Abstract
The Purulia carbonatite of West Bengal, India is a dyke occurring within the 100 km long South Purulia Shear Zone (SPSZ), which marks the boundary between the Singhbhum Group of Rocks and the Chhotanagpur Granite Gneissic Complex (CGGC). It is composed essentially of calcite with apatite, amphibole, phlogopite, biotite, magnetite and ilmenite as common accessories. Based on optical properties and mineral chemistry two varieties of the amphibole are recognized: magnesiokatophorite and richterite. The latter is characterized by a relatively high content of Si and Na, while the former is enriched in Al and Ca. Such a composition of the amphibole is characteristic for the intermediate to the late stage carbonatite development. These two co-existing amphiboles reflect a sudden variation in total pressure within the magma chamber during the intrusion of the carbonatite dyke. It is inferred that the magnesiokatophorite started crystallizing first along with calcite and apatite. Subsequently, the ascent of carbonatitic magma to a more shallow depth (hypabyssal) resulted in the formation of the richterite. The difference in amphibole composition reflects a variation in the total pressure within the magma chamber that took place during the formation of the Purulia carbonatite. However, an alternative explanation, such as wallrock contamination, or liquid immiscibility, followed by carbonate magma segregation or magma mixing, may also be possible. There is, however, no evidence corroborating such an interpretation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diversity in mineralogical and chemical composition of carbonatites depends on a large number of geological factors (Bell 1989). The formation of carbonatites has often been divided into stages (Kapustin 1973; Sokolov 1985; Hogarth 1989) based on the evolution of carbonatite mineralogy and mineral compositions. Particularly useful for this study are the observations that the calcite from the early stages of carbonatite evolution often has higher Sr contents compared to calcites from later stages (Sokolov 1985). Moreover, the amphibole compositions noted by Hogarth (1989), where amphibole is associated with the initial stage of carbonatite evolution, is relatively enriched in Ca and Al and later stage amphibole is relatively more enriched in alkali metals and low in Al content. The present study builds on the compositions of distinct amphibole accessory phases in the Purulia carbonatite to present a hypothesis on the evolution of the carbonatite dyke.
Geological setting
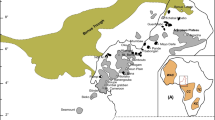
The Purulia carbonatite is a dyke, close to Beldih village, within the chlorite–phyllite of the Singhbhum Group. The other lithological units of this Group are quartzite, mica–schist, and amphibolite. The carbonatite is associated with an apatite–magnetite rock, which is mined commercially for the production of phosphate fertilizer (Basu 1988, 1993; Bhattacharya et al. 1991; Dasgupta and Bhattacharya 1992). An association of apatite–magnetite rock and minor carbonatite has also been reported from the nearby areas of the Mednitanr (M), the Kutni (K) and the Chirugora (C) villages (Fig. 1). A body of alkali–syenite is exposed in the Sushina Hill (S) area, which lies about 25 km away from the Beldih (B) village (Basu 1993). All of these localities are situated within a tectonically disturbed zone, known as the South Purulia Shear Zone (SPSZ), where the rocks of both the Chhotanagpur Granite Gneiss Complex (CGGC) and the Singhbhum Group are in contact with each other (Fig. 1). Satellite images of the area reveal the presence of two sets of lineaments: WNW–ESE and NW–SE. The WNW trending lineament or SPSZ extends for about 100 km through the villages of Beldih, Mednitanr, Kutni, Chirugora and Sushina from west to east (Basu 1993). The other set of lineaments appears to represent extensional tectonic activities and is responsible for the emplacement of the Purulia carbonatite (close to Beldih village) and other alkaline–carbonatite intrusions along the SPSZ. So far, exposures of rocks, such as ijolite and nepheline–syenite, which are known as common associates of carbonatite, have not been recorded from the area.
Regional geological map of the Purulia carbonatite (modified after Basu 1993). Localities: B Beldih, the study area, M Mednitanr, K Kutni, C Chirugora, S Sushina
Petrography
The Purulia carbonatite is a light coloured, medium-grained rock, composed essentially of calcite (about 90–95%, by volume) with an appreciable amount (about 2–5%, by volume) of fluorapatite. Amphibole (Fig. 2a), phlogopite, biotite, magnetite and ilmenite are present as accessories (max. 2–5%, by volume). The calcite grains are subhedral to euhedral, whereas the amphibole and apatite (Fig. 2b) are subhedral. At places clustering of biotite and phlogopite flakes occurs. Magnetite and ilmenite are evenly distributed throughout the rock. Higher concentration of phlogopite, biotite, and amphibole occur in places as continuous or discontinuous bands.
a Carbonatite showing two different types of amphibole: magnesiokatophorite and richterite within a calcite matrix. b Apatite within the calcite matrix. Calcite grains exhibit mosaic texture. c Well developed subhedral crystal of magnesiokatophorite in polygonal calcite matrix along with apatite and magnetite. d BSE image of magnesiokatophorite with calcite inclusion within calcite matrix. e Richterite in polygonal calcite matrix. f BSE image of richterite within calcite matrix which is highly fractured. MK magnesiokatophorite, R richterite, Cal calcite, Apt apatite, Mt magnetite
Although the amount of amphibole rarely exceeds 1–2% by volume, it can easily be distinguished by its green colour in the white calcite matrix. Based on petrographic evidence the amphiboles may be divided into two groups: (1) dark green variety (Fig. 2c) showing dark green to dark brown pleochroism. A BSE image (Fig. 2d) of this amphibole lacks zoning but carries inclusions of apatite and calcite and (2) light green amphibole (Fig. 2e) shows pale-green to light green pleochroism. A BSE image of this type of amphibole is marked by the presence of relatively few inclusions. The first variety of amphibole is coarser grained than the second one. Both these varieties of amphibole, show corroded margins indicating post-crystallization reaction with the carbonate melt.
Analytical techniques
EPMA-WDS analyses was performed on polished thin sections. The EPMA study was done using a JEOL 8600M Superprobe, at the IIC (Institute Instrumentation Centre), IIT Roorkee, India. The analytical conditions were 15 kV acceleration voltage, 50 nA beam current (cup) and point beam mode with 2 μm probe diameter. Calibration of the instrument was done using well-characterized silicate and oxide standards (multistandard), supplied by SPI Supplies Division of Structure Probe Inc., Canada. The precision of replicate measurements of the standards was better than ±1% (relative). Matrix corrections were done using the JEOL 8600 ZAF (oxide method) software. Total-Fe is calculated as FeO and later converted into FeO and Fe2O3 using the AX Mineral End-Member Activity Models as given by Holland and Blundy (1994).
Chemical composition of amphibole
The general formula of amphibole may be written as A0–1B2 VIC5 IVT8O22(OH, F, CI)2 (roman superscripts represents co-ordination number of the C and T sites, respectively). In terms of nomenclature and cation distribution we use the recommendations of the International Mineralogical Association (IMA) (Leake 1978; Leake et al. 1997, 2004). Three varieties of amphibole—calcic, sodic–calcic and sodic, are generally reported from carbonatite and a comprehensive review of their mineralogy and composition is given by Hogarth (1989). Pioneer work on amphibole from carbonatite was done by Bulakh (1965), Samoylov et al. (1974), Samoylov and Gormasheva (1975), Samoylov (1977), Fabriés (1978) and Kapustin (1980). Giret et al. (1980) and Mitchell (1990) demonstrated the compositional diversity of amphiboles from different types of alkaline rocks. Recent work on the composition and morphology of amphiboles from an alkaline–ultramafic complex from Montana, USA by Meeker et al. (2003) has been used for assessing the impact of the same on human health. Chakhmouradian and Zaitsev (2002) used amphibole, along with the calcite and pyroxene from the ultramafic veins of Kola peninsula, Russia to establish its genetic linkage with the associated carbonatite.
The chemical composition and structural formula of the amphibole (Table 1) from the Purulia carbonatite falls into the broad group of sodic–calcic amphibole. This group is defined as monoclinic amphibole with (Ca+Na)B ≥ 1.00 and 0.50 < NaB < 1.50. It is further classified into two sub categories: (Na+K)A ≥ 0.50 and (Na+K)A < 0.50. Based on (Na+K) in the A site the amphibole composition of the Purulia carbonatite falls into the first group in a ivSi–Mg/(Mg+Fe2+) plot (Leake et al. 1997) (Fig. 3) and is represented by magnesiokatophorite (Fig. 2c) and richterite (Fig. 2e).
Classification of the sodic–calcic amphiboles as per IMA norm (Leake et al. 1997). Diagram parameters: (Na+K)A ≥ 0.50; (Ca+NaB) ≥ 1.00; 0.50 < NaB < 1.50. R richterite, MK magnesiokatophorite, MT magnesio-taramite, FR ferro-richterite, K katophorite, T taramite. The compositions of the amphiboles from Purulia carbonatite fall into the R and MK fields. Open triangles amphibole of richterite composition and open squares amphibole of magnesiokatophorite composition from the study area. For comparison the amphibole composition taken from Hogarth (1989) from Iron Hill (IH), Colorado—solid circle; Gatineau (G), Quebec—solid triangle; Homa Bay (HB), Kenya—hexagon; Turii peninsula (TP), Russia—solid hexagon and Goldray (GR), Ontario—solid diamond are plotted
Compared to richterite, the magnesiokatophorite is relatively poor in SiO2 (49.4–51.9 wt.%) and MgO (14.9–15.8 wt.%), and it is enriched in Al2O3 (3.4–4.3 wt.%), TiO2 (0.66–0.42 wt.%) and CaO (5.0–5.5 wt.%). The reverse trend is noticed in the richterite variety, which is characterized by relatively high contents of SiO2 (52.7–55.1 wt.%) and MgO (15.7–17.2 wt.%) but low values of Al2O3 (1.3–2.1 wt.%), CaO (3.4–3.7 wt.%) and TiO2 (0.09–0.50 wt.%). The total iron as FeO, does not show any significant variation. The amounts of Na2O and K2O are rather similar in both the varieties ranging from 6.9 to 8.1 wt.% and 0.42 to 0.66 wt.%, respectively. The total alkali (Na2O+K2O) content is, nevertheless, slightly higher in richterite. The composition of the two varieties is consistent with the cationic distribution in different crystallographic sites (Table 1).
Other than the amphibole, only a few grains of calcite and ilmenite were analyzed. The calcite is found almost pure with low contents of FeO, MgO and MnO, together constituting about 1–3.0 wt.%. A random check shows that the Sr-content of the calcite is about 0.8–1.0 wt.%. The ilmenite is also FeO-rich and contains a very small quantity of MgO and MnO, the two constituents making up to 1.5–2.0 wt.%. Overall the MgO/MnO ratio is about 2:1.
Discussion
The amphibole of the carbonatite descend can be divided into two major groups depending on Al- and Ti-content. Early crystallized amphibole is rich in Al and Ti and best developed at the apical parts of the carbonatite complexes. On the other hand, low-Al amphibole is also poor in Ti and characteristic of the intermediate to late stages of carbonatite evolution (Samoylov 1977). The three end members, of representative amphibole compositions are: magnesio-richterite, magnesio-arfvedsonite and riebeckite (Fabriés 1978). On a R3+VI versus R++R2+ (where Rn + represents a cation of valence n) plot, the composition of low-Al amphibole falls into a narrow triangle, bounded by the above three mineral phases (Fig. 4a). The different compositional trends of amphibole from carbonatite can also be illustrated conveniently in the Ca+AlIV versus Si+Na+K plot and two main domains: one for low-Al amphibole and the other for high-Al amphibole, can easily be discerned (Fabriés 1978) (Fig. 4b).
a R3+VI versus R++R2+ plot of low-aluminium amphibole (Fabriés 1978) from Purulia carbonatite. The compositions of the amphiboles from the study area cluster between MA and R. b Ca+AlIV versus Si+Na+K plot of amphiboles (Fabriés 1978) from the Purulia carbonatite. The compositions of amphiboles (open triangles richterite and open squares magnesiokatophorite) from the study area fall on the line joining R and MK. MR magnesio-riebeckite, MA magnesio-arfvedsonite, R richterite, T tremolite, FW ferri-winchite, H hal, MH magnesio-hastingsite, E edenite, MK magnesiokatophorite, R richterite, W winchite, T tremolite, I high Al field, II low Al field. Other reported amphibole composition (Hogarth 1989) from Iron Hill (IH), Colorado—solid circle; Gatineau (G1 and G2), Quebec—solid triangle (up and down); Homa Bay (HB), Kenya—hexagon; Turii peninsula (TP), Russia—solid hexagon and Goldray (GR), Ontario—solid diamond are also plotted
The amphibole composition of the Purulia carbonatite is in parity to other well known carbonatite complexes of the world such as Iron Hill, Colorado; Gatineau, Quebec; Homa Bay, Kenya; Turii peninsula, Russia and Goldray, Ontario (Hogarth 1989) (Fig. 3). Moreover, the amphiboles of the Purulia carbonatite classify as low-Al amphibole (Fig. 4a) when plotted in a R3+VIversus R++R2+ diagram (Fabriés 1978). In the Ca+AlIV versus Si+Na+K diagram the amphibole compositions (Fabriés 1978) plot between richterite and magnesiokatophorite within the field of low-Al amphibole (Fig. 4b). Thus the intermediate to late stage formation of the Purulia carbonatite under hypabyssal conditions is indicated by the presence of low Al-amphibole, magnetite as a common accessory mineral (Kapustin 1973) and further supported by its mode of occurrence as dyke and by its texture.
The substitution of Si4+ by Al3+ in the amphibole T-site depends on total pressure, prevailing during the crystallization (Hammarstrom and Zen 1986; Hollister et al. 1987). The similar analogy is also used by Hammarstrom and Zen (1986) and Hollister et al. (1987) for geobarometric calculations using hornblende from calc-alkaline plutons. If other physico-chemical parameters remain unchanged, the Al-content in amphibole increases with increasing pressure (Schmidt 1992).
The two varieties of amphiboles (magnesiokatophorite and richterite) from the Purulia carbonatite differ from each other. Compared to richterite, the magnesiokatophorite, here, is Al-rich in the T-site, with 0.65 Al atoms on the T-site per formula unit. This is by a factor 3–4 higher than in richterite. The higher proportion of Al in the T-site of magnesiokatophorite is attributed to the formation of this amphibole species at relatively higher pressure. The high Al-content in the T-site is expected to influence the cation occupancy of the C-site, which is expected to contain more tetra- or tri-valent cations, such as Al3+, Ti4+ and Fe3+. The contents of divalent cations such as Mg2+ in the C-site should be relatively low, accordingly. This is in line with the chemical composition of magnesiokatophorite (Table 1). A clear positive correlation is observed between Al3+ and Ti4+ and between Al3+ and Fe3+, which indicate Ti- and Fe3+-enrichment with increasing Al-content (Fig. 5a,b). On the other hand, a negative correlation is present between Al3+ and Mg2+ (Fig. 5c). However, the higher content of CaO in magnesiokatophorite can be explained by coupled substitution between Si4++NaB and Al3++CaB (Fig. 6a). A similar feature is also observed between CaB and NaB (Fig. 6b). In both cases a strong negative correlation is documented. The high CaO-content of magnesiokatoporite correlates with the substitution of Si4+ by Al3. It is also indicated by relatively poorer CaO content of richterite compared to the magnesiokatophorite.
a AlIV versus Ti4+, b AlIV–Fe3+ and c AlIV–Mg plots of amphiboles from the Purulia carbonatite. AlIV shows a positive correlation with Ti4+ (a) and Fe3+ (b) and negative correlation with Mg (c) in richterite (open triangles) and magnesiokatophorite (open squares) species. For comparison the amphibole compositions taken from Hogarth (1989) from Iron Hill (IH), Colorado—solid circle; Homa Bay (HB), Kenya—hexagon; Turii peninsula (TP), Russia—solid hexagon and Goldray (GR), Ontario—solid diamond are plotted
a (Si4++Na+ B)–(Al3+ T+CaB) plot for the two varieties of amphibole—richterite (open triangles) and magnesiokatophorite (open squares). There are two distinct fields of these two types of amphibole with correlation coefficient of −0.9652 and −0.9805 for richterite and magnesiokatophorite, respectively. b NaB–CaB plot of richterite (open triangles) and magnesiokatophorite (open squares), showing strong negative correlation between them in B-site
We infer that the presence of sodic–calcic amphibole with two distinct compositional modes in the Purulia carbonatite can possibly be attributed to the sudden change in pressure within the magma chamber. Relatively Al3+ and Ca2+ rich magnesiokatophorite started crystallizing along with calcite and apatite at a greater depth. Then, due to rejuvenation of the South Purulia Shear Zone, the carbonatite melt, along with the already-formed magnesiokatophorite crystals intruded into the shallower zone of the crust. This would lead to a sudden drop of the total pressure, and further cooling of this magma seems to have caused crystallization of the richterite, with comparatively low Al-content. In richterite, Al3+ is substituted by Si4+ and to maintain the charge balance Ca2+ is substituted by Na+. Thus, this small change in physico-chemical condition could be recorded in the amphibole mineral as they are very sensitive to pressure variations. It is, therefore, assumed that the difference of the Al-content of the two varieties of the amphibole was due to a sudden change in the total pressure. In contrast, the effect of the temperature changes on the crystallization process was more gradual.
The above phenomenon, however, can also be explained by the sudden changes in the magma composition by wallrock assimilation. No evidence has been found to support the changes in the magma composition by such wall-rock assimilation. Assimilation of the country rocks should, normally, introduce both Al and Si, into the magma without any major change in its composition. Therefore, assimilation is not considered to alter the amphibole composition, i.e., the Al/Si ratio as this is mainly controlled by the total pressure. Another possibility may be silicate–carbonate immiscibility in the parent magma; if we assume the original magma (alkaline in composition) with innumerable droplets of immiscible carbonate melt. With progressive differentiation, immiscible carbonate droplets are likely to start segregating and eventually may generate a number of small pools of segregated carbonate magma at different levels within the magma chamber. The composition of the amphibole crystals developed within individual pools of carbonate magma will, nevertheless, depend upon P–T-conditions prevailing within individual carbonate magma pools. The P–T condition would largely depend on the depth. Once these small carbonate magma pools combine to a large carbonate magma body, the already-formed amphibole crystals of different composition will get mixed. But also this process cannot satisfactorily explain the co-existence of the two varieties of amphibole. Instead, it is expected that amphibole crystals thus formed should have a range of compositional variation.
Conclusions
The amphibole in the Purulia carbonatite is sodic–calcic and overall low-Al in composition, which is characteristic of the intermediate to late stages of carbonatite development, at shallow depth. Two varieties of amphibole: richterite and magnesiokatophorite are discerned, where the magnesiokatophorite is relatively rich in Al in the T-site, indicating substitution of Si4+ by Al3+. This implies that the total pressure has been higher during the formation of magnesiokatophorite than during crystallization of richterite. The co-existence of magnesiokatophorite and richterite, within the same carbonatite body indicates sudden change in the total pressure condition, and thus could be related to the intrusive nature of the carbonatite melt emplaced in the SPSZ.
It is, therefore, concluded that the amphibole occurring as an accessory mineral phase in carbonatite, can serve as a good petrogenetic indicator, providing valuable information on the stages of carbonatite development.
References
Basu SK (1988) Investigation of apatite and other associated minerals in the southern Shear Zone, Purulia District, West Bengal. Extended Abstracts, Rec Geol Surv India 122: Part 2, 42
Basu SK (1993) Alkaline–carbonatite complex in precambrian of South Purulia Shear Zone, Eastern India: its characteristics and mineral potentialities. Indian Miner 47:179–194
Bell K (1989) Carbonatites genesis and evolution. Unwin Hyman, London
Bhattacharya C, Chakraborty A, Banerjee PK (1991) Petrology–geochemistry of apatite–magnetite amphibolites and their role in phosphate mineralisation along the southern shear zone, Puruliya Dt., West Bengal. Indian J Earth Sci 18:94–109
Bulakh AG (1965) Amphibole group. In: Kukharenko AA (ed) Kaledonskii kompleks ul’traosnovnykh, shchelochnykh porod i karbonatitov Kol’skogo Poluostrova i severnoi Karelia. Nedra, Moscow, pp 453–463 (in Russian)
Chakhmouradian AR, Zaitsev AN (2002) Calcite–Amphibole–Clinopyroxene rock from the Afrikanda Complex, Kola Peninsula, Russia: mineralogy and a possible link to carbonatites. III. Silicate minerals. Can Mineral 40:1347–1374
Dasgupta S, Bhattacharya DK (1992) Apatite mineralisation along Singhbhum and Purulia–Bankura shear zones: their nature and physico-chemical characteristics. Indian Miner 46:123–132
Fabriés J (1978) Les types paragénétiques des amphiboles sodiques dans les roches magmatiques. Bull Minéral 101:155–165
Giret A, Bonin B, Leger JM (1980) Amphibole compositional trends in oversaturated and understurated alaklaine plutonic ring-complexes. Can Mineral 18:481–495
Hammarstrom JM, Zen E (1986) Aluminium in hornblende: an empirical igneous geobarometer. Am Mineral 71:1297–1313
Hogarth DD (1989) Pyrochlore, apatite and amphibole: distinctive minerals in carbonatite. In: Bell K (eds) Carbonatites: genesis and evolution. Unwin Hyman, London, pp 105–148
Holland T, Blundy J (1994) Non-ideal interactions in calcic amphiboles and their bearing on amphibole–plagioclase thermometry. Contrib Mineral Petrol 116:433–447
Hollister LS, Grissom GC, Peters EK, Stowell HH, Sisson VB (1987) Confirmation of the emperical correlation of Al in hornblende with pressure of sloidification of calc-alkaline plutons. Am Mineral 72:231–239
Kapustin YL (1973) Mineralogiya Kory Vyvetrivaniya Karbonatitov. Nedra, Moscow (in Russian)
Kapustin YL (1980) Mineralogy of carbonatites. Amerind, New Delhi, India (translated by DK Biswas)
Leake BE (1978) Nomenclature of amphiboles. Mineral Mag 42:533–563
Leake BE, Woolley AR, Arps CES, Birch WD, Gilbert MC, Grice JD, Hawthorne FC, Kato A, Kisch HJ, Krivovichev VG, Linthout K, Larid J, Mandarino JA, Maresch WV, Nickel EH, Rock NMS, Schumacher JC, Smith DC, Stephenson NCN, Ungaretti L, Whittaker EJW, Youzhi G (1997) Nomenclature of amphiboles: report of the Subcommittee on Amphiboles of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Can Mineral 35:219–246
Leake BE, Woolley AR, Birch WD, Burke EAJ, Ferraris G, Grice JD, Hawthorne FC, Kisch HJ, Krivovichev VG, Schumacher JC, Stephenson NCN, Whittaker EJW (2004) Nomenclature of amphiboles: additions and revisions to the International Mineralogical Associations amphibole nomenclature. Min Mag 68(1):209–215
Meeker GP, Bern AM, Brownfield IK, Lowers HA, Sutley SJ, Hoefen TM, Vance JS (2003) The composition and morphology of amphiboles from the Rainey Creek Complex, Near Libby, Montana. Am Mineral 88:1955–1969
Mitchell RH (1990) A review of the compositional variation of amphiboles in alkaline plutonic complexes. Lithos 26:135–156
Samoylov VS (1977) Karbonatity (fatsii i usloviya obrazovaniya). Nauka, Moscow (in Russian)
Samoylov VS, Gormasheva GS (1975) Alkali amphiboles of carbonatites and genetically related rocks. Zapiski Vsesoyuznogo Mineralogicheskogo Obshchetva 104:145–159 (in Russian)
Samoylov VS, Gormasheva GS, Chernysheva EA (1974) Low-aluminum alkalic amphiboles of carbonatites. Yearbook Institute of Geochemistry, Irskutsk, pp 189–193
Schmidt MW (1992) Amphibole composition in tonalite as a function of pressure: an experimental calibration of the Al-in-hornblende barometer. Contrib Mineral Petrol 110:304–310
Sokolov SV (1985) Carbonates in ultramafic alkali-rock, and carbonatite intrusions. Geochem Int 22:150–166
Acknowledgements
We express sincere thanks to CSIR, New Delhi for funding the project (no. 24(0286)/05/EMR-II). The help rendered by Mr. Sandeep Banerjee (Durgapur Govt. College) during the fieldwork, Head, Earth Sciences and IIC, IIT Roorkee for providing laboratory facilities and Prof. O. P. Varma, and Prof. R. P. Gupta for improving the manuscript is gratefully acknowledged. The manuscript has been substantially improved by the suggestions of the reviewers Dr. Keith Bell and Dr. Frances Wall. We sincerely thank both of them. We also express our sincere gratitude to Prof. L.G. Gwalani for his excellent editorial handling.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: L.G. Gwalani
Rights and permissions
About this article
Cite this article
Chakrabarty, A., Sen, A.K. & Ghosh, T.K. Amphibole—a key indicator mineral for petrogenesis of the Purulia carbonatite, West Bengal, India. Miner Petrol 95, 105–112 (2009). https://doi.org/10.1007/s00710-008-0024-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-008-0024-3