Abstract
Fifty years ago Max Kleiber described what has become known as the “mouse-to-elephant” curve, i.e., a log–log plot of basal metabolic rate versus body mass. From these data, “Kleiber’s 3/4 law” was deduced, which states that metabolic activity scales as the three fourths-power of body mass. However, for reasons unknown so far, no such “universal scaling law” has been discovered for land plants (embryophytes). Here, we report that the metabolic rates of four different organs (cotyledons, cotyledonary hook, hypocotyl, and roots) of developing sunflower (Helianthus annuus L.) seedlings grown in darkness (skotomorphogenesis) and in white light (photomorphogenesis) differ by a factor of 2 to 5 and are largely independent of light treatment. The organ-specific respiration rate (oxygen uptake per minute per gram of fresh mass) of the apical hook, which is composed of cells with densely packaged cytoplasm, is much higher than that of the hypocotyl, an organ that contains vacuolated cells. Data for cell length, cell density, and DNA content reveal that (1) hook opening in white light is caused by a stimulation of cell elongation on the inside of the curved organ, (2) respiration, cell density and DNA content are much higher in the hook than in the stem, and (3) organ-specific respiration rates and the DNA contents of tissues are statistically correlated. We conclude that, due to the heterogeneity of the plant body caused by the vacuolization of the cells, Kleiber’s law, which was deduced using mammals as a model system, cannot be applied to embryophytes. In plants, this rule may reflect scaling phenomena at the level of the metabolically active protoplasmic contents of the cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Five decades ago, the Swiss-American scientist Max Kleiber (1893–1976) published his seminal book entitled The Fire of Life, wherein he summarized the available information relating metabolic rate R (oxygen uptake per min) to body mass M (kilograms per individual) in mammals. Based on these data, he presented what has become known as the “mouse-to elephant-curve” or “Kleiber’s law”, i.e., R = βM α, where β is a normalization constant and α (~3/4) is a scaling exponent (Kleiber 1961). This log–log scaling relationship appears to hold more or less true across a broad spectrum of protists (Lewis 1989), arthropods, such as insects (Niven and Scharlemann 2005) and vertebrates (Niklas 1994; Wang et al. 2001; Kutschera and Niklas 2009). However, a controversy exists concerning the numerical value of α with respect to the monophyletic group of the land plants (embryophytes) (Niklas and Kutschera 2009, 2010), even as to whether the relationship R~M α is allometric or isometric (i.e., α larger or smaller than 1.0, or α = 1.0, respectively).
West et al. (1997, 1999) hypothesize that α = 3/4 across plant as well as animal species based on theoretical grounds, whereas Reich et al. (2006) report α = 1.0 for comparatively young and small plants. In keeping with these two studies, Mori et al. (2010) report that α changes from 1.0 to three fourths with increasing M across nine orders of magnitude in body mass (i.e., M~10−5 to 103 kg) based on an examination of R directly measured for 271 whole plants, and they conclude that “the transition from a linear to a three fourths-power scaling may indicate fundamental physical and physiological constraints on the allocation of plant biomass between photosynthetic and non-photosynthetic organs over the course of ontogenetic plant growth” (Mori et al. 2010).
Recently, we presented evidence that R changes during the germination and early development of sunflower (Helianthus annuus L.) seedlings raised in the dark (scotomorphogenesis) and subsequently grown under white light (photomorphogenesis) (Kutschera and Niklas 2011). Based on preliminary data and simple biophysical principles, we hypothesized that “the metabolic rates of plant tissues undoubtedly differ or change as the volume fractions of individual cells and tissues change ontogenetically as a consequence of the accumulation of secondary cell wall layers and/or secondary tissues as plants increase in size” (Kutschera and Niklas 2011). Recently, Armstrong and Beckett (2011) analyzed changes in the oxygen consumption rate of root segments when the availability of O2 gradually decreases and thereby showed that it is possible to measure metabolic activity at very small anatomical level. However, we are not aware of a study wherein organ-specific rates of cellular respiration in developing tissues or parts of plants are reported or one in which respiration rates are correlated with critical cytological differences among tissue or organ types. This lack of information motivated the present study.
Here, we present a detailed analysis of how metabolic activity changes ontogenetically by examining the organ-specific respiration rates (R m, oxygen uptake per minute per gram fresh mass) of the cotyledons, cotyledonary hooks, hypocotyls, and roots of sunflower seedlings grown in the dark and subsequently under white light (Fig. 1). Since the hook was found to have the highest R m, we analyzed in detail the cytological basis of the light-mediated opening response, a process that is part of photomorphogenesis (Briggs and Olney 2001; Briggs and Spudich 2005). We also measured the cell densities, cell lengths, and DNA contents of this organ and compared these data with those of the hypocotyl, which had consistently much lower R m values. The correlations among these features and organ-specific respiration rates are statistically robust and thus have far-reaching implications for metabolic scaling theory as it relates to multicellular animals as well as plants (Niklas 1994, 2004; Kutschera and Niklas 2009; West et al. 1997, 1999; Reich et al. 2006; Wang et al. 2001; Mori et al. 2010; White 2010). In particular, we argue that ontogenetic changes in the volume fraction of the living substance in the plant body (i.e., the symplast) result in log-log nonlinear relationships between R and M. Because R m is shown to correlate with organ DNA content, which in turn provides a measure of the potential for metabolic activity, we further argue that this hypothesis could apply to all types of organisms.
Germination and development of sunflower seedlings (H. annuus) and separation of the juvenile plants into four different parts (a–d). Achenes were sown in moist vermiculite and raised in darkness (D; days 0 to 6; skotomorphogenesis) or grown for 3 days in the dark and subsequently irradiated for 1 to 3 days with continuous white light (WL) (photomorphogenesis). Note that the hook (b) is open in the 6-day-old light-grown seedling and closed in the majority of dark controls. The seedlings were kept in 99% relative humidity at 25°C
Materials and methods
Plant material and growth conditions
Sunflower (H. annuus L. cv. Giganteus) achenes (containing seeds) were germinated in moist vermiculite in closed, transparent plastic boxes at 25°C in darkness (ca. 99% relative humidity) as described by Kutschera et al. (2010). On day 3 after sowing, half of the seedling trays were transferred into an irradiated growth chamber (continuous white light (WL); photosynthetically active radiation, ~120 μmol m−2 s−1 at plant level, see Kutschera and Niklas 2007). The other trays were left in darkness as controls. One to 6 days after sowing, imbibed embryos or germinated seedlings, respectively, were removed from the vermiculite, washed, and blotted dry. After mechanical removal of the attached seed coat (with pericarp), representative seedlings (and dry embryos at day 0) were analyzed (Fig. 1).
Determination of organ-specific cellular respiration
Two-to 6-day-old sunflower seedlings were removed from the vermiculite, washed, and separated into four parts: (a) cotyledons, (b) hook, (c) hypocotyls, and (d) root system. In some experiments, entire embryos were used (days 0 and 1 after sowing) (Fig. 1). In a control experiment, hypocotyls were excised from 3-day-old etiolated seedlings and peeled (i.e., separated into outer and inner tissues, cortex/vascular bundles and pith, as described by Kutschera and Niklas 2007). Oxygen uptake (i.e., respiration of the cells of the isolated organs in darkness) was measured polarographically in O2-saturated water using a Clark-type oxygen electrode as described by Björkman and Demmig (1987) and Kutschera (1999). The rate of O2 depletion of the living samples (ca. 0.5 g) in air-saturated water was recorded over a period of 30 min in darkness (25°C). Thereafter, the O2 uptake of killed (boiled-cooled down) samples was determined as a control (i.e., physical O2 absorption of the tissues unrelated to metabolic activity of the cells). At the end of these measurements, the embryos or isolated organs were removed from the water, blotted dry, and their fresh mass determined with a micro-balance. These data were used to calculate the rates of cellular respiration per gram of material (R m; unit, nmol O2 min−1 g fresh mass−1). All experiments were repeated six times using independent batches of fresh seeds (ten individuals each) and the average values of these replicates were calculated. Thereafter, the standard errors of the means were calculated. Since these values were not larger than the sizes of the symbols, they are not shown in the figures.
Determination of cell length, cell density, and DNA content
The lengths of the epidermal cells on the flank of the closed (and opened) hooks and in the middle of the hypocotyls were measured as described by Hodick and Kutschera (1992) (50 cells per plant and 12 replicates each). For determination of cell density, the hook and hypocotyl were excised from 2- to 6-day-old seedlings. The organ segments were cut into smaller pieces. Samples consisting of ca. 0.1 g of tissue were incubated for 3 days in a solution of pectinase (10 vol.%), 0.1 M Na-EDTA in 0.01 M Na citrate buffer (pH 5.0) at 45°C. After the complete separation of the cells, these units were counted under a light microscope using a Thoma-Chamber (depth, 0.1 mm; volume, 0.0025 mm2). Based on these data, cell densities (unit, 106 cells per gram of fresh mass) were calculated for the apical hook and the stem, respectively.
The DNA contents of cotyledonary hooks and hypocotyls were determined using the protocol for extraction and quantification of genomic DNA as described by Sambrook et al. (1989). Samples (ca. 0.1 g of fresh mass) were homogenized in the presence of 0.3 ml of DNA extraction reagent (Sigma-Genosys, The Woodlands, TX). Thereafter, 0.7 ml of reagent was added to the homogenate and the samples shaken gently for 18 h at 25°C in darkness. After centrifugation (3,000 rpm, 15 min), the viscous aqueous phase was transferred to a new test tube and chloroform (0.2 ml) added. After removal of the phase that contains RNA and protein, DNA was precipitated with ethanol (100%, p.a.). The white mass (genomic DNA) was washed in ethanol (80%, p.a.), dried, and dissolved in 1 ml of double-distilled H2O. As a control, samples of salmon DNA (Sigma-Genosys) were treated as described above and the extinction coefficient (E) determined spectrometrically at 256 nm. The absorbance maximum at 256 nm and the E value were used to calculate DNA contents of the tissue samples (unit, milligrams per milliliter). Based on these results and the corresponding fresh mass data, the DNA contents of the hooks and stems were calculated (unit: milligrams DNA per gram of fresh mass).
Statistical protocols
Standard parametric tests (e.g., Tukey–Kramer all pair-wise comparisons) among the respiration rates of the four seedling body parts could not be performed because these measurements were not independent, because they violated the assumptions of normality and homoscedasticity, and because there exists variation among the plants sampled each day during the course of experimental measurements. With these considerations in mind, a blocked (according to the identification number assigned to each plant, factor A) two-way analysis of variance (ANOVA) with day (factor B), organ type (factor C), and BC interaction for each of the four organ types (i.e., cotyledons, cotyledonary hook, hypocotyl, and root system) was employed. This ANOVA was followed by a one-way ANOVA (using factors C and BC) and Tukey–Kramer comparisons to determine whether the respiration rates of the four organ types differed for each day and whether they differed for each organ type during seedling ontogeny. The results of these statistical comparisons were subsequently compared using two non-parametric tests, i.e., the Wilcoxon/Kruskal–Wallis and the van der Waerden test (Sokal and Rohlf 1981). Both of these tests gave the same results, which did not differ significantly from those given by the ANOVA tests.
Ordinary least squares (OLS) regression was used to determine the relationship between non-transformed and log10-transformed data for respiration and all other variables of interest. Reduced major axis (RMA; also called standard major axis) regression protocols were employed to calculate the slopes and Y-intercepts of log–log linear relationships (i.e., α RMA and log β RMA, respectively). These parameters were computed as described previously (Kutschera and Niklas 2011). The protocols used in the software package “Standardized Major Axis Tests and Routines” were used to determine whether the numerical values of α RMA and log β RMA differed among the data collected from plants raised in the dark and those subsequently grown in white light (Kutschera and Niklas 2011; Warton and Weber 2002; Niklas 1992). Heterogeneity in α RMA and log β RMA was rejected in all cases if P > 0.05.
Light and transmission electron microscopy
Light micrographs of hooks and hypocotyls cut from 3-day-old etiolated sunflower seedlings were prepared and analyzed as described by Hodick and Kutschera (1992). For transmission electron microscopy, sections, 2 mm in length, were cut in the hook or sub-apical region of the plant axis, using 3-day-old dark-grown seedlings of average size (see Fig. 1). The samples were fixed for 2 h in 4% glutaraldehyde, washed for 30 min in sodiumcacodylate buffer (0.1 M, pH 7.2), and fixed for 2 h in OsO4 (2%). After de-hydration in an alcohol series, the samples were embedded in Spurr’s epoxyresin. Thereafter, ultrathin transverse sections were cut with a diamond knife and viewed in an electron microscope (Hodick and Kutschera 1992).
Results
Tissue damage and metabolic activity
Two series of experiments were carried out to analyze a possible wound-induced enhancement (or reduction) in metabolic activity of the cells. In the first set, R m was measured on entire, intact 3-day-old sunflower seedlings grown in the dark. Thereafter, R m was quantified on the four isolated parts shown in Fig. 1. In the second series, R m was determined using entire (excised) hypocotyls and isolated outer and inner tissues, respectively. Our data show that neither dissection nor peeling (mechanical removal of the peripheral 2 to 3 cell layers) has an effect on R m of the sunflower tissues (Table 1). Hence, at least over the time period O2-uptake measurements were taken (30 min), mechanical wounding (excision and peeling) does not have an effect on the rate of oxygen consumption.
Metabolic activity of different plant organs
The R m values were measured for dry embryos isolated from the seed coat (day zero), embryos with an elongated radicle (day 1), and four different organs cut from seedlings that were harvested between days 2 and 6 after sowing (Fig. 1). On day 3 after sowing, half of the seedling trays were irradiated for the next 3 days with continuous WL; the other trays were left in darkness as controls. The rate of oxygen uptake of isolated dry embryos was very low (0.40 ± 0.01 nmol O2 min−1 g fresh mass−1, n = 6). After the emergence of the radicle, R m increased ca. 350-fold (Fig. 2). Between days 2 and 4, the R m of the cotyledons reached a maximum of ca. 230 nmol O2 min−1 g fresh mass−1 and thereafter declined. The cotyledonary hook consistently had the highest metabolic activity (i.e., R m ranged between 540 and 320 nmol O2 min−1 g fresh mass−1). A comparison between the cotyledonary hook and the hypocotyl revealed a 4- to 5-fold higher metabolic rate in the former compared with the latter. Light had no effect on the metabolic activity of the hook or the cotyledons, which opened and unfolded normally. However, minor WL-induced enhancements in cellular respiration were recorded in the roots and hypocotyls of the developing seedlings (Fig. 2).
Changes in the organ-specific rates of cellular respiration (R m) during seedling development in darkness (closed symbols) and white light (WL, open symbols). Sunflower seedlings were grown for up to 6 days and separated into four different parts as shown in Fig. 1. R m values were measured over a time period of 30 min after organ separation (days 2 to 6) or on dry seeds (isolated embryos, day 0) and entire juvenile embryos (day 1), respectively. Arrows, onset of WL treatment. Fm fresh mass
Cytological basis of light-induced hook opening
The enlargement of axial plant organs is caused by water uptake by the cells and a concomitant anisotropic expansion of the growth-controlling cell walls (Boyer and Silk 2004; Cosgrove 2005). Microscopy revealed that the volume fraction of cytoplasm per cell (and thus the amount of oxygen-consuming mitochondria per cell) appears to be much higher in the hook than in the hypocotyl of the plant axis. This observation provides a proximate explanation for the 4- to 5-fold higher respiratory rate of the hook vs. the hypocotyl. Specifically, microscopy shows that the cells in the closed apical hook are small, isodiametric, and contain pro-vacuoles (Fig. 3a, b), whereas those in the hypocotyl are elongated, highly vacuolated tubes (Fig. 3c, d).
Light and transmission electron micrographs of the epidermal cells from 2- versus 6-day-old (a–d) sunflower seedlings grown in the dark. The epidermal cells on the flank of the hook (a) are isodiametric, whereas those of the hypocotyl (c) are elongated. Transverse sections of the cells revealed a low (b) and high (d) degree of vacuolation in the hook and stem region of young and older seedlings, respectively. C cytoplasm, V vacuole, W cell wall. Bars: 100 (c) and 10 μm (d), respectively
These qualitative cytological observations are in accordance with the cell-length data summarized in Fig. 4. In the dark-grown hypocotyl, the average length of the epidermal cells increases from 70 to 245 μm between days 2 and 6 after sowing. Upon irradiation with WL, an inhibition of cell elongation occurs that corresponds to the reduction in stem growth depicted in Fig. 1. The epidermal cells of the hook, measured on the flat side of the organ, were 40 to 50 μm long; WL had no effect on cell length. However, WL-induced hook opening (Fig. 1) was associated with an enhancement in epidermal cell length on the inside and a reduction of cell length on the opposite outer surface of the hook (Fig. 4a, b). These differential changes in cell size provide the mechanical hook-opening mechanism in developing sunflower seedlings.
Changes in the lengths of the epidermal cells during seedling development in sunflower. Average cell lengths were determined on the flanks of the hook (Ho 1), the outer surface of the organ (Ho 2), and the hypocotyl (Hy) (a). In a second set of experiments using the same batch of seedlings, cell length measurements were performed on the inside of the hook (Ho 3) (b). Dark symbols, seedlings raised in the dark; white symbols, plants grown in WL. Arrows, onset of WL treatment
Changes in cell density and DNA content
In accord with cytological observations (Fig. 3), quantitative analyses revealed that the average density of the cells per g fresh mass is much higher in the hook than in the hypocotyl (Fig. 5a). WL had a minor effect on cell density, particularly in the hypocotyl, which was insensitive to light treatment. The DNA contents of these two organs changed considerably during plant development (Fig. 5b). In the hook, the amount of DNA per g fresh mass was ca. 3-fold higher than in the hypocotyl. During WL-induced hook opening, the DNA content declined, but no change was observed in the stem.
Changes in cell density (a) and DNA content (b) during seedling development in the dark or white light. Two plant organs, the hook and the hypocotyl, were analyzed separately, using the same population of seedlings. Dark symbols, seedlings raised in the dark; white symbols, plants grown in WL. Arrow, start of WL treatment. Fm fresh mass
Statistical analyses of the data
Two- and one-way ANOVA followed by Tukey–Kramer comparisons among the four organ types revealed that R m differed significantly in the majority of cases among the four organ types for each day during the course of the experiments and in the majority of cases for each organ type during seedling maturation (Fig. 2; Table 2). Regardless of the age of seedlings, the cotyledonary hook had the highest respiration rate, reached its maximum on the second day of germination, subsequently declined, and reached its minimum on days 5 and 6. Tukey–Kramer comparisons among seedlings grown in the dark and those irradiated with WL during days 4, 5, and 6 after sowing found no statistically significant differences in the respiration rates of cotyledons and the cotyledonary hook, in contrast to differences of R m of the hypocotyl and roots (Fig. 2; Table 2).
OLS regression analyses of log10-transformed data for the R m and DNA contents of the hook indicated a significant correlation between these two variables of interest measured for seedlings grown in the dark (i.e., r 2 = 0.909, n = 30, F = 278.4, P < 0.0001) and for seedlings grown in the dark and subsequently grown in WL (i.e., r 2 = 0.633, n = 18, F = 27.6, P < 0.0001). OLS regression also showed that DNA contents and cell densities were significantly correlated among all plants from both treatments (e.g., for the hook, r 2 = 0.839, n = 48, F = 240.6, P < 0.0001). The relationship between DNA contents and cell length was log–log nonlinear; DNA contents decreased with increasing cell length. OLS regression additionally revealed statistically significant correlations between R m and cell density in the cotyledonary hook of seedlings grown in the dark (i.e., r 2 = 0.643, n = 30, F = 50.5, P < 0.0001) and of the cotyledonary hooks of seedlings subsequently grown in the light (i.e., r 2 = 0.607, n = 18, F = 24.8, P = 0.0001). Two-way ANOVA blocked for treatment (plants grown in the dark versus those subsequently grown in the light) indicated that DNA content was the only significant factor contributing to the variance observed for respiration rates, i.e., DNA content accounted for 78.5% of the variance in R m.
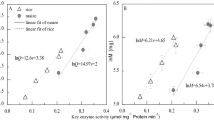
RMA regression analyses indicated that the R m of the cotyledonary hooks of plants grown in the dark, scaled nearly isometrically with respect to DNA content (i.e., scaling exponent α RMA = 0.956; 95% confidence intervals = 0.919, 0.992) and differed significantly from the R m of the opened hooks of seedlings grown subsequently in the light (i.e., α RMA = 0.186; 95% confidence intervals = 0.166, 0.206) (Fig. 6). Similar results were obtained from RMA regression analysis of R m and DNA content of the hypocotyl (data not shown). Finally, regression analysis showed that the respiration rates of plants grown in the dark were negatively correlated with dry mass. In the case of the cotyledonary hook, R m scaled as the −23.7 power of dry mass (95% confidence intervals = −24.1, −23.3), which is consistent with the hypothesis that dry mass is largely composed of metabolically inert materials (e.g., cellulose, hemicellulose, and other cell wall polysaccharides as well as glycoproteins).
The relationship between the log10-transformed data for organ-specific respiration rate (R m) and DNA content determined for the hook of dark- and light-grown sunflower seedlings (see inserts; note that in dark-grown seedlings, the hook is closed and in irradiated plants open. The arrow points to the organ segments that were excised and analyzed). Regression analyses indicate that R m and DNA content are statistically robustly correlated for seedlings subjected to each of the two treatments, that the regression slopes statistically significantly differ between the two treatments, and that both variables of interest decrease as seedlings mature and increase in size
Discussion
Our detailed study of the respiration rates of sunflower seedlings demonstrates a statistically strong correlation between R m and the DNA content of the upper seedling body parts. The data show that the closed cotyledonary hook, which protects the seedling during growth below the surface of the earth, has the highest R m, DNA content and cell density among the four organ types. The results also document that R m is strongly correlated with DNA contents and cell density (and that R m is negatively correlated with dry mass as a consequence of metabolically inert materials predominantly in the cell wall) over the entire course of seedling development, both for the cotyledonary hook and the hypocotyl. Finally, our results demonstrate that even very small and comparatively immature multicellular organisms, such as the sunflower seedling, are spatiotemporally highly dynamic in terms of respiration rates, cytology, and DNA contents.
These findings have a particular bearing on empirical and theoretical studies that attempt to relate growth or respiration rates with differences in body size (Kleiber 1961; Niklas 1994, 2004; Kutschera and Niklas 2009; White 2010). Specifically, they indicate that different parts of an organism can have significantly different growth and respiration rates, that these rates change during development, and that they correlate with the metabolic activity and capacity of body parts as gauged directly by DNA content (or indirectly by cell density). If it is reasonable to argue that growth rates across all aerobic organisms depend on metabolic activity and that cellular respiration depends on the number of mitochondria per cell, it follows that growth and respiration rates are correlated with the DNA contents of cells and tissues. This extended syllogism, in tandem with the data presented here, provides a proximal explanation for why metabolic rates scale nearly isometrically across very small, juvenile plants and subsequently declines to a minimum with increasing body sizes as plants mature and increase to adult size (Reich et al. 2006; Mori et al. 2010; Kutschera and Niklas 2011). The explanation lies in the ontogenetic decrease in the volume fraction of metabolically active cells/tissues. Previously, the ontogenetic decline in plant respiration rates was ascribed to the accumulation of metabolically “inert” cell wall materials and secondary tissues in herbaceous and woody plants (Niklas 1994, 2004; Gillooly et al. 2001, 2002; Price et al. 2010; Enquist et al. 2007). However, the data presented here provide a more wide-ranging explanation for the dependency of respiration rates on body mass, both across conspecifics differing in size and species with different adult body masses since DNA content and cell density are biological variables that span the entire spectrum of multicellular eukaryotes.
The hypothesis that intra- and interspecific allometries relating respiration rates and body size are controlled by, or at the very least correlated with cell- or tissue-specific DNA content (that can change ontogenetically) clearly requires extensive experimental data drawn from different species, particularly since recent meta-analysis of respiration rates across diverse organisms shows conclusively that respiration rates are predictably correlated with body temperature (Dell et al. 2011). An alternative, albeit not mutually exclusive approach would be to use cell density as a surrogate measure of DNA content since these two variables appear to be highly correlated in the case of plants. Clearly, the pursuit of either research agenda is a time-consuming task. However, based on the data presented here and related studies on mammals (Wang et al. 2001), it is reasonable to argue that all organisms (unicellular or multicellular, animals as well as plants) are “spatiotemporally heterogeneous” at the cellular or tissue, organ, and organ-system levels of organization and that cellular DNA content can change ontogenetically (even for unicellular eukaryotes). It is therefore reasonable to argue that Kleiber’s law, which was based originally on experiments using mammals (Kleiber 1961) but has been extended to encompass all kinds of organisms (see Lewis 1989; Wang et al. 2001; Niven and Scharlemann 2005) is the result of scaling phenomena operating at the level of the protoplasmic contents of the cells. We conclude that Kleiber’s law is a manifestation of how the volume fractions of the metabolically active and non-living body parts change during ontogenesis and how they differ among the adult forms of uni- and multicellular, oxygen-consuming (aerobic) organisms.
Abbreviations
- M :
-
Body mass
- R :
-
Rate of cellular respiration
- R m :
-
Organ-specific respiration rate
- WL:
-
White light
References
Armstrong W, Beckett PM (2011) Experimental and modelling data contradict the idea of respiratory down-regulation in plant tissues at an internal [O2] substantially above the critical oxygen pressure for cytochrome oxidase. New Phytol 190:431–444
Björkman O, Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 170:489–504
Boyer JS, Silk WK (2004) Hydraulics of plant growth. Funct Plant Biol 31:761–773
Briggs WR, Olney MA (2001) Photoreceptors in plant photomorphogenesis to date: Five phytochromes, two cryptochromes, one phototropin, and one superchrome. Plant Physiol 125:85–88
Briggs WR, Spudich J (eds) (2005) Handbook of photosensory receptors. Wiley, Weinheim
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Dell AI, Pawar S, Savage VM (2011) Systematic variation in the temperature dependence of physiological and ecological traits. Proc Natl Acad Sci USA 108:10591–10596
Enquist BJ, Allen AP, Brown JH, Gillooly JF, Kerkoff AJ, Niklas KJ, Price CA, West GB (2007) Biological scaling: does the exception prove the rule? Nature 445:E9–E10
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251
Gillooly JF, Charnov EL, West GB, Savage VM, Brown JH (2002) Effects of size and temperature on developmental time. Nature 417:70–73
Hodick D, Kutschera U (1992) Light-induced inhibition of elongation growth in sunflower hypocotyls. Biophysical and ultrastructural investigations. Protoplasma 168:7–13
Kleiber M (1961) The fire of life: an introduction to animal energetics. Wiley, New York
Kutschera U (1999) Fusicoccin-induced growth and dark respiration in rye coleoptiles. J Plant Physiol 154:554–556
Kutschera U, Niklas KJ (2007) The epidermal-growth-control theory of stem elongation: an old and a new perspective. J Plant Physiol 164:1395–1409
Kutschera U, Niklas KJ (2009) Evolutionary plant physiology: Charles Darwin’s forgotten synthesis. Naturwissenschaften 96:1339–1354
Kutschera U, Niklas KJ (2011) Ontogenetic changes in the scaling of cellular respiration with respect to size among sunflower seedlings. Plant Signal Behav 6:72–76
Kutschera U, Deng Z, Oses-Prieto JA, Burlingame AL, Wang Z-Y (2010) Cessation of coleoptile elongation and loss of auxin sensivity in developing rye seedlings. A quantitative proteomic analysis. Plant Signal Behav 5:509–517
Lewis WM (1989) Further evidence for anomalous size scaling of respiration in phytoplankton. J Phycol 25:359–397
Mori S, Yamaji K, Ishida A, Prokushkin SG, Masyagina OV, Hagihara A, Hoque A, Suwa R, Osawa A, Nishizono T, Ueda T, Kinjo M, Miyagi T, Kajimoto T, Koike T, Matsuura Y, Toma T, Zyryanova OA, Abaimov AP, Awaya Y, Araki MG, Kawasaki T, Chiba Y, Umari M (2010) Mixed-powered scaling of whole-plant respiration from seedlings to giant trees. Proc Natl Acad Sci USA 107:1447–1451
Niklas KJ (1992) Plant biomechanics. An engineering approach to plant form and function. University of Chicago Press, Chicago
Niklas KJ (1994) Plant allometry. The scaling of form and process. University of Chicago Press, Chicago
Niklas KJ (2004) Plant allometry: is there a grand unifying theory? Biol Rev 79:871–889
Niklas KJ, Kutschera U (2009) The evolutionary development of plant body plans. Funct Plant Biol 36:682–695
Niklas KJ, Kutschera U (2010) The evolution of the land plant life cycle. New Phytol 185:27–41
Niven JA, Scharlemann PW (2005) Do insect metabolic rates at rest and during flight scale with body mass? Biol Lett 1:346–349
Price CA, Gillooly JF, Allen AP, Weitz JS, Niklas KJ (2010) The metabolic theory of ecology: prospects and challenges for plant biology. New Phytol 188:696–710
Reich PB, Tjoelker MG, Machado J-L, Oleksyn J (2006) Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature 439:457–461
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Press, Cold Spring Harbor, New York
Sokal RR, Rohlf FJ (1981) Biometry, 2nd edn. W. H. Freeman, New York
Wang Z, O’Conner TP, Heshka S, Heymsfield SB (2001) The reconstruction of Kleiber’s law at the organ-tissue level. J Nutr 131:2967–2970
Warton DI, Weber NC (2002) Common slope tests for bivariate errors-in-variables models. Biometrical J 44:161–174
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276:122–126
West GB, Brown JH, Enquist BJ (1999) A general model for the structure and allometry of plant vascular systems. Nature 400:664–667
White CR (2010) Physiology: there is no single p. Nature 464:691–692
Acknowledgments
We thank Prof. W. R. Briggs (Department of Plant Biology, Carnegie Institution for Science, Stanford, CA 94305, USA) for the provision of laboratory space and consultation, and helpful comments of an earlier version of the manuscript. This work was supported by the Alexander von Humboldt-Stiftung (AvH, Bonn, Germany; Fellowships Stanford/USA 2009–2011 to U. K.).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Rights and permissions
About this article
Cite this article
Kutschera, U., Niklas, K.J. Organ-specific rates of cellular respiration in developing sunflower seedlings and their bearing on metabolic scaling theory. Protoplasma 249, 1049–1057 (2012). https://doi.org/10.1007/s00709-011-0338-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-011-0338-6