaxic relationship is broken and spinel shows more scattered and more random pattern of orientations (Fig. 2b, points (b) and (d); Fig. 5 (b), (d)). Such changes of crystallographic relationship seem to be gradational from one to the other within the same domain although the crystallographic orientation of the host Opx is constant (Fig. 5(a)).
Pole figure plots of Opx and spinel from domain 3 of kelyphite II. a Opx; b spinels from outer margin of the domain; c spinels from the central part of the domain; d spinels from deep inner part close to kelyphite III. Approximate areas where these data were obtained are indicated as (b), (c) and (d), respectively, in Fig. 2b. Small circles in (c) represent approximate areas of Opx (100) and (010), respectively, defined by the Opx data in (a)
In kelyphite III, the amphiboles have nearly the same crystallographic orientation with the adjacent Opx, shearing their (100) and (010) and [001] axis (compare Fig. 6(a) with Fig. 5(a)); whereas, spinel shows a random pattern as is observed in the immediate adjacency in kelyphite II (Fig. 6(b)).
Pole figure plots for amphibole (a) and spinel (b) from kelyphite III adjacent to kelyphite II domain 3. Note that the orientation of amphibole is nearly coincidental with that of Opx in the adjacent kelyphite II (Fig. 5(a))
Mineral and bulk chemical compositions of kelyphites
Bulk compositions of the three kinds of kelyphites, I, II, and III, were obtained with EPMA using a defocused electron beam and the results are listed and compared with the garnet composition in Table 1; other microprobe analyses of minerals are listed in Table 2. It was confirmed that kelyphite II is nearly isochemical, at least in term of major elements, with the adjacent (i.e., precursor) garnet. In more details, however, kelyphite II contains slightly more Na (0.04 Na2O wt%) than the garnet. Amphibole bearing margins and the vicinity of the fracture veins cutting across the kelyphite (‘f’ in Fig. 4a), where slight depletion of Ca and enrichment of Na are recognized (Fig. 3), were avoided in the bulk analyses of kelyphite II. The garnet is homogenous except near the grain edges facing to kelyphite I (within a few tens of microns from the boundary), where a significant rim-ward increase in the Fe-Mg ratio is noted (Table 2). Such zoning does not occur at the edges facing to kelyphite II (or, more precisely, kelyphite III). Kelyphite I, on the other hand, is significantly higher in MgO and lower in Al2O3 in bulk contents than the garnet, which is typical for this type of kelyphite (Obata 2011). Kelyphite III is distinct from kelyphite II in that it has higher CaO and Na2O contents (Table 2). It is noted that transition subzones, both B and C, are not isochemical to garnet but their heterogeneities hindered us to obtain good average compositions even using a defocused electron beam. Plagioclase in Zone C is very calcic; its anorthite content being 99 mol%. Although accurate analysis was not possible for the plagiolcase in kelyphite II, semi-quantitative analysis using EDS attached to FE-SEM revealed that the plagioclase in kelyphite II is highly calcic as in Zone C. The amphibole in transition subzone B and kelyphite III is variable but all quite aluminous (13.3 to 17.5 in Al2O3 wt.%) and sodic, being pargasitic (Table 2). The Al2O3 content of Opx is 3.7 wt.% in kelyphite I and that of kelyphite II (the large grains) is 4.0 wt.%. The composition of spinel in kelyphite III is inferred by semi-quantitative analysis using EDS attached to TEM to be: Cr# (Cr/(Cr+Al) in mole) = 0.05~0.16 and Mg# (Mg/(Mg+Fe) in mole) = 0.61–0.73, which covers the compositional range of that in kelyphite II (Table 2). The plagioclase in kelyphite III is more sodic than those in kelyphite II, being in the range of An80–90. The bulk analysis obtained for the Opx-spinel symplectite in Zone B (marked ‘s’ with a white circle in Fig. 4e) using a defocused electron beam (of 5 μm diameter) (No. 11, Table 2) may satisfactorily be modeled in terms of a mechanical mixture of Opx and spinel, of 3:7 in weight (equivalent to 1:2 in volume), using the microprobe analyses of respective phases in kelyphite II (i.e., Nos. 4 and 6, Table 2).
Discussion
A sequence of kelyphitization processes
It is inferred from textural relationships that kelyphite I first formed and then kelyphite II, and finally terminated with the formation of kelyphite III. The question to be raised here is how these events are related in physical conditions and in time, and what the critical factors that controlled the kelyphitization processes were. The key to understand the mode of transition in the formation of kelyphite I to kelyphite II may be contained in the transition zone.
It is conceivable that kelyphite I was formed, when garnet peridotite was subjected to a decompression and brought into the spinel-lherzolite stability field, by a well-studied reaction between garnet and olivine (e.g., Kushiro and Yoder 1966) :
The temperature at which this reaction took place has been estimated to be 730–770 °C by employing the two-pyroxene geothermometer of Taylor (1998) to the microprobe analyses of pyroxenes in kelyphite I (Naemura et al. 2009). Note that garnet itself can still be stable as a single phase although the garnet became thermodynamically incompatible with olivine at this stage. The inner, fine-grained symplectite (i.e., kelyphite I) is interpreted to be after garnet, whereas the outer, coarse Opx rim (COR) be after olivine (Obata and Ozawa 2011; Obata 2011). These replacement reactions for garnet and olivine, respectively, are both open-system reactions and there must have been significant long-range material transfer across the reaction zone between the two reaction fronts (Obata 2011). The presence of the remnant garnet (i.e., kelyphite II in this case) implies that reaction (1) did not proceed to completion but ceased at some point. It is conceivable that the reaction had slowed down as the reaction zone widened probably because of the decrease of the chemical potential gradients in the reaction zone. A progressive increase of the internal stress due to the volume-increase nature of the reaction (Obata 2011) may also be a factor in decelerating the process (Milke et al. 2009; Schmid et al. 2009), and thereby punctuating the formation stage of kelyphite I.
Upon further ascent and decompression in the rocks, garnet itself will eventually become unstable, and beyond some critical level of decompression, the remaining garnet will start to break down by itself as follows:
The proportion of the product phases, Opx, Sp, and Pl, in the studied kelyphite is calculated to be 52:16: 32 (in wt%), from a mass balance calculation using mineral microprobe analyses and the bulk composition of the kelyphite (Table 2). A relevant reaction that may define the lower stability limit of garnet in mafic systems would be:
whose equilibrium position has been experimentally determined in the CaO-MgO-Al2O3-SiO2 system (Kushiro and Yoder 1966; Gasparik 1984; Fig. 7). In their model, the garnet composition was assumed to be Py2Gr1 (i.e., Ca/(Ca+Mg) = 0.333 in mole). The garnet studied here is less calcic than this and its Ca/(Ca+Mg+Fe) value is 0.17 (in mole), which may account for the absence of Cpx in the assemblage of the studied kelyphite II. Theoretically, such less-calcic garnet can become unstable at higher pressures than that of the equilibrium position of reaction (3). It should be noted here that reaction (2) must have taken place irreversibly far from equilibrium because it does not appear to have been accompanied by a diffusion-aided compositional adjustment of garnet (to an equilibrium composition). The pressure at which the irreversible reaction (2) actually took place, therefore, is indeterminable; it could be higher (but below the pressure of reaction (1))(Point 2 in Fig. 7) or lower than the equilibrium pressure of reaction (3)(Point 2’ in Fig. 7). If the former were the case, i.e., kelyphite II formed at relatively higher pressure (Point 2 in Fig. 7), the formation of kelyphite II may not have been significantly separated in time from the kelyphite I formation and the kelyphitization processes, from I to II, may have been a single continuous event. If the latter were the case, there must have been a considerable time interval between the two events of kelyphitization in order for a considerable cooling (and decompression) is allowed to occur (from Point 1 to Point 2’, Fig. 7). In either case it is important to note that the isochemical breakdown of garnet started to occur at some points on the grain boundaries, not inside the crystal, and the kelyphite grew inward replacing the garnet, forming a sharp and migrating reaction front.
Inferred relative timing of the formation of kelyphites I and II marked on a P-T path for the Plešovice peridotite (modified from Naemura et al. 2009). Reaction boundaries (1) for Ol+Grt=Opx+Cpx+Sp; (3) for Grt=Opx+Cpx+Sp+An; and (5) for Ol+An=Opx+Cpx+Sp, are theoretical calculations for the system CaO-MgO-Al2O3-SiO2, compliled by Gasparik (1984) and Green et al. (2012). Boundary (1’) is that for the garnet lherzolite to spinel lherzolite transition experimentally determined for a Cr-bearing natural lherzolite, after O’Hara et al. (1971). Kelyphite I formed at Point 1 in the ariegite-facies field. Two possibilities are indicated for the kelyphite II formation: Point 2 at slightly higher pressure than equilibrium position (3) (thus in the ariegite-facies field but below Point 1) or; Point 2’, which is plotted at lower pressures than reaction (3) in the seiland-facies field (see text for more details). GL, garnet-lherzolite facies; Ar, ariegite subfacies of spinel-lherzolite facies; Se, seiland subfacies of spinel-lherzolite facies; PL, plagioclase-lherzolite facies (O’Hara 1967)
A question then arises as to why reaction (2) did not proceed to a completion, thereby eliminating all the garnet. The argument of volume-increase and stress building-up as made for kelyphite I above may also be applicable here. Unlike reaction (1), reaction (2) does not require a long-range material transfer and may be regarded as a kind of polymorphic transition in the sense that long-range element diffusion is not required. The situation may be analogous to the occurrence of metastable coesite surrounded by polycrystalline quartz (the ‘palisade quartz’) that occurs within a garnet crystal (Chopin 1984). The reaction is said to have ceased because of the elevation of internal stress brought about by the progress of the volume-increase, coesite-to-quartz transformation reaction in a restricted volume within the garnet host (Gillet et al. 1984). Unlike the ‘palisade’ quartz, however, kelyphite II is typically developed only at one side of garnet—the “asymmetry”. (The origin of the asymmetry is discussed below.) Moreover, the relict garnet is sheltered from kelyphite II by another thin film of hydrous symplectite—kelyphite III. Such occurrence implies that the formation of kelyphite III may have played some role for the cessation of reaction (2). As opposed to reaction (2), however, the formation of kelyphite III, although the reaction has not been identified yet, must have been accompanied by a significant long-range material transfer, as well as the introduction of water. The fracture veins running through the kelyphite II region (Fig. 4a) might represent relicts of such a fluid pathway. The observed geochemical anomalies around the vein may be the result of fluid-rock interaction. Admittedly it sounds paradoxical that an introduction of fluid leads to a cessation of the breakdown reaction of garnet. More detailed processes and exact mechanisms of such a hypothetical hydration reaction are the subject for future research.
Origin of the transition zone
Considering the microstructural continuity, subzone B of the transition zone is thought to represent a very late stage of kelyphite I. It is conceivable that, as temperature decreases during the advance of kelyphitization, amphibole replaces clinopyroxene if the activity of H2O is sufficiently high, where reaction (1) switches to another, hydration reaction as,
This inference is consistent with the local and incipient development of Zone B partially enveloping the garnet crystal where kelyphite II is not developed (Fig. 4a, e). On the other hand, subzone C may represent the onset of reaction (2), where a nucleation of a new phase plagioclase had occurred. Opx, spinel and amphibole, on the other hand, did not have to nucleate and probably simply continued to grow from subzone B on the expense of garnet, thereby maintaining the microstructural continuity as observed. At very early stages of reaction (2), the system may not have been completely closed and there may have been some chemical interactions via material transfer with the surroundings probably with the aid of intergranular fluids. The presence of amphibole and the coarser-grained nature of subzone C implies the presence of some intergranular aqueous fluids at this stage, which may have promoted some long-range material transfer and grain coarsening and thus causing a local deviation from the original garnet composition. As subzone C grows replacing garnet, the limited amount of water will be consumed by the formation of amphiboles and the system will become desiccated and closed chemically, grading rapidly into the anhydrous, isochemical kelyphite – kelyphite II.
Another possibility to be considered is that of the secondary origin for the transition zone. The transition zone, particularly subzone C, might represent a secondary alteration zone that was developed around a primary interface between the kelyphite I and kelyphite II zones. Such a locally-selective secondary recrystallization could have occurred if some aqueous fluid was introduced along the primary zone boundary. Lack of lateral continuity of subzone B may be ascribed to such a secondary modification but the microstructural continuity observed across the transition zone is not favorable to this hypothesis.
Considerations on reaction kinetics and the origin of topotaxy
A comparison with other occurrences of isochemical kelyphite reported in literature would give some insights to reaction kinetics for the garnet breakdown. The isochemical kelyphite from the Delegate garnet pyroxenite xenoliths consists of clinoferrosilite, anorthite and spinel (Keankeo et al. 2000). The clinoferrosilite is said to be after protoenstatite, which implies considerably high temperatures for the isochemical breakdown of garnet, being definitely above 1000 °C. The fine-grained nature of the Delegate samples was ascribed to a rapid growth of kelyphite probably resulting from a rapid ascent and rapid decompression of the xenolith brought up by volcanics (Keankeo et al. 2000). The fine-grained nature of the studied Czech sample, however, cannot be ascribed to a rapid ascent like the xenoliths but rather has to be ascribed to a rapid growth, which is not necessarily related with the ascent rate. It should be noted that the first kelyphitization (kelyphite I) took place at considerably lower temperatures (< 800 °C) for the Czech sample than the Delegate xenoliths. The temperature of the second kelyphitization (kelyphite II) cannot be determined precisely because of the lack of an appropriate geothermometer for this mineral assemblage, but it is likely to be below that of kelyphite I considering the inferred P-T history and the geotectonic setting (Naemura et al. 2009; Fig. 7). The inferred rapid growth of kelyphite II, therefore, must be ascribed to a high degree of super-saturation (i.e., far from equilbrium) and a strong irreversibility of the reaction, which may become possible at relatively low temperatures as discussed for kelyphite I by Obata and Ozawa (2011).
The partial topotaxy observed in kelyphite II is puzzling. It was suggested that Zone C marks the beginning of kelyphite II, where the nucleation of plagioclase took place. It is natural that non-topotaxic relationship between Opx and spinel in kelyphite I is simply inherited to the transition zone and further to the kelyphite II. Observation indicates that spinel gradually gained the topotaxic relationship with its host Opx within the kelyphite II as the kelyphitization advanced. It is puzzling then why spinel gained topotaxic relationship with Opx at such low temperatures, while such relationship is not realized in kelyphite I at earlier stages. The answer to this question may lie in the fact that the degree of supersaturation for reaction (2) may have been smaller than that of reaction (1) because the equilibrium position of the former lies at considerably lower pressures than the latter (Fig. 7). The loss of topotaxic relationship as is seen closer to the garnet, i.e., at later stages, may be ascribed to a further decrease in temperature.
Obata and Ozawa (2011) interpreted the presence or absence of topotaxic relationship between Opx and spinel (for kelyphite I) in terms of the degree of supersaturation of the transformation reactions, which may further be related to the transformation temperature (or more accurately, the temperature of nucleation), for the garnet peridotite to spinel peridotite transition. They demonstrated that kelyphite I may be classified into two categories: the high-temperature type, where topotaxy is perfect and the low-temperature type, where topotaxy is imperfect or none. The lack of topotaxic relationship in kelyphite I of the studied sample indicates that this kelyphite belongs to the ‘low-temperature type’ of Obata and Ozawa (2011), which is consistent with the inferred P–T path (Naemura et al. 2009) and the inferred kelyphite-I formation temperature, 730–770 °C. Considering all these occurrences and observations, it appears that the boundary between the high- and low-temperature kelyphites lies somewhere between 850 °C (for the Czech Mohelno peridotite, Obata and Ozawa 2011) on one hand and 740–760 °C (for the Norwegian peridotite, Obata and Ozawa 2011) or 730–770 °C for the present Czech sample, on the other, and therefore, we tentatively set the boundary temperature at 800 ± 50 °C (according to the Taylor’s pyroxene geothermometric calibration). It should be noted that this criterion, however, may not apply to kelyphite II, which is a product of a different reaction, as emphasized above.
Origin of the asymmetry of kelyphite II
An important morphological feature of kelyphite II in the Czech sample is that, as emphasized above, it does not form a corona enveloping garnet like ordinary kelyphites in garnet peridotites or eclogites (e.g., Obata 2011) but it only occurs on one side of garnet. Other isochemical kelyphites reported in the literature (Keankeo et al. 2000; Sapienza et al. 2001; Degi et al. 2010), however, occur surrounding relict garnets and so the asymmetric configuration seems to be a unique feature of the Czech sample. The corona structure indicates that the breakdown of garnet occurs from outside of garnet, and more importantly, being initiated by multiple nucleation scattered around the garnet grain surface (see Fig. 20 in Obata 2011). Domain structures typically observed in those kelyphites (Obata and Ozawa 2011) is considered to be a result of such a multiple nucleation. If nucleation is restricted for some reason or another to one side of the garnet, the symmetry breaks down, resulting in a “unidirectional growth” of symplectite as observed for the spinel-pyroxene symplectite from the Horoman peridotite (Odashima et al. 2008). Odashima et al. (2008) argued that the first nucleation would suppress further nucleation because of the high kinetic barrier and high degree of supersaturation of reaction (1). A similar account may be applied to the unidirectional growth of the Czech kelyphite II but, unlike the Horoman sample, kelyphite II is much finer-grained and of lower temperature in origin, and furthermore, the reaction responsible for the formation is different, being reaction (2). Moreover, it is difficult to explain why unidirectional growth occurred only in kelyphite II and not in kelyphite I.
We interpret that the unidirectional growth of kelyphite II is the result of suppression of nucleation as advocated by Odashima et al. (2008) but consider the reason for the suppression being different. It is noted that the volume increase for reaction (2) is greater than that for reaction (1). (ca. 5 % for reaction (1); whereas ca. 15 % for reaction (2) relative to garnet; Obata 2011). So the effect of internal stress is considered to be even greater for the latter than for the former. It is conceivable, therefore, that the initiation of a breakdown of garnet from one side, by reaction (2), will build up a significant internal stress around the garnet, which will suppress further nucleation on other sites. If, however, there were enough time for the built-up stress to relax by deformation of the surroundings, either plastic or brittle deformation, more nucleation may have occurred at other sites, as is the case for kelyphite I (and probably for other isochemical kelyphites in the xenoliths). The observed asymmetric nature of kelyphite II, therefore, implies that the process was so rapid that there was no time allowed for the stress relaxation. This view is consistent with the very fine-grained and dry nature of kelyphite II. The site of the first nucleation may be dictated by some other factors such as fluid infiltration or deformational effects (Odashima, et al. 2008).
Other possible occurrences of isochemical kelyphite
As mentioned in the Introduction, isochemical kelyphite is not uncommon in xenolithic garnet pyroxenites or mafic granulites that lack olivine as a reactant counterpart of garnet (Keankeo et al. 2000; Sapienza et al. 2001; Degi et al. 2010). From orogenic peridotites, one occurrence of “isochemical kelyphite” has been reported in the literature from an orogenic garnet peridotite of Vosges Mts. (France) that also belongs to the Moldanubian zone (Altherr and Kalt 1996). The lack of detailed description, however, hinders a judgment of whether it really belongs to the same type as we describe here. Although some analyses are said to be “approximately isochemical”, the presence of amphibole in the assemblage and a significant amount of Na in the bulk analysis (1.12 %, Table 6 in Altherr and Kalt 1996) casts a doubt if it does. Regardless of such uncertainties, we think that there is a good chance that true isochemical kelyphites may be found from those rocks. It may be, to some extent, a matter of chance to encounter isochemical kelyphites in randomly-cut rock thin sections and so, we expect to find more isochemical kelyphites from other localities of orogenic peridotite as well as pyroxenite xenolithes. In fact we are finding isochemical kelyphites from a well-studied orogenic peridotite—the Ronda peridotite, Spain (Obata 1980) that has the same features as described above including the asymmetry (work in progress). They are so fine grained that they would be easily overlooked without careful examinations using X-ray compositional mapping and high-resolution electron microscopy. There must also be cases, however, that such isochemical breakdown did not take place at all and that the kelyphitization ceased entirely at the first stage (i.e., formation of kelyphite I). More detailed comparative studies of both cases would be required in order to identify the key factors that control the kelyphitization processes, particularly that of isochemical breakdown of garnet, in terms of P-T paths, cooling and/or ascent rates and availability of fluids in various geotectonic settings.
Summary and concluding remarks
Isochemical kelyphite (kelyphite II) has been described from a garnet peridotite, Czech Moldanubian Zone. Important observations and conclusions may be summarized as follows.
-
(1)
Isochemical kelyphite – kelyphite II – occurs with a transitional zone within ordinary non-isochemical kelyphites – kelyphite I. Furthermore, the kelyphite II is separated from a relict garnet by a thin zone (10–20 μm thickness) of very fine-grained amphibole-spinel symplectite – kelyphite III.
-
(2)
An asymmetric configuration of kelyphite II with respect to relict garnets and kelyphite III was emphasized as a unique structural feature for the studied locality.
-
(3)
It was concluded that kelyphite I was first formed by the reaction between garnet and olivine. This reaction was apparently ceased leaving some relict garnet in the center of kelyphite I. Upon a further decompression, when the garnet itself became unstable, it got partially broken down forming an isochemical kelyphite – kelyphite II.
-
(4)
The transition zone is divided into two subzones: B (Opx+Sp+Amp) and C (Opx+Sp+Pl+Amp). We interpret that subzone B represents an extension of kelyphite I, whereas subzone C is interpreted to mark the onset of the breakdown reaction of garnet, which was followed by the formation of isochemical kelyphite.
-
(5)
The asymmetric nature of kelyphite II may be ascribed to a stress build-up due to the volume-increase nature of the kelyphite II-formation reaction that took place rapidly enough not to allow for stress relaxation by deformation.
-
(6)
It was hypothesized that the second reaction (i.e., isochemical breakdown of garnet) has been stopped by the third, hydration reaction that formed a thin hydrous zone, kelyphite III, at the reaction front of kelyphite II.
-
(7)
The absence of topotaxic relationships between Opx and spinel in kelyphite I implies that the studied Czech sample belongs to the ‘low-temperature type’ kelyphite of Obata and Ozawa (2011); whereas partial topotaxy observed in kelyphite II suggests that the degree of supersaturation of the relevant reaction was not that great, despite of the inferred low temperatures of the transformation.
-
(8)
It is not conclusive whether the formation of kelyphite I and that of kelyphite II form a consecutive single process or represents distinct events separated in both time and physical conditions.
-
(9)
It is proposed that the boundary between the high- and low-temperature kelyphites (for kelyphite I) lies around 800 ± 50 °C.
Isochemical kelyphite may be more widespread in orogenic peridotites than previously thought. More comparative studies of cases in which isochemical kelyphite is formed and other cases in which kelyphitization has terminated at the kelyphite I stage is needed to clarify the factors controlling the kelyphitization processes and its dynamics.
References
Altherr A, Kalt A (1996) Metamorphic evolution of ultrahigh-pressure garnet peridotites from the Variscan Vosges Mts. (France). Chem Geol 134:27–47
Bailey SW, Frank-Kamenetskii VA, Goldsztaub SA, Kato A, Pabst H, Schlz H, Taylor HFW, Wilson AJC (1977) Report of the International Mineralogical Association (IMA)-International Union of Crystallography (IUCr) Joint Committee on Nomenclature. Acta Cryst A33:681–684
Chopin C (1984) Coesite and pure pyrope in high-grade blueschists of the Western Alps; a first record and some consequences. Contrib Mineral Petrol 86:107–118
Degi J, Abart R, Kalman T, Bali E, Wirth R, Rhede D (2010) Symplectite formation during decompression induced garnet breakdown in lower crustal mafic granulite xenoliths: mechanisms and rates. Contrib Mineral Petrol 159:293–314
Gasparik T (1984) Two-pyroxene thermobarometry with new experimental data in the system CaO-MgO-Al2O3-SiO2. Contrib Mineral Petrol 87:87–97
Gillet P, Ingen J, Chopin C (1984) Coesite in subducted continental crust: P–T history deduced from an elastic model. Earth Planet Sci Lett 70:426–436
Green ECR, Holland TJB, Powell R, White RW (2012) Garnet and spinel lherzolite assemblages in MgO-Al2O3-SiO2 and CaO-MgO-Al2O3-SiO2: thermodynamic models and an experimental conflict. J Metamorphic Geol 30:561–577
Keankeo W, Taylor WR, FitzGerald JD (2000) Clinoferrosilite-bearing kelyphite: a breakdown product of xenolithic garnet, Delegate breccia pipes, New South Wales, Australia. Mineral Mag 64(3):469–479
Kushiro I, Yoder HS Jr (1966) Anorthite-forsterite and anorthite-enstatite reactions and their bearing on the basalt eclogite transformation. J Petrol 7:337–362
Medaris LG, Wang HF, Jelínek E, Jakeš P (2005) Characteristics and origins of diverse Variscan peridotites in the Gföhl Nappe, Bohemian Massif, Czech Republic. Lithos 82:1–23
Milke R, Abart R, Kunze K, Koch-Müller M, Schmid D, Ulmer P (2009) Matrix rheology effects on reaction rim growth I: evidence from orthopyroxene rim growth experiments. J Metamorphic Geol 27:71–82
Naemura K, Hirajima T, Svojtka M (2009) The pressure-temperature path and the origin of phlogopite in spinel–garnet peridotites from the Blansky Les Massif of the Moldanubian Zone, Czech Republic. J Petrology 50:1795–1827
O’Hara M (1967) Mineral paragenesis in ultrabasic rocks. In: Wyllie PJ (ed) Ultramafic and Related Rocks. John Wiley & Sons, New York, pp 393–401
O’Hara M, Richardson SW, Wilson G (1971) Garnet peridotite stability and occurrence in crust and mantle. Contrib Mineral Petrol 32:48–68
Obata M (1980) The Ronda peridotite - garnet-lherzolite, spinel-lherzolite, and plagioclase-lherzolite facies and the P-T trajectories of a high temperature mantle intrusion. J Petrology 21:533–572
Obata M (2011) Kelyphite and symplectite: textural and mineralogical diversities and universality, and a new dynamic view of their structural formation. in New Frontiers in Tectonic Research, Sharkov, E. V. (Ed.), ISBN: 978-953-307-595-2, InTech, pp. 350
Obata M, Ozawa K (2011) Topotaxic relationships between spinel and pyroxene in kelyphite after garnet in mantle-derived peridotites and their implications to reaction mechanism and kinetics. Mineral Petrol 101:217–224
Odashima N, Morishita T, Ozawa K, Nagahara H, Tsuchiyama A, Nagashima R (2008) Formation and deformation mechanisms of pyroxene-spinel symplectite in an ascending mantle, the Horoman peridotite complex, Japan: An EBSD (electron backscatter diffraction) study. J Miner Petrol Sci 103:1–15
Sapienza G, Scribano V, Calvari S (2001) Kelyphitic breakdown of garnets from pyroxenite xenoliths, south-easten Sicily, Italy. Periodico de Mineraligia 70(3):377–386
Schmid DW, Abart R, Podladchikov YY, Milke R (2009) Matrix rheology effects on reaction rim growth II: coupled diffusion and creep model. J Metamorphic Geol 27:83–91
Stipska P, Powell R (2005) Constraining the P–T path of a MORB-type eclogite using pseudosections, garnet zoning and garnet-clinopyroxene thermometry: an example from the Bohemian Massif. J Metamorphic Geol 23:725–743
Taylor WR (1998) An experimental test of some geothermometer and barometer formulation for upper mantle peridotites with application to the thermobarometry of fertile lherzolite and garnet websterite. Neues Jahrbuch fur Mineralogie Abhandlungen 172:381–408
Acknowledgments
We are grateful to Prof. T. Hirajima (Kyoto Univ.) and Dr. M. Svojtka (Academy of Science, Czech Republic) for their cooperations in the field and for discussions. We are also grateful to Prof. H. Nagahara for her permission of the use of FE-SEM and EBSD facilities at Univ. Tokyo, and to Dr. H. Yoshida for his technical assistance and to Professor Emeritus I. Kushiro for his discussions on the garnet breakdown reactions. Thanks are extended to Dr. T. Kawakami and T. Ueda for their assistance in the microprobe work, to Dr. Y. Seto (Kobe Univ.) for his assistance with the TEM analytical work and to Mr. Tutumi for his thin section preparations. The paper was benefitted by constructive criticisms of Petr Jerabek and an anonymous reviewer and their efforts are acknowledged. Editorial handling by R. Abart is greatly acknowledged. We thank Oxford University Press for letting us reuse Fig. 1 in Naemura et al (2009) published in Journal of Petrology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: R. Abart
Rights and permissions
About this article
Cite this article
Obata, M., Ozawa, K., Naemura, K. et al. Isochemical breakdown of garnet in orogenic garnet peridotite and its implication to reaction kinetics. Miner Petrol 107, 881–895 (2013). https://doi.org/10.1007/s00710-012-0260-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-012-0260-4