Abstract
Despite that there is some literature on pollen morphology of Rhamnaceae, studies addressing general aspects of the microsporogenesis, microgametogenesis, and anther development are rare. The aim of this paper is to describe the ultrastructure of pollen grain ontogeny with special attention to tapetum cytology in Hovenia dulcis. Anthers at different stages of development were processed for transmission and scanning electron microscopy, bright-field microscopy, and fluorescence microscopy. Different histochemical reactions were carried out. The ultrastructural changes observed during the development of the tapetal cells and pollen grains are described. Large vesicles containing carbohydrates occur in the tapetal cell cytoplasm during the early stages of pollen development. Its origin and composition are described and discussed. This is the first report on the ontogeny and ultrastructure of the pollen grain and related sporophytic structures of H. dulcis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rhamnaceae is a family of about 55 genera and 900 species of cosmopolitan distribution (Medan and Schirarend 2004; Perveen and Qaiser 2005). Hovenia dulcis Thunb. is a member of the Rhamneae Tribe, known as an oriental raisin tree, and usually found in China, Korea, Japan, and India (Yun and Lee 2002). The fleshy rachis of the infructescence is sweet, fragrant, and edible. This edible peduncle contains polysaccharides among other nutrition constituents. The total amount of sugar exceeds that from general fruits, since it can reach 42 % (Xiang et al. 2012). The fruit, seeds, leaves, roots, and bark of H. dulcis are used for medical purposes in some cultures (Okuma et al. 1995; Cho et al. 2004).
There is some literature on pollen morphology of Rhamnaceae (Papagiannes 1974; Schirarend and Köhler 1993; Perveen and Qaiser 2005). Schirarend and Köhler (1993) described 12 types of pollen grains based mainly in characters of tectum architecture. In spite of all these reports on pollen morphology, studies addressing general aspects of the microsporogenesis, microgametogenesis, and anther development are rare (Gotelli et al. 2012).
Tapetum is an anther tissue that plays a very important function in the pollen grain development (Johri et al. 1992; Raghavan 1997). Changes on the ultrastructure of tapetal cells during pollen ontogeny provide key information for understanding the importance of this tissue’s function in the development of viable pollen. In Rhamnaceae, only Colletia paradoxa and Discaria americana, both belonging to the tribe Colletieae, have been studied from this aspect (Gotelli et al. 2012).
The aim of this paper is to describe the ultrastructure of pollen grains and microsporangium development with special attention to tapetum ontogeny in H. dulcis in order to broaden the current embryological knowledge of Rhamnaceae.
Materials and methods
Samples of H. dulcis were collected from individuals cultivated in the campus of the Facultad de Agronomía, Universidad de Buenos Aires. Voucher specimens were deposited in the Herbarium Gaspar Xuarez (BAA).
For transmission electron microscopy, anthers at different developmental stages were prefixed overnight in 2.5 % glutaraldehyde in phosphate buffer (pH 7.2) and then postfixed in OsO4 at 2 °C in the same buffer for 3 h. Following dehydration in an ethanol series, the material was embedded in Spurr’s resin. Ultrathin sections (750–900 nm) were made on a Reichert-Jung ultramicrotome and then stained with uranyl acetate and lead citrate (Zarlavsky 2014). The sections were observed and photographed with a JEOL-JEM 1200 EX II TEM at 85.0 kV.
For scanning electron microscopy studies, the material was dehydrated in an ethanol series (70, 80, 90, 100 %), critical-point dried with liquid CO2, and sputter-coated with gold-palladium for 3 min (Zarlavsky 2014). Scanning micrographs were taken with a Philips XL 30 microscope.
Sections of 1 μm thick were made on a Reichert-Jung ultramicrotome and then stained with toluidine blue, sudan black B (Pearse 1961) to localize lipids, periodic acid-Schiff (PAS) (Jensen 1962) to find insoluble polysaccharides, lugol to find starch, picric acid and eosin for proteins (Zarlavsky 2014). Photomicrographs were taken using a Motic bright field microscope with digital camera, DMWB1-223ASC.
Results
The anther is tetrasporangiate, and its wall consists of the following: epidermis (ep), endothecium (en), two middle layers (ml), and a secretory type tapetum (t). The vascular bundle that reaches the connective tissue presents less amount of xylem than phloem. This last one has connections with the tapetal cells of each of the four locules (Fig. 1).
Stage 1: microsporocytes
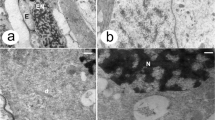
The microsporocytes are uninucleate and with a very dense cytoplasm. Abundant mitochondria, lipid globules, and endoplasmic reticulum (ER) are present (Fig. 2a). A thick callosic wall is formed between the plasmalemma and the primary wall (Fig. 2a).
TEM photomicrographs. a Microsporocyte and tapetal cell (c callose, m mitochondria, lg lipidic globule, fv fibrillar vesicle, cw cell wall, m mitochondria, lg lipidic globule, er endoplasmic reticulum. b Detail of a tapetal cell with developing fibrillar vesicles (dfv) connected by a tubular network (tn), vacuoles (v), and dictyosomes (d). c Fibrillar vesicles (fv) connected by the tubular network. d Fibrillar vesicles and the tubular network are highlighted in green. Scale bars: a, c, d 1 μm, b 500 nm
Tapetal cells are uninucleate and contain a few small vacuoles, mitochondria, lipid globules, and plastids (Fig. 2a). Numerous dictyosomes are present (Fig. 2b), and dictyosomic vesicles fill the cytoplasm (Fig. 2b). An anastomosing tubular network (tn) connects adjacent cisternal stacks of dictyosomes with secretory vesicles (Fig. 2b–d). Some vesicles connected with the tubular network are larger and with a slightly fibrillar content (Fig. 2c, d). In this stage, vesicles of different sizes are present, in the range of 0.25 μm × 0.1 μm (developing fibrillar vesicle, dfv) up to 4 μm × 2 μm (fibrillar vesicle, fv) (Fig. 2b–d).
Stage 2: young microspore tetrads
Microsporocytes undergo simultaneous meiosis, forming tetrahedral tetrads.
Tapetal cells show characteristics similar to those of the previous stage, but the cytoplasm is filled with fibrillar vesicles connected by the tubular network (Fig. 2c, d).
Stage 3: mature microspore tetrads
The protectum, the probacula, and the future basal layer are formed into a fibrillar primexine matrix in the mature tetrads (Fig. 3a). The microspore cytoplasm shows mitochondria, dictyosomes, lipid globules, and plastids (Fig. 3a).
TEM photomicrographs. a Microspore of a tetrad and tapetum, p-te pro-tectum, it infratectum, fl foot layer, d dyctiosome, m mitochondria, lg lipidic globule, p plastid, fv fibrillar vesicle. Arrow showing the disintegration of the tapetal cell wall. b Detail of a tapetal cell with globular depositions of moderate electron density (arrow) and mitochondria (m), fibrillar vesicles (fv), and vacuoles (v). Scale bars a, b 500 nm
As the microspore tetrad matures, tapetal cells begin to lose their walls (Fig. 3a, b). Only a few globular depositions of moderate electron density occur between the plasmalemma and the wall remains. The anastomosing tubular network is no longer present. The fibrillar vesicles are close to each other and occupy most of the cytoplasm. Mitochondria are peripheral in the cytoplasm near the inner tangential face of the tapetal cell (Fig. 3b).
Stage 3: free microspores
Microspores have a conspicuous nucleus, and their cytoplasm, including a few mitochondria, is limited to a parietal position due to the presence of a large vacuole (Fig. 4a). The thickness of the tectum and basal layer increased, and the infratectum is now reduced and has a granular appearance (Fig. 4a).
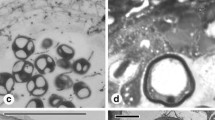
TEM photomicrographs. a Free microspore (te tectum, it infratectum, fl foot layer, v vacuole, n nucleus). b Tapetum at free microspore stage with wall disintegrated (arrows), vacuoles (v), fibrillar vesicles (fv), and plastids (p). c Detail of a pollen grain showing the vegetative nucleus (vn) surrounding the generative cell (gc). c’ Detail of microtubules in generative cell cytoplasm. d Detail of pollen cell wall and tapetal cell (en endexine, in intine, fl foot layer, it infratectum, t tectum) and tapetal cell with a new cell wall (cw) and homogeneous fibrillar cytoplasmatic content. e Detail of a tapetal cell with the new cell wall (cw) and orbicule-like structures (o). Scale bars: a 2 μm, b–d 500 nm, c 125 nm, e 200 nm
Tapetal cells lose all their walls, and some globular and fibrillar depositions of moderate electron density appear near the plasmalemma. Fibrillar material with the same electron density occurs within the loculus and on the developing pollen exine. The tapetal cells present similar characteristics to the previous stage (Fig. 4b). Plastids and small vacuoles are present (Fig. 4b).
Stage 4: pollen grain
The generative cell that is formed by a mitotic division of the microspore nucleus first occupies a parietal position. Then, it migrates toward a central position and appears to be surrounded by the conspicuous and lobed nucleus of the vegetative cell (Fig. 4c) as a consequence of the generative cell having a thin and sinuous wall transparent to the electrons. The cytoplasm of the generative cell presents many microtubules (Fig. 4c’). In the cytoplasm of the vegetative cell, mitochondria and RER are abundant (Fig. 4c). The pollen is shed at bicellular stage.
At this stage, the tapetal cells are still present. Fibrillar vesicles lose their integrity, and the cytoplasm has a homogeneous fibrillar appearance. A material with low electron density and similar to a cell wall appears surrounding the tapetal cells. Globular depositions with electron density similar to that of the sporopollenin are attached to it, coating the inner loculus surface (Figs. 4d, e and 5a). Some of these depositions are more conspicuous (0.5 μm), similar to orbicules without central cores.
The pollen wall has a granular infratectum, lamellar endexine, and thin intine (Fig. 4d). Pollen grains are oblate-spheroidal, three-colporate, triangular shape in polar view with lightly convex mesocolpia. The exine is striate-rugulate (Fig. 5b–d).
Histochemical reactions
The fibrillar vesicles of the tapetal cells colored bright blue with toluidine blue and react positively with periodic acid-Schiff (PAS) (Fig. 6a, b). Fibrillar vesicles in tapetal cells do not react with Sudan Black B, picric acid, nor eosin (Fig. 6c–e).
Summary of the major events in polysaccharide vesicle development
At young microsporocyte stage, numerous dictyosomes are present in the cytoplasm of tapetal cells (Fig. 7a). As microsporocytes form the callosic wall, developing fibrillar vesicles fill the cytoplasm of tapetal cells. An anastomosing tubular network connects adjacent cisternal stacks of dictyosomes with these vesicles (Fig. 7b). At the mature microspore tetrads stage, tapetal cells with developed fibrillar vesicles are connected by the tubular network (Fig. 7c). At the free microspore stage, the tubular network is no longer present, and mitochondria are arranged in the periphery of the tapetal cell which cell wall is degraded (Fig. 7d). At pollen grain stage, tapetal cells have a reduced volume and present a new lax cell wall and a homogeneous fibrillar content (Fig. 7e).
Schematic development of polysaccharide vesicles related with the pollen ontogeny stage. a Microspore mother cell (mmc) stage. Tapetal cell with nucleus (n), vacuoles (va) numerous dictyosomes (d) and mitochondria (m); cw cell wall. b Mmc with callosic wall (mmc/c) stage. Tapetal cell with numerous dictyosomes, mitochondria, and developing fibrillar vesicles (dfv) connected by a tubular network (tn) associated to dictyosomes. c Mature microspore tetrads (mte) stage. Tapetal cell with fibrillar vesicles (fv) called polysaccharide vesicles connected by the tubular network (tn). d Free microspore (mi) stage. Tapetal cell with fibrillar vesicles (fv), the tubular network is not present and the mitochondria (m) are arranged in the periphery of the cell. Tapetal cell wall is degraded (arrow). e Pollen grain (pg) stage. Tapetal cell has a reduced volumen and presents a new lax cell wall (lcw) and a cytoplasm with homogeneous fibrillar content (cy)
Discussion
It is generally assumed that the tapetum may be involved in different aspects of pollen development (Johri et al. 1992). Maheshwari (1950) regarded the tapetum to be of considerable physiological significance. Several authors described in detail the tapetum cytology in cytoplasmic male steriles and their fertile counterparts and showed that male sterility is temporally linked with tapetal abnormalities (Raghavan 1997). The cytology of the tapetum of H. dulcis presents two characteristics not yet described for other species of angiosperms: the presence of the anastomosing tubular network (tn) and large fibrillar vesicles (lfv) that react positively with PAS.
According to Mollenhauer and Morré (1966), each dictyosome consists of a stack of cisternae (usually 5 or 6) and associated vesicles. In the maize root cap, these authors observed tubular proliferations forming an anastomosing network of tubules extending from the central structure. These cisternal tubules possibly provide a channel for transfer of materials and may serve to segregate the activities of the cisternal lumen from those of the forming vesicles. In this study, the anastomosing tubular network was observed for the first time in tapetal cells of H. dulcis. No reports of such structure in tapetal cells of other angiosperm species were found.
Histochemical tests indicate that the large fibrillar vesicles of H. dulcis accumulate insoluble polysaccharides since they present a positive reaction with the PAS reactive. Therefore, we can now identify them as large polysaccharide vesicles.
Observations made in the different stages of development of H. dulcis show that large polysaccharide vesicles are originated from dictyosomic secretory vesicles. These small vesicles accumulate fibrillar material and increase in volume probably by the transfer of material through the anastomosing tubular network. When vesicles reach their maximum size, the tubular network is no longer present. Polysaccharide vesicles are not present at the pollen grain stage, where remarkably the tapetal cells are still present and show a PAS+ cytoplasm with a homogeneous fibrillar appearance. At this stage, a new structure resembling a lax cell wall is present in the tapetal cells.
There is a general difficulty in assigning a role for the tapetum based on static electron micrographs alone (Raghavan 1997). However, the observations made in this investigation suggest that one of the possible functions of the large polysaccharide vesicles in H. dulcis is the synthesis of a new structure similar to a cell wall in the tapetal cells by providing the precursors of it. It is known that one of the most important functions of the Golgi apparatus in plants is the ability to synthesize complex matrix polysaccharides of the cell wall (Driouich et al. 1993, 2012; Lerouxel et al. 2006). The presence of this new cell wall and the persistence of the tapetal cells accumulating many polysaccharides until advanced development stages suggest that the tapetum could be acting as a reservoir of sugar which are possibly translocated to the inflorescence rachis while the fruit is formed. This reservoir of sugars in the anthers could be necessary for the quick development of the fleshy peduncles, since the content of sugars of these last ones can reach 42 %, which exceeds general fruits far away (Xiang et al. 2012). The vascular bundle that reaches the anther presents phloematic connections with the tapetal cells. Therefore, the polysaccharides from tapetal cells may be reabsorbed and transported by the phloem to the edible peduncles. Reabsorption of sugary solutions is known for nectaries (Bieleski and Redgwell 1980; Stpiczynska 2002; Radice and Galati 2003).
An organelle unique to the tapetum, the tapetosome, was described in Brassicaceae (Wu et al. 1997; Hernández-Pinzón et al. 1999; Hsieh and Huang 2005, 2007). Tapetosomes are relatively large lipid-rich organelles that contain oleosin, flavonoids, and alkanes, usually considered as cisternae-like vesicles derived from the ER (Ross and Murphy 1996; Ruiter et al. 1997; Wu et al. 1997; Murphy and Ross 1998; Hsieh and Huang 2005, 2007; Suzuki et al. 2013). The large polysaccharide vesicles described in H. dulcis cannot be regarded as tapetosomes since they present a different origin and composition.
Orbicules are corpuscles of sporopollenin lining the inner tangential and sometimes also the radial tapetal cell walls, which appear during pollen grain development (Huysmans et al. 1998; Galati et al. 2010). Ubisch (1927) gave a detailed description of them. Their size is variable, ranging from 0.14 to 20 μm (Galati et al. 2010). The average size of orbicules in H. dulcis is 0.40 μm. Huysmans et al. (1998) studied the distribution of orbicules in Angiosperms, but they failed to report them in Rhamnales. Their presence in this order was first indicated by Gotelli et al. (2012). Orbicules of Colletia paradoxa and Discaria americana were described following Galati’s classification (Galati 2003) as having each a central core transparent to electrons and a sporopollenin wall with a spheric to subspheric shape and superficial invaginations (Gotelli et al. 2012). In H. dulcis, orbicule-like structures without central cores are present. However, their distribution in this species is too unusual to qualify as typical orbicules. These orbicule-like structures are part of a deposition with the same electron density of the sporopollenin precursors that is coating the inner loculus surface. This deposition is not as continuous as a tapetal membrane, but since it occurs over the newly synthesized wall of the tapetal cells, it could be acting in the same way as a continuous layer of sporopollenin.
The male germ unit is known as the physical association of the generative cell to the vegetative cell nucleus that forms a linkage of the genetic material in the pollen grain. According to McCue et al. (2011), this cytoplasmic connection is formed by the development of vesicles and the elongation of microtubules after the formation of the generative cell. In H. dulcis, the generative cell has a cytoplasm rich in microtubules. Moreover, the present ultrastructural study showed that the generative cell and the vegetative nuclei are in close contact in a central position in the grain. The lobed vegetative nucleus surrounds the generative cell forming a physical association or male germ unit as described in Nicotiana tabacum by Yu et al. (1989).
Pollen grain morphology of H. dulcis is in accordance with the general morphological pattern found in Rhamnaceae (Perveen and Qaiser 2005; Johri et al. 1992; Schirarend 1996) and is classified as Hovenia-type following Perveen and Qaiser (2005) descriptions.
This is the first report on the ontogeny and ultrastructure of the pollen grain and related sporophytic structures of H. dulcis and of the presence of vesicles containing carbohydrate. Ultrastructural studies in other species of Rhamnaceae should be performed to determine the presence of these polysaccharide vesicles and to provide a more precise interpretation of their function.
References
Bieleski RL, Redgwell RJ (1980) Sorbitol metabolism in nectaries from flowers of Roseaceae. Aust J Plant Physiol 7:15–25
Cho JY, Moon JH, Eun JB, Chung SJ, Park KH (2004) Isolation and characterization of 3(Z)-dodecenedioic acid as an antibacterial substance from Hovenia dulcis THUNB. Food Sci Biotechnol 13:46–50
Driouich A, Faye L, Staehelin LA (1993) The plant Golgi apparatus: a factory for complex polysaccharides and glycoproteins. Trends Biochem Sci 18:210–214
Driouich A, Cannesan MA, Dardelle F et al (2012) Unity is strength: the power of border cells and border like cells in relation with plant defense. In: Vivanco JM, Baluska F (eds) Secretions and exudates in biological systems. Springer, Heidelberg, pp 91–108
Galati BG (2003) Ubisch bodies in Angiosperms. In: Pandey K, Dhakal MR (eds) Advances in plant reproductive biology, vol II. Narendra Publishing House, Delhi, pp 1–20
Galati BG, Gotelli MM, Rosenfeldt S, Torretta JP, Zarlavsky G (2010) Orbicules in relation to the pollination modes. In: Kaiser BJ (ed) Pollen: structure, types and effects. Nova, New York, pp 1–15
Gotelli M, Galati B, Medan D (2012) Pollen, Tapetum, and Orbicule Development in Colletia paradoxa and Discaria americana (Rhamnaceae). ScientificWorldJournal: Article ID 948469. doi:10.1100/2012/948469
Hernández-Pinzón I, Ross JHE, Barnes KA, Damant AP, Murphy DJ (1999) Composition and role of tapetal lipid bodies in the biogenesis of the pollen coat of Brassica napus. Planta 208:588–598
Hsieh K, Huang AHC (2005) Lipid-rich tapetosomes in Brassica tapetum are composed of oleosin-coated oil droplets and vesicles, both assembled in and then detached from the endoplasmic reticulum. Plant J 43:889–899
Hsieh K, Huang AHC (2007) Tapetosomes in Brassica tapetum accumulate endoplasmic reticulum-derived flavonoids and alkanes for delivery to the pollen surface. Plant Cell 19:582–596
Huysmans S, El-Ghazaly G, Smets E (1998) Orbicules in Angiosperms: morphology, function, distribution, and relation with tapetum types. Bot Rev 64:240–272
Jensen WA (1962) Botanical Hystochemistry. Freeman, San Francisco
Johri BM, Ambegaokar KB, Srivastava PS (1992) Comparative embryology of angiosperms, vol 1–2. Springer, Berlin
Lerouxel O, Cavalier DM, Liepman AH, Keegstra K (2006) Biosynthesis of plant cell wall polysaccharides—a complex process. Curr Opin Plant Biol 9:621–630
Maheshwari P (1950) An introduction to the embryology of angiosperms. McGraw-Hill, New York
McCue AD, Cresti M, Feijo JA, Slotkin RK (2011) Cytoplasmic connection of sperm cells to the pollen vegetative cell nucleus: potential roles of the male germ unit revisited. J Exp Bot 62:1621–1631
Medan D, Schirarend C (2004) Rhamnaceae. In: Kubitzki K (ed) The families and genera of vascular plants VI. Flowering plants – dicotyledons: Celastrales, Oxalidales, Rosales, Cornales, Ericales. Springer Verlag, Heidelberg
Mollenhauer HH, Morré DJ (1966) Tubular connections between dictyosomes and forming secretory vesicles in plant Golgi apparatus. J Cell Biol 29:373–376
Murphy DJ, Ross JHE (1998) Biosynthesis, targeting and processing of oleosin-like proteins, which are major pollen coat components in Brassica napus. Plant J 12:1–16
Okuma Y, Ishikawa H, Ito Y, Hayashi Y, Endo A, Watanabe T (1995) Effects of extracts from Hovenia dulcis Thunb. on alcohol concentration in rats and men administered alcohol. J Jpn Soc Food Sci Technol 48:167–172
Papagiannes E (1974) Pollen studies of selected genera of Rhamnaceae, Master Thesis. University of Illinois at the Chicago Circle, Chicago, Illinois
Pearse AGE (1961) Histochemistry, theoretical and applied, 2nd edn. Little Brown, Boston
Perveen A, Qaiser M (2005) Pollen Flora of Pakistan –XLIV. Rhamnaceae. Pakistan J Bot 37:195–202
Radice S, Galati BG (2003) Floral nectary ultrastructure of Prunus persica (L.) Batch cv. Forastero (Newcomer), an Argentine peach. Plant Syst Evol 238:23–32
Raghavan V (1997) Molecular embryology of flowering plants. Cambridge University Press, UK, pp 357–393
Ross JHE, Murphy DJ (1996) Characterization of antherexpressed genes encoding a major class of extracellular oleosinlike proteins in the pollen coat of Brassicaceae. Plant J 9:625–637
Ruiter RK, van Eldik GJ, van Herpen RMA, Schrauwen JAM (1997) Characterization of oleosins in the pollen coat of Brassica oleracea. Plant Cell 9:1621–1631
Schirarend C (1996) Pollen morphology of the genus Paliurus (Rhamnaceae). Grana 35:347–356
Schirarend C, Köhler E (1993) Rhamnaceae Juss. World Pollen and Spore Flora 17(18):1–53
Stpiczynska M (2002) Nectar resorption in the spur of Platanthera chloranta Custer (Rchb.) Orchidaceae. Nectar and Nectary: from biology to biotechnology. Montalcino, Siena, pp 28–31
Suzuki T, Tsunekawa S, Koizuka C et al (2013) Development and disintegration of tapetum-specific lipid-accumulating organelles, elaioplasts and tapetosomes, in Arabidopsis thaliana and Brassica napus. Plant Sci 207:25–36
Ubisch G (1927) Kurze mitteilungen zur entwicklungsgeschiehte der antheren. Planta 3:490–495
Wu SSH, Platt KA, Ratnayake C, Wang TW, Ting JTL, Huang AHC (1997) Isolation and characterization of novel neutrallipid- containing organelles and globuli-filled plastids from Brassicanapus tapetum. Proc Natl Acad Sci U S A 94:12711–12716
Xiang J, Zhu W, Han J, Li Z, Ge H, Lin D (2012) Analysis of organic acids in Chinese raisin tree (Hovenia dulcis) peduncle and their changes in liquid fermentation process. Food Sci Biotechnol 21:1119–1127
Yu HS, Hu SY, Zhu C (1989) Ultrastructure of sperm cells and the male germ unit in pollen tubes of Nicotiana tabacum. Protoplasma 152:29–36
Yun CW, Lee BC (2002) Vegetation structure of Hovenia dulcis community in South Korea. Korean J Ecol 25:93–99
Zarlavsky GE (2014) Histología Vegetal: Técnicas Simples y Complejas. Sociedad Argentina de Botánica, Buenos Aires
Funding
This research was financially supported by a grant (UBACyT 2013–2016 GC 20020120100056BA) to B. Galati. M. Gotelli and D. Medan are affiliated with CONICET, Argentina.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Alexander Schulz
Rights and permissions
About this article
Cite this article
Gotelli, M.M., Galati, B.G., Zarlavsky, G. et al. Pollen and microsporangium development in Hovenia dulcis (Rhamnaceae): a different type of tapetal cell ultrastructure. Protoplasma 253, 1125–1133 (2016). https://doi.org/10.1007/s00709-015-0870-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-015-0870-x