Abstract
Plant cell wall secretion is the result of dynamic vesicle fusion events at the plasma membrane. The importance of the lipid bilayer environment of the plasma membrane and its interactions with the endomembrane system through vesicle traffic are well recognized. Recent advances in yeast molecular biology and biochemistry lead us to re-examine the hypothesis that non-vesicular traffic of lipids through close contact sites of the plasma membrane and endoplasmic reticulum could also be important in plant cell wall biosynthesis. Non-vesicular traffic is the extraction and transfer of individual lipid molecules from a donor bilayer to a target bilayer, usually with the assistance of lipid transfer proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agriculture, forestry, and carbon sequestration into the terrestrial biosphere all rely on plant growth. Growth occurs by the coordinated action of both cell division and cell expansion. During expansion, cell wall biosynthesis is required, in the form of cellulose deposition by cellulose synthase at the plasma membrane (PM) and production of cell wall matrix polysaccharides, which are synthesized in the Golgi apparatus and secreted by exocytosis (Cosgrove 2005; Burton et al. 2010).Vesicle traffic at the plant PM is important for delivery of extracellular matrix cargo and for the controlled delivery and removal of vesicles containing cellulose synthase complexes during wall synthesis (Crowell et al. 2009; Gutierrez et al. 2009; Wightman et al. 2009; Wightman and Turner 2010). In growing plant cells, the cortical cytoplasm is rich in vesicles that are fusing and budding with the PM, cortical endoplasmic reticulum (ER), and arrays of microtubules that lie parallel to the PM (Fig. 1).
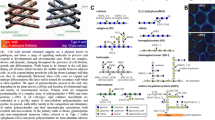
Cortical cytoplasm of an Arabidopsis inflorescence stem epidermal cell during cell elongation when both cell wall polysaccharide and cuticular lipid secretion are high. a The cell wall (CW) and plasma membrane (PM) have been removed from the top of the cell to expose the underlying cortical cytoplasm. Note the abundant array of cortical microtubules, the variety of budding/fusing vesicles including clathrin-coated pits (arrowhead), and the complex network of cortical endoplasmic reticulum (ER). ER–PM contact sites are marked by arrows. b Cross section of another stem epidermal cell. Scale bars = 500 nm. Samples were high pressure frozen, freeze substituted, and imaged as described by McFarlane et al. (2010)
It is increasingly clear that dynamic events at the nanoscale in the cell cortex must be elucidated in order to properly understand cell wall synthesis. However, these analyses must extend beyond monitoring protein complexes, such as cellulose synthases and microtubules, to include the different lipid bilayer environments as well. The major challenge is to obtain both nanometer spatial resolution in three dimensions and to integrate structural information with dynamics (Gutierrez et al. 2010). Our focus is on the lipid macromolecular assemblies in the plant cell cortex, specifically sites of contact between the ER and the PM.
Hypothesis: ER–PM contact sites act in non-vesicular lipid traffic
Given the large volume of cell wall material secreted by exocytosis and the high surface area to volume ratio of secretory vesicles, it is clear that more lipid is added to the plasma membrane bilayer than is required for growth (Robinson et al. 2008; Ketelaar et al. 2008). The cell wall limits the ability of the plasma membrane to expand when membrane is added. This necessitates significant lipid recycling from the plasma membrane to internal membrane compartments during cell wall expansion (Battey et al. 1999; Zarsky et al. 2009). In animal cells, the prevailing view is that membrane recycling is accomplished primarily by endocytosis. For plant systems, the effect of turgor pressure on the energetic costs of endocytosis has been modeled and debated (Cram 1980; Saxton and Breidenbach 1988). More recent studies of the uptake of styryl dyes such as FM4-64 and characterization of clathrin and related components have clearly demonstrated roles for endocytosis in membrane protein traffic (Robinson et al. 2008; Zarsky et al. 2009; Viotti et al. 2010; Konopka et al. 2008). The proportion of membrane lipid recycling following exocytosis that is due to clathrin vesicle-mediated endocytosis is difficult to estimate. Recently, developments in understanding of non-vesicular lipid traffic in mammals and yeast have led us to reconsider membrane recycling following exocytosis, specifically the hypothesis of Staehelin, who postulated that non-vesicular trafficking of lipids at ER–PM contact sites could also be a mechanism for membrane lipid recycling (Staehelin and Chapman 1987; Staehelin 1997).
Non-vesicular lipid trafficking is defined as movement of substances throughout the cell without use of the canonical vesicular trafficking machinery, including coat proteins, SNAREs, RABs, ARFs, and their related GTP exchange proteins (Holthuis and Levine 2005). Non-vesicular lipid trafficking connects organelles that are isolated from vesicle trafficking, such as mitochondria and plastids (Benning 2009), but it can also connect organelles through which abundant vesicle exchange occurs, e.g. ER–PM or ER–Golgi non-vesicular lipid trafficking (Voelker 2009). Non-vesicular lipid transfer would allow lipid recycling even under high turgor conditions (Staehelin and Chapman 1987; Craig and Staehelin 1988; Saxton and Breidenbach 1988). Although non-vesicular lipid recycling could potentially occur between the plasma membrane and any organelle within the secretory pathway, the ER is the best candidate destination because it plays a central role in lipid metabolism, including lipid synthesis and remodeling (Staehelin 1997; Melser et al. 2011). For example, when fluorescently labelled lipids are applied to living suspension-cultured plant cells, sphingolipids are retained in the PM, while phosphatidylcholine is cleaved to diacylglycerol and moved into the ER (Grabski et al. 1993). Similarly, under phosphate starvation, significant remodeling of PM lipids occurs, and the first step of this process is cleavage of a phospholipid into phosphatidic acid or diacylglycerol, before recycling to the ER (Tjellström et al. 2008).
Membrane contact sites are subcellular nanospaces where two biological membranes with distinct lipid and protein compositions are separated by a narrow (about 10 nm) layer of aqueous cytoplasm. Membrane contact sites limit diffusion of trafficked substrates within the confined nanospace, thus reducing loss to the bulk cytoplasm. Close associations between membranes provide a mechanism to limit diffusion time of a molecule between the origin and destination membranes, thereby allowing much higher rates of targeted transfer (Holthuis and Levine 2005; Voelker 2009; Prinz 2010). Lipid transfer proteins (LTPs) are found in all eukaryotes and are broadly defined as soluble proteins with a hydrophobic pocket that are potentially capable of lipid transfer in vitro (D’Angelo et al. 2008). This definition includes both the plant-specific family of secreted eight-cysteine motif proteins known as “non-specific LTPs” (Yeats and Rose 2008), as well as intracellular lipid transfer proteins capable of transferring phospholipids, sterols, or sphingolipids (reviewed by Lev 2010 for mammalian systems; Jouhet et al. 2007 for plants). Intracellular LTPs may play a role in non-vesicular lipid trafficking by binding a lipid substrate and shielding it from the hydrophilic cellular environment during its short diffusion between the origin and destination membranes.
In yeast (Saccharomyces cerevisiae), preliminary functional characterization of ER–PM contact sites has shown that non-vesicular lipid traffic can occur at these sites (Li and Prinz 2004; Raychaudhuri et al. 2006; Schulz et al. 2009), but whether they participate in bulk membrane recycling remains an open question. If ER–PM contact sites are participating in non-vesicular lipid trafficking, then the protein composition of ER–PM contact sites likely includes lipid transfer proteins, as well as tethering proteins to stabilize the interaction between the two membranes. In yeast and mammals, integral ER proteins, e.g. Scs2 in yeast, encoded by the VAP gene family (vesicle-associated membrane protein (VAMP)-associated proteins) have been implicated in contact site formation. In yeast, scs2∆ mutants displayed defects in cortical ER attachment to the PM in both light and electron microscopy, indicating that this ER-localized protein may be a component of ER–PM contact sites (Loewen et al. 2007). Scs2 interacts with Osh1 and Osh2, LTPs of the oxysterol-binding related protein (ORP) family, and Osh2 localization to cortical ER is dependent on the presence of Scs2 (Loewen et al. 2003). Recent studies using yeast Osh4 as a model demonstrated that the ORP lipid binding domain contained two membrane binding sites, potentially allowing them to bridge both the origin and destination membrane, and that sterol transport was dependent on membrane binding at the distal site (Schulz et al. 2009). Overexpression of mammalian ORPs led to increased non-vesicular sterol transport from PM to lipid droplets in HeLa cells (Jansen et al. 2011). Preliminary characterization of VAP and OSH homologs in plants demonstrated that an ORP from Arabidopsis, ORP3a, can bind sitosterol in vitro and that its ER localization is dependent on binding to the VAP homolog PVA12 (Saravanan et al. 2009). However, whether potential molecular components of membrane contact sites are acting in lipid traffic was not tested, and whether contact sites are important in general lipid homeostasis, such as membrane lipid recycling during cell wall secretion, remains unknown.
Both transmission electron microscopy and freeze-fracture electron microscopy have revealed ER–PM contact sites in a variety of plant species and cell types (Staehelin and Chapman 1987; Staehelin 1997). However, from these studies it was unclear whether ER–PM contact sites were due to chance associations or whether they were actively maintained to play a functional role within the cell. For many years, attempts to biochemically purify plasma membranes resulted in co-purification of ER membranes; however, these were dismissed as contamination. Recent careful biochemical analysis has demonstrated that this is not contamination but rather co-purification of the ER with the plasma membrane, representing genuine ER–PM contact sites (Larsson et al. 2007). Further evidence for ER–PM contact sites comes from detailed fluorescence microscopy studies of ER dynamics. While most of the cortical ER is highly motile, stationary puncta of ER have been observed, and these have been interpreted as contact sites with the plasma membrane (Oparka et al. 1994; Sparkes et al. 2009). Taken together, these data indicate that ER–PM contact sites are not chance associations between the two membranes. Instead, they are actively maintained, and this suggests that they play an important biological role.
The membrane lipid components that could be recycled from the PM to the ER are not known, although most direct transport assays, e.g. in yeast and mammals, have tested sterol flux. Membrane lipids added by vesicles would contain phospholipids, sphingolipids, and sterols, and any or all of these could be removed from the PM by lipid transfer proteins and trafficked through the ER–PM contact site to the cortical ER. However, the enrichment of sterols and sphingolipids in the plasma membrane, relative to the endoplasmic reticulum, suggests that phospholipids could be recycled from the phospholipid-poor plasma membrane to the phospholipid-rich ER in order to maintain this asymmetry. While the most probable hypothesis is that ER–PM contact sites are involved in retrograde non-vesicular lipid recycling, it is also possible that lipid traffic at ER–PM contact sites could be both retrograde membrane recycling and anterograde non-vesicular secretion of lipid products.
Anterograde lipid traffic includes export of essential oils, secreted by many economically important plants such as mint, as well as secretion of the lipids that form protective plant cell wall structures: waxes, suberin, and sporopollenin. Rapidly expanding epidermal cells can produce and secrete protective cell wall lipids at extraordinarily high flux rates. For example, in the stem epidermis of Arabidopsis, more than half of the fatty acid chains produced by these cells end up in the cuticle that overlays the cell wall (Suh et al. 2005). The protective wax cover on the plant surface limits water loss; sheds water, dirt, and spores; and mediates interactions with insects and fungi. The lipids of the cuticle are saturated very long chain hydrocarbons such as alkanes, fatty alcohols, and fatty acids. These specialized lipids are synthesized by ER-localized enzymes and secreted through the plasma membrane and the cell wall to the cell surface (Kunst and Samuels 2009). The export of lipids to the plant cuticle requires ATP binding cassette (ABC) transporters located in the plasma membrane. Mutants that carry defects in these ABC transporters accumulate cuticular lipids in the ER, but not in the Golgi apparatus (McFarlane et al. 2010). This indirect evidence suggests that cuticular lipids may move directly from the ER to the PM via a non-vesicular route, supporting a role for anterograde lipid secretion at ER–PM nanospaces.
Major limitations and ways forward: resolution tradeoffs, specific probes for and composition of lipid nanospaces
The major limitation in studying any cellular nanospace is the limit of resolution of a light microscope, which is the most useful tool for studying dynamic processes in live cells. Though electron microscopy can give much higher resolution images, it provides only static images that cannot be used to reconstruct a time course of events, and it provides images that are essentially two-dimensional. Thus, a major challenge in studying cellular nanospaces, such as ER–PM contact sites, comes in combining the temporal and 3D resolution of live cell imaging and the 2D resolution of electron microscopy. Three key techniques are emerging as possible solutions to these limitations: correlative microscopy (Mironov and Beznoussenko 2009), super-resolution microscopy (Gutierrez et al. 2010), and electron tomography (Haas and Otegui 2007).
A second limitation is the lack of specific probes for these lipid nanospaces. While fluorescently tagged lipids do exist, they often display unusual dynamics or undergo modification upon uptake by the cell (Grabski et al. 1993). No reliable lipid probes exist for lipid labeling at the electron microscopy level. An ideal lipid probe would be detectable in both live cell imaging and TEM, allowing for correlative localization studies to be performed.
To build useful fluorescent protein probes for PM–ER nanospaces, knowledge of specific protein components is required. Unfortunately, the protein composition of ER–PM contact sites has not been determined in any organism. Biochemical isolation of these sites has demonstrated that the bulk protein composition of the subfraction of ER that is associated with the plasma membrane is distinct from bulk ER or bulk PM (Larsson et al. 2007), and this is consistent with results from yeast (Pichler et al. 2001).
Dynamic events at the nanoscale in the cell cortex link cell wall biosynthetic processes such as secretory and endocytic vesicle traffic, lipid flux and, potentially, cellulose synthesis, and the cytoskeleton. The lipid macromolecular assemblies in the plant cell cortex such as ER–PM contact sites and the microdomains of the PM add structural complexity with functional implications for lipid homeostasis and traffic. The plasma membrane must be considered a three-dimensionally diverse bilayer with areas of stability and areas that are very dynamic and constantly changing. Studying the mechanism of specialized export of cuticular lipids focuses attention on the importance of the nanospaces of the plant cell cortex, and understanding these fine structures will also have implications for the broader field of cell wall biosynthesis.
References
Battey NH, James NC, Greenland AJ, Brownlee C (1999) Exocytosis and endocytosis. Plant Cell 11:643–660
Benning C (2009) Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Annu Rev Cell Dev Biol 25:71–91
Burton RA, Gidley MJ, Fincher GB (2010) Heterogeneity in the chemistry, structure and function of plant cell walls. Nat Chem Biol 6:724–732
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Craig S, Staehelin LA (1988) High pressure freezing of intact plant tissues. Evaluation and characterization of novel features of the endoplasmic reticulum and associated membrane systems. Eur J Cell Biol 46:81–93
Cram WJ (1980) Pinocytosis in plants. New Phytol 84:1–17
Crowell EF, Bischoff V, Desprez T, Rolland A, Stierhof YD, Schumacher K, Gonneau M, Hofte H, Vernhettes S (2009) Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell 21:1141–1154
D’Angelo G, Vicinanza M, De Matteis MA (2008) Lipid-transfer proteins in biosynthetic pathways. Curr Opin Cell Biol 20:360–370
Grabski S, De Feijter AW, Schindler M (1993) Endoplasmic reticulum forms a dynamic continuum for lipid diffusion between contiguous soybean root cells. Plant Cell 5:25–38
Gutierrez R, Lindeboom JJ, Paredez AR, Emons AMC, Ehrhardt DW (2009) Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat Cell Biol 11:797–806
Gutierrez R, Grossmann G, Frommer WB, Ehrhardt DW (2010) Opportunities to explore plant membrane organization with super-resolution microscopy. Plant Physiol 154:463–466
Haas TJ, Otegui MS (2007) Electron tomography in plant cell biology. J Integr Plant Biol 49:1091–1099
Holthuis JC, Levine TP (2005) Lipid traffic: floppy drives and a superhighway. Nat Rev Mol Cell Biol 6:209–220
Jansen M, Ohsaki Y, Rita Rega L, Bittman R, Olkkonen VM, Ikonen E (2011) Role of ORPs in sterol transport from plasma membrane to ER and lipid droplets in mammalian cells. Traffic 12:218–231
Jouhet J, Maréchal E, Block MA (2007) Glycerolipid transfer for the building of membranes in plant cells. Prog Lipid Res 46:37–55
Ketelaar T, Galway ME, Mulder BM, Emons AM (2008) Rates of exocytosis and endocytosis in Arabidopsis root hairs and pollen tubes. J Microsc 231:265–273
Konopka CA, Backues SK, Bednarek SY (2008) Dynamics of Arabidopsis dynamin-related protein 1C and a clathrin light chain at the plasma membrane. Plant Cell 20:1363–1380
Kunst L, Samuels L (2009) Plant cuticles shine: advances in wax biosynthesis and export. Curr Opin Plant Biol 12:721–727
Larsson KE, Kjellberg JM, Tjellstrom H, Sandelius AS (2007) LysoPC acyltransferase/PC transacylase activities in plant plasma membrane and plasma membrane-associated endoplasmic reticulum. BMC Plant Biol 7:64
Lev S (2010) Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nat Rev Mol Cell Biol 11:739–750
Li Y, Prinz WA (2004) ATP-binding cassette (ABC) transporters mediate nonvesicular, raft-modulated sterol movement from the plasma membrane to the endoplasmic reticulum. J Biol Chem 279:45226–45234
Loewen CJ, Roy A, Levine TP (2003) A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP. EMBO J 22:2025–2035
Loewen CJ, Young BP, Tavassoli S, Levine TP (2007) Inheritance of cortical ER in yeast is required for normal septin organization. J Cell Biol 179:467–483
McFarlane HE, Shin JJ, Bird DA, Samuels AL (2010) Arabidopsis ABCG transporters, which are required for export of diverse cuticular lipids, dimerize in different combinations. Plant Cell 22:3066–3075
Melser S, Molino D, Batailler B, Peypelut M, Laloi M, Wattelet-Boyer V, Bellec Y, Faure JD, Moreau P (2011) Links between lipid homeostasis, organelle morphodynamics and protein trafficking in eukaryotic and plant secretory pathways. Plant Cell Rep 30:177–193
Mironov AA, Beznoussenko GV (2009) Correlative microscopy: a potent tool for the study of rare or unique cellular and tissue events. J Microsc 235:308–321
Oparka KJ, Prior DAM, Crawford JW (1994) Behaviour of the plasma membrane, cortical ER, and plasmodesmata during plasmolysis of onion epidermal cells. Plant, Cell and Environ 17:163–171
Pichler H, Gaigg B, Hrastnik C, Achleitner G, Kohlwein SD, Zellnig G, Perktold A, Daum G (2001) A subfraction of the yeast endoplasmic reticulum associates with the plasma membrane and has a high capacity to synthesize lipids. Eur J Biochem 268:2351–2361
Prinz WA (2010) Lipid trafficking sans vesicles: where, why, how? Cell 143:870–874
Raychaudhuri S, Im YJ, Hurley JH, Prinz WA (2006) Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein–related proteins and phosphoinositides. J Cell Biol 173:107–119
Robinson DG, Jiang L, Schumacher K (2008) The endosomal system of plants: charting new and familiar territories. Plant Physiol 147:1482–1492
Saravanan RS, Slabaugh E, Singh VR, Lapidus LJ, Haas T, Brandizzi F (2009) The targeting of the oxysterol-binding protein ORP3a to the endoplasmic reticulum relies on the plant VAP33 homolog PVA12. Plant J 58:817–830
Saxton MJ, Breidenbach RW (1988) Receptor-mediated endocytosis in plants is energetically possible. Plant Physiol 86:993–995
Schulz TA, Choi MG, Raychaudhuri S, Mears JA, Ghirlando R, Hinshaw JE, Prinz WA (2009) Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologues. J Cell Biol 187:889–903
Sparkes I, Runions J, Hawes C, Griffing L (2009) Movement and remodeling of the endoplasmic reticulum in nondividing cells of tobacco leaves. Plant Cell 21:3937–3949
Staehelin LA (1997) The plant ER: a dynamic organelle composed of a large number of discrete functional domains. Plant J 11:1151–1165
Staehelin LA, Chapman RL (1987) Secretion and membrane recycling in plant cells: novel intermediary structures visualized in ultrarapidly frozen sycamore and carrot suspension-culture cells. Planta 171:43–57
Suh MC, Samuels AL, Jetter R, Kunst L, Pollard M, Ohlrogge J, Beisson F (2005) Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis. Plant Physiol 139:1649–1665
Tjellström H, Andersson MX, Larsson KE, Sandelius AS (2008) Membrane phospholipids as a phosphate reserve: the dynamic nature of phospholipid-to-digalactosyl diacylglycerol exchange in higher plants. Plant Cell Environ 31:1388–1398
Viotti C, Bubeck J, Stierhof YD, Krebs M, Langhans M, van den Berg W, van Dongen W, Richter S, Geldner N, Takano J, Jurgens G, de Vries SC, Robinson DG, Schumacher K (2010) Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell 22:1344–1357
Voelker DR (2009) Genetic and biochemical analysis of non-vesicular lipid traffic. Annu Rev Biochem 78:827–856
Wightman R, Turner S (2010) Trafficking of the plant cellulose synthase complex. Plant Physiol 153:427–432
Wightman R, Marshall R, Turner S (2009) A cellulose synthase-containing compartment moves rapidly beneath sites of secondary wall synthesis. Plant Cell Physiol 50:584–594
Yeats TH, Rose JKC (2008) The biochemistry and biology of extracellular plant lipid-transfer proteins (LTPs). Protein Sci 17:191–198
Zarsky V, Cvrckova F, Potocky M, Hala M (2009) Exocytosis and cell polarity in plants—exocyst and recycling domains. New Phytol 183:255–272
Acknowledgements
The authors thank the UBC Bioimaging Facility for technical assistance, the Peter Wall Institute for Advanced Research for funding the Nanospaces Exploratory Workshop, and Anna Stina Sandelius, Dr. Mats Andersson and Chris Loewen for stimulating discussions.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Geoffrey O. Wasteneys
Rights and permissions
About this article
Cite this article
Samuels, L., McFarlane, H.E. Plant cell wall secretion and lipid traffic at membrane contact sites of the cell cortex. Protoplasma 249 (Suppl 1), 19–23 (2012). https://doi.org/10.1007/s00709-011-0345-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-011-0345-7