Abstract
The formation of viable angiosperm seeds involves the co-ordinated growth and development of three genetically distinct organisms, the maternally derived seed coat and the zygotic embryo and endosperm. The physical relationships of these tissues are initially established during the specification and differentiation of the female gametophyte within the tissues of the developing ovule. The molecular programmes implicated in both ovule and seed development involve elements of globally important pathways (such as auxin signalling), as well as ovule- and seed-specific pathways. Recurrent themes, such as the precisely controlled death of specific cell types and the regulation of cell–cell communication and nutrition by the selective establishment of symplastic and apoplastic barriers, appear to play key roles in both pre- and post-fertilization seed development. Much of post-fertilization seed growth occurs during a key developmental window shortly after fertilization and involves the dramatic expansion of the young endosperm, constrained by surrounding maternal tissues. The complex tissue-specific regulation of carbohydrate metabolism in specific seed compartments has been shown to provide a driving force for this early seed expansion. The embryo, which is arguably the most important component of the seed, appears to be only minimally involved in early seed development. Given the evolutionary and agronomic importance of angiosperm seeds, the complex combination of communication pathways which co-ordinate their growth and development remains remarkably poorly understood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production of seeds was a major innovation in the evolution of vascular plants, playing an important role in the success of all seed plants, and in particular, the angiosperms (reviewed in (Linkies et al. 2010). In addition to their interest as an evolutionary innovation, seeds, and the plants which produce them, also underlie the development of human agricultural practices and, thus, civilization. Today, seeds constitute a major proportion of the diet in many human cultures, and a drive to improve seed yield and quality thus provides much of the impetus behind research into seed biology. This agronomic interest has led to a marked bias in seed research in favour of specific areas, such as the control of seed dormancy, the regulation of metabolic processes during grain filling and the epigenetic control of endosperm development. Other areas of seed biology, however, remain relatively poorly understood. Angiosperm seed development is an extremely complex process necessitating the strictly co-ordinated growth and development of three closely juxtaposed but genetically distinct organisms, in order to assure the survival and establishment of viable progeny. This makes the developing seed an attractive system in which to study the control and co-ordination of growth. This review will aim to summarise current knowledge of the molecular and cellular pathways via which the three main seed compartments, the seed coat, the endosperm and the embryo, communicate to co-ordinate their growth and development. Evidence from a range of model angiosperms will be integrated, with the aim of providing an overview of the types of molecular pathways and mechanisms which could be involved in this complex process. Later interactions, especially those involved in seed maturation, the establishment and maintenance of dormancy, and germination have been widely reviewed elsewhere and will not be considered here. For reference, Fig. 1 shows the main tissues under discussion as they appear at two key stages in seed development in the model species Arabidopsis thaliana.
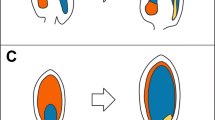
The mature ovule (a) and young developing seed (b) of A. thaliana. a The mature ovule contains the female gametophyte (blue), composed of the egg cell (dark), the two synergids (mid) and the central cell (light). The degenerating antipodal cells (A) are situated next to the maternal chalazal tissues (C). The gametophyte is enclosed by the inner integument (pink) and the outer integument (red). The micropyle (M) is indicated. b Post fertilization the egg cell gives rise to the embryo (dark green), which develops a stalk like suspensor. The central cell gives rise to the triploid endosperm (pale green). The specialized ESR is indicated by a dotted line, and the chalazal endosperm is labelled CE. The integuments expand in a co-ordinated fashion as the endosperm grows, with the inner integument developing a specialized internal cell layer, the endothelium (lighter pink), adjacent to the endosperm
The ovule
Sexually derived angiosperm seeds develop to maturity following the double fertilization of ovules, which can be considered as arrested “protoseeds”. Ovules are generated from projections of placental ovary tissue, inside the tip of which (the nucellus), a single archesporial cell, is selected. Although in some angiosperms this cell may divide prior to megasporocyte selection and differentiation, in model species such as maize and Arabidopsis, differentiation occurs directly to form the megasporocyte/megaspore mother cell (MMC), which subsequently undergoes meiosis. The developmental fate of the resulting megaspores varies in some basal angiosperms, where more than one can contribute to reproductive structures. However, in most model species including Arabidopsis and cereals, following the programmed death of three of its siblings, only one of the megaspores undergoes mitotic divisions to produce a mature, multicellular, female gametophyte (egg sac). The egg sac contains two fertilization-competent cells, one of which, the egg cell, will, upon fertilization, produce the zygotic embryo. The second cell is destined, upon fertilization, to give rise to a unique innovation of the angiosperms; the endosperm. During its development, sheets of maternal tissue called integuments (the precursors of the seed coat/testa) grow out from the chalazal region of the ovule to sheath the nucellus and female gametophyte. The structure of the mature, fertilization-competent ovule of the model species A. thaliana is schematized in Fig. 1a. Once integument outgrowth and gametophyte maturation are complete, the ovule enters a period of effective dormancy until either fertilization or degeneration occurs. If successful, fertilization triggers a suite of developmental programmes, which lead ultimately to the production of a mature, dormant seed containing a germination-competent embryo.
Seed development and ovule development are often considered in isolation. However, because all important seed tissues are directly derived from ovule cells/structures and the interactions between these tissues are initiated during ovule morphogenesis, it is impossible to fully divorce developmental pathways acting during post-fertilization seed development from those involved in ovule development.
Act 1. Setting the scene: cell and tissue interactions during ovule development
Specification of the megaspore mother cell
The interaction of the female gametophyte with surrounding nucellar tissues initiates prior to its inception, during the selection and differentiation of the MMC. Mutants have been reported in both rice and maize in which multiple cells acquire MMC fate. The MULTIPLE SPOROCYTE1 (MSP1) locus of rice encodes a leucine-rich repeat receptor-like kinase (LRR-RLK) expressed in nucellus cells surrounding the megasporocyte and is presumed to mediate feedback inhibition from the megasporocyte preventing neighbouring cells from entering the germline (Nonomura et al. 2003). The extracellular domain of the MSP1 protein may bind a putative peptide ligand, OsTDL1A, which is also involved in germline specification (Zhao et al. 2008). However, both MPS1 and OsTDL1A are expressed in the nucellus, but not the germline. It, therefore, seems likely that another as yet unidentified germline-expressed component could also be involved in ensuring that only one cell acquires megasporocyte fate.
Recent research has also shown that the activity of ARGONAUTE 9 (AGO9) and other components of 24-nucleotide small interfering RNA (siRNA) biosynthetic pathways implicated in the silencing of transposable elements is necessary for restricting the acquisition of gametic identity by nucellus cells (Olmedo-Monfil et al. 2010). AGO9 expression is, again, not detected in the germline but restricted to neighbouring somatic cells, suggesting that non-autonomous movement of siRNAs into the gamete precursors may be implicated in controlling their specification. Thus, inter-tissue movement of signals both apoplastically (peptide ligands) and symplastically (small RNAs) may control the capacity of somatic cells to acquire gametic cell fates.
Selection of the functional megaspore: the role of MMC polarization
In most higher angiosperms, the egg sac is monosporous, i.e. formed from only one of the four megaspores produced by meiosis. In Arabidopsis, rice and many other species, the functional megaspore (FM) appears to be selected based on position, with the most proximal (chalazal) surviving and the remaining three (situated near the eventual micropyle (micropylar) undergoing cell death. The molecular mechanisms underlying selection of the FM remain elusive, possibly because the genetic components involved are also necessary for other aspects of plant development. Histological studies, particularly in maize, show that the MMC has a distinct polarity prior to meiosis. In maize, plasmodesmata are restricted to the chalazal pole of the MMC, and most mitochondria are also located in this zone. Meiotic cell divisions are also highly asymmetric, with the chalazal megaspore occupying around half the total volume of the MMC by the end of meiosis (Russell 1979). The cell wall of the MMC of maize, and other species, has been found to contain electron-dense deposits which stain with aniline blue (probably callose), particularly towards the micropylar end, suggesting that in the absence of plasmodesmal connections, the three micropylar megaspores could simply be starved of nutrients or energy after cytokinesis, triggering cell death. This hypothesis is generally supported by the analysis of mutants in which cytokinesis during meiosis is incomplete, in which more than one megaspore can survive, presumably through the acquisition of energy from neighbouring cells (Kennell and Horner 1985).
The determinants of MMC polarity could be the same as those involved later in determining polarity in the early female gametophyte. Recent work from Pagnussat and colleagues (2009) has shown that a sporophytically derived auxin maximum exists at the tip of the nucellus during early post-meiotic gametophyte development and that a distal–proximal auxin gradient is necessary for normal patterning of the female gametophyte (see below). Auxin is known to be involved in establishing the polarity of several plant tissues, and an auxin gradient could therefore be implicated in FM selection, for example by polarizing cytoplasmic/membranous components in the megasporocyte prior to meiosis. This role, however, remains to be tested.
Signalling cell identity in the female gametophyte
Although the role of the auxin maximum located in nucellar cells at the micropylar tip of the developing ovule in polarizing the MMC has not been investigated, recent research has provided compelling evidence that this could be important in patterning the female gametophyte.
The female gametophyte of Arabidopsis has been extensively studied and shows, from its inception, a marked chalazal/micropylar (C/M) polarity. The post-meiotic divisions of the haploid gametophyte nucleus occur without cytokinesis and are followed by nuclear migrations along the C/M axis. Division 1 gives two nuclei, situated at opposite poles of the gametophyte. After division 2, the two M-pole nuclei tend to lie next to each other, whereas the C-pole nuclei often lie one behind the other along the C/M axis. By this stage, a large central vacuole is observed. After the final round of mitotic divisions, one of the four C-pole nuclei migrates towards the M-pole and cellularisation is initiated, giving the seven cells of the mature gametophyte. Cellularisation and the appearance of cell fate-specific characteristics appear to go hand in hand, indicating that specification of cell/nuclear identity may already be at least partially fixed by this point. Upon cellularisation, the two most micropylar nuclei are incorporated into the synergids, and a third micropylar nucleus is enclosed in the egg cell. These cells have very specific polarities, with the nuclei of the synergids localized towards their micropylar pole, and the egg cell nucleus localized to its chalazal pole. The cells also express specific markers and develop characteristic structural features, such as the filliform apparatus of the synergids. The fourth M-pole nucleus (a so-called polar nucleus) fuses with the migrating C-pole nucleus (the second polar nucleus) to give the central cell nucleus. The remaining three chalazal nuclei are incorporated into the antipodal cells, which degenerate prior to fertilization (Robinson-Beers et al. 1992; reviewed in Drews et al. 1998; Drews and Yadegari 2002; Yadegari and Drews 2004). The development of female gametophytes in other commonly studied species including cereals basically resembles that in Arabidopsis, although differences in timing, nuclear positioning and final number and longevity of the antipodal cells have been reported (Diboll and Larson 1966; Haig 1990).
As previously described, a sporophytically derived auxin maximum is generated during megaspore development, at the micropylar pole of the nucellus (Pagnussat et al. 2009). As the female gametophyte starts to divide, auxin synthesis is initiated at the micropylar pole of the gametophyte and is maintained there until gametophyte maturity. Thus, the various nuclei of the female gametophyte are likely to be exposed to different auxin concentrations throughout their development. Pagnussat and colleagues elegantly tested the role of this auxin gradient in cell fate specification, by ectopically expressing the auxin biosynthetic protein YUCCA1, which is usually only expressed in the micropylar tip of the nucellus/egg sac, throughout the egg sac. They were able to show that this ectopic expression caused cells which would normally become egg cells, central cells and antipodal cells to express synergid cell fate markers. They also observed expression of egg cell markers in antipodal cells. Occasionally, the central cell was found to produce a zygote-like structure upon fertilization, suggesting a switch to egg cell fate. Conversely, cells in the “synergid” position of the egg sacs of mutants in auxin signalling, where responses to auxin are compromised, were found to occasionally acquire “egg” fate, sometimes leading to production of two zygotes upon fertilization. Thus, it seems possible that cell-fate specification in the female gametophyte is dependent, at least partially, upon auxin concentration, with high levels of auxin leading to synergid cell fate, slightly lower levels to egg cell fate, and so on along the M/C axis. However, it is as yet unclear whether auxin is the primary patterning element in this process or whether localized auxin biosynthesis occurs downstream of a second, as yet unidentified, patterning factor.
Female gametophyte cell fate is also disturbed in other mutants including lachesis (lis) clotho (clo) and atropos (ato). In lis mutants, synergids and the central cell take on egg cell identity, and antipodal cells acquire the characteristics of the central cell (Gross-Hardt et al. 2007). LIS, CLO and ATO all encode potential component of the spliceosome, with the production of CLO being necessary for the restriction of LIS expression to the two cells normally developing egg cell and central cell fate (Moll et al. 2008). These results indicate that just as a lateral inhibition is required for selection of a single MMC early in germline selection, acquisition of egg/central cell identity by one cell may cause it to repress the ability of its neighbours to acquire gamete cell fate. LIS appears to be a seminal component of the pathway sharpening cell fate decisions in the female gametophyte, although it is as yet unclear how pre-RNA splicing components could be implicated in lateral inhibition. Studies in Torenia fournieri, as well as other species, show that the cells of the female gametophyte are connected by plasmodesmata (Han et al. 2000). It is therefore possible that symplastic cell–cell movement of small peptides or RNAs is implicated in fine-tuning these cell fate decisions.
Thus, cell fate decisions in the female gametophyte appear to be intrinsically linked to an auxin gradient established early in the sporophyte and then maintained in gametophytic tissue. However, cell identities loosely established based on position within this auxin gradient also require a second “fixing” step, involving some form of lateral inhibition, in order to ensure that the correct number of gametic and accessory cells are produced in the female gametophyte.
Finally, there is considerable evidence that cytokinin signalling may play an important role in the development of the female gametophyte. Although this role is less well characterized than that of auxin, it is worth mentioning. In particular, a specific role for the cytokinin sensor histidine kinase CKI1 in allowing the completion of female gametophyte development has been noted by several groups (Deng et al. 2010; Hejatko et al. 2003; Pischke et al. 2002). These studies suggest that cytokinin is necessary for the formation of the central vacuole of the gametophyte, with defects in this process causing pleiotropic phenotypes, including arrest, at later stages of gametophyte development. Hejatko et al. investigated the expression of CKI1 in ovules and showed as strong and specific signal in the gametophyte throughout its development. Interestingly, a study of the expression of isoprenyl transferase (IPT)-encoding genes in Arabidopsis indicates that the cytokinin perceived in the gametophyte could come from maternal tissues since AtIPT1 is very strongly expressed in the chalazal zone of the developing ovule (Miyawaki et al. 2004).
Gametophytic interactions with sporophytic tissues
It is clear that the development of the nucellus and the megasporocyte/egg sac are integrally associated from the very start of germline inception. Nucellar cells are linked via plasmodesmata to the chalazal pole of the MMC and presumably support the development of the FM post meiosis. As described above, the regulation of interactions with nucellar cells is also seminal in MMC selection and likely also in FM selection and subsequent polarization and development of the egg sac. In addition, the development of the nucellus appears to be influenced by female gametophyte development. In Arabidopsis, the MMC originates from the hypodermal cells of the nucellus, and the female gametophyte thus develops surrounded by a single layer of nucellar cells which separate it from the enveloping integuments. By the time female gametophyte development arrests at ovule maturity, the single nucellar cell layer surrounding the micropylar pole of the egg sac has degenerated, leaving most of the gametophyte cells in direct contact with integument tissue. This cell degeneration appears to be dependent upon female gametophyte development, since in mutants such as sporocyteless (spl), in which sporogenesis is arrested, the nucellus remains viable and even undergoes periclinal cell divisions which are never observed in the nucellar tissues of wild-type ovules (Yang et al. 1999). The mechanism underlying nucellar cell degradation in Arabidopsis remains unclear. Interestingly, however, other plant species, including many cereals, cucurbits and Ricinus communis, show a proliferation of nucellar tissue prior to fertilization. The degradation of this tissue early during post fertilization development is thought to play a role in supporting the developing embryo sac. In some species, nucellar degradation has been analysed and shown to exhibit many of the characteristics associated with programmed cell death, including nuclear condensation and DNA fragmentation, membrane blebbing, vacuole collapse and the expression of caspase-like proteases (Dominguez and Cejudo 2006; Dominguez et al. 2001; Greenwood et al. 2005; Hiratsuka et al. 2002; Lombardi et al. 2007; Lombardi et al. 2010). However, it still remains unclear how nucellar degradation is triggered. Could degradation be a physical response to “squashing” by the expanding embryo sac? Or is some more subtle signalling pathway involved?
The mechanisms underlying communication of the female gametophyte with integuments also remain somewhat opaque. In general, the development of integuments appears to proceed normally in the absence of female gametophyte development. In spl mutants, for example, no major defects in integument growth are apparent despite the complete lack of a female gametophyte (Yang et al. 1999). Interestingly, however, recent observations in clo mutants suggest that defects in female gametophyte development can lead to gametophyte expulsion through the micropyle during ovule development. Moll and colleagues suggest that this could be a mechanism preventing allocation of maternal resources to abnormal/sterile ovules (Moll et al. 2008). This could indicate that integument tissues can in fact sense defects in the gametophyte, but this possibility remains to be explored.
Although little evidence for signalling from the female gametophyte to the developing integuments exists, it is clear that integument development is critically important for the normal development of the female gametophyte. Mutants in which integument outgrowth is defective, including bell1 and aintegumenta, almost invariably lead to arrested female gametophyte development (Elliott et al. 1996; Klucher et al. 1996; Reiser et al. 1995). However, since integument cells play important roles in physically protecting and possibly also providing nutrients to the developing female gametophyte, then this might not be a particularly surprising observation. The exact path of nutrients into the developing female gametophyte during its development has not been traced. Early in female gametophyte development, multiple plasmodesmal connections are observed to nucellar cells, through which nutrients are presumably assimilated. Towards the chalazal pole of the egg sac, these connections appear to be maintained until relatively late in ovule development, at least in cereals (Engell 1994). However, post cellularisation, especially in species like Arabidopsis where some nucellar cells degenerate, nutrient assimilation by micropylar cells of the gametophyte could either occur via symplastic movement of nutrients within the gametophyte or via the integuments. Since no direct plasmodesmal connections between integument cells and the female gametophyte have been described, it seems likely that movement of molecules between these two tissues, if it occurs, involves active transport across the apoplast, mediated by membrane-localized proteins such as sucrose transporters. Based on the analysis of the sterile apetala (sap) mutant, it has been proposed that the integuments could also produce developmentally important signals, other than nutrients, which are necessary for gametophyte development. Although SAP, which encodes a unique protein involved in a wide variety of developmental processes, is expressed early in the nucellus and MMC, its later expression is mainly restricted to the integuments, which apparently develop normally. Despite this, female gametophyte development is arrested in sap mutants (Byzova et al. 1999). However, the exact nature of the defect in this mutant is, as yet, unclear.
In summary, the development of a fully fertile ovule containing a functional female gametophyte, is critically dependent upon the co-ordinated development and communication of a wide range of tissue and cell types. This communication likely involves a whole suite of pathways including symplastic movement of RNAs and proteins, apoplastic signalling through receptor kinases and auxin movement and perception. Some of the cell types produced during this process, especially the synergids (Higashiyama et al. 2001; Marton et al. 2005; Okuda et al. 2009), the central cell (Chen et al. 2007; Rotman et al. 2008) and integument tissues (Palanivelu et al. 2003), play critical roles in attracting pollen tubes to the micropyle and in pollen tube reception prior to fertilization (reviewed in (Dresselhaus and Marton 2009). Huge breakthroughs in our understanding of these signalling events, and the important contribution of signalling components expressed in the male gametophyte, have been made and thus widely reviewed in recent years (Boisson-Dernier et al. 2008, 2009; Chapman and Goring 2010; von Besser et al. 2006). Likewise, the state of current understanding regarding actual events underlying sperm cell fusion with the egg cell and central cell, and subsequent nuclear fusion, have been widely and critically discussed (Berger 2009; Sprunck 2010). We will therefore now jump swiftly to post fertilization seed development, skipping the drama of the love scene and passing on to the complex interactions involved in family life.
Act2: a breakdown in communication
Fertilization definitively changes the genetic landscape within the developing seed. In higher angiosperms, the switch from a diploid mother (sporophyte) nurturing an arrested haploid gametophyte to a situation in which the same mother houses an embryo sac containing two dynamically developing and genetically distinct organisms is the most dramatic in the plant life cycle. Here, we will examine the co-ordinated development of the diploid embryo (product of fusion between the egg cell and one sperm nucleus), the (usually) triploid endosperm, produced by fusion of the second sperm nucleus with the central cell nucleus, and the surrounding maternal tissues. The structure of the young developing seed of the model species A. thaliana is schematized in Fig. 1b. However, before discussing how different tissues in a developing seed could communicate, it is important to discuss barriers which could affect the communication pathways linking the different tissues involved in seed development.
Symplastic isolation: slamming the door on a shared cytoplasm
Cytoplasmic connections have been shown to exist between the cells of the female gametophyte and also between the female gametophyte and the sporophytic nucellar cells at various stages during ovule development. These connections likely act to co-ordinate the development of the female gametophyte and the ovule. Although the sizes of molecules which can pass through these apertures may be extremely restricted in some cases, these connections undoubtedly serve to permit the movement of solutes and water between neighbouring cells. This has important implications in physiological terms because, at the very least, it influences the maintenance of major differences in turgor pressure and/or solute concentrations between the different cells of the developing ovule.
The point at which the female gametophyte becomes symplastically isolated from surrounding sporophytic cells is unclear. In Arabidopsis and Capsella, for example, it seems quite clear that after cellularisation of the female gametophyte and degeneration of the micropylar nucellus, there are no direct plasmodesmal connections between cells at the micropylar pole of the gametophyte and the integuments or between the central cell and the more chalazal nucellus (Schulz and Jensen 1971, 1973; Stadler et al. 2005); however, whether connections remain between the antipodal cells and neighbouring nucellar cells is unclear. This question may also be largely immaterial given that antipodal cells degenerate either shortly before, or during, fertilization, which would serve to break any remaining cytoplasmic contact between the gametophyte and nucellus (Mansfield et al. 1991; Murgia et al. 1993). In some grass species, however, antipodal cells continue to divide after fertilization. In maize, for example, around 20 cells are derived from mitotic division of the three antipodal cells, and the number in bamboo can be as high as 100 (Diboll and Larson 1966). These cells appear to play a nutritive role. Histological studies in maize and barley indicate that during pre-fertilization development, the number of plasmodesmatal connections between the chalazal pole of the gametophyte and nucellar cells decrease so that by egg sac maturity, no plasmodesmata remain (Diboll and Larson 1966; Engell 1994). Thus, although evidence is somewhat fragmentary, it is probably safe to assume that either before or at the latest during fertilization, in most angiosperm species, the female gametophyte becomes symplastically isolated from the maternal sporophyte, a state which permits a new autonomous and tight regulation of assimilate and water uptake, and also has profound implications in terms of signalling and growth co-ordination.
Although the mature gametophyte appears to be symplastically isolated from the sporophyte, plasmodesmata are still observed between most of the gametophytic cells. However, fertilization and the onset of embryo and endosperm development coincide with a sealing off of the embryo from the endosperm cytoplasm and the deposition of new cell wall material between these cells. In T. fournieri, tracer studies have shown that solute movement between the central cell and egg cell ceases at around the same time as nuclear fusion in the egg cell is complete, i.e. at the time when the zygote initiates its independent existence as the new sporophyte generation (Han et al. 2000). Although the precise timing of this event is, again, poorly documented, most sources appear to agree that the embryo and endosperm represent independent symplastic fields from very early in embryogenesis. This again has important implications both in terms of developmental signalling and nutrient uptake. From the point of fertilization, the embryo must actively import all its nutrients from the surrounding endosperm tissues and is thus totally reliant upon endosperm function. It is also easy to assume that this new symplastic isolation could be extremely important in the process of differentiating the two organisms, although this hypothesis remains, as yet, untested.
Thus, by the time the embryo has started to divide, it seems likely that two symplastic boundaries separate it from the maternal sporophyte. In fact, recent work in Arabidopsis suggests that phloem unloading from the female sporophyte occurs principally into the outer integument during early seed development, with the inner integument representing an independent symplastic field (Stadler et al. 2005). The proposed symplastic barriers present in the Arabidopsis seed are indicated in Fig. 2a. If, as many authors suggest, nutrient uptake into the developing endosperm can occur via the endothelium (derived from the inner cell layer of the inner integument), then this would suggest that three symplastic barriers (outer–inner integument, endothelium–endosperm and endosperm–embryo) could separate the two sporophyte generations. However, as will be seen below, the exact point at which different nutrients enter the endosperm remains unclear and may vary between species.
Potential barriers to cell–cell communication in the developing Arabidopsis seed. a Symplastic barriers. Both histological and tracer studies suggest that a symplastic barrier exists between the inner and outer integumenta during seed development (solid blue line). In addition, no symplastic connections are believed to exist either between the maternal and zygotic tissues (dotted blue line) or between the embryo and endosperm (green line). b Potential barriers/gating in the apoplast. Histological studies in many species have shown that cuticular material is deposited around the developing embryo proper (solid red line) and between the inner integument and the endosperm (dotted red line). However, the properties of this potential barrier to solute movement at different developmental stages are unknown. For discussion, see main text
Cuticles: gating the apoplast?
The symplastic isolation between embryo and endosperm, at least in some species, is rapidly followed by the initiation of cuticle deposition on the outside of the embryo proper. Several studies have noted the deposition of a cuticle on the early globular embryo, and in some species, cuticular deposition is even detectable in the fertilized zygote, before the first embryonic cell division (Bruck and Walker 1985; Chamberlin et al. 1994). Given its lipidic nature, one might assume that cuticular material would act as a barrier to the movement of apoplastic solutes, including photo-assimilates, proteins, peptides and amino acids. This assumption is borne out to some extent by the proposed function of the embryonic suspensor in nutrient assimilation from surrounding tissues (reviewed in Kawashima and Goldberg 2009).
Although the suspensor, like the rest of the embryo, is thought to be symplastically isolated from the endosperm and maternal sporophytic tissue, it shows a remarkably consistent lack of cuticle deposition in every species in which this character has been studied. In some families, especially many legumes, the suspensor develops into an extremely large and complex structure early in embryogenesis and differentiates characteristics associated with nutrient transfer capacities. Even in Arabidopsis, where the suspensor remains rather modest, up-regulation of sucrose transporters in suspensor cells suggests that nutrient uptake via this tissue is likely to be important for early embryo nutrition. However, later in embryo development, nutrient uptake cannot be entirely dependent upon active transport via the suspensor. In Arabidopsis, for example, the suspensor undergoes programmed cell death at the torpedo stage of embryo development, well before embryo growth and storage compound sequestration are terminated. Moreover, epidermal cells of some species, particularly legumes, differentiate transfer-cell-like characteristics which suggest that they are involved in the active uptake of both sugars and amino acids from the endosperm, despite being covered with a cuticular membrane. In addition transporters, such as those involved in the uptake of some amino acids, have been found to be expressed through-out the embryo, suggesting that at least some polar molecules must move across the embryonic cuticle. Indeed, experimental evidence for such polar paths for movement across cuticles has been generated (reviewed in (Schreiber 2005), although whether these pathways, the molecular basis for which is unclear, are particularly abundant in embryonic cuticle has not been tested. However, the presence of such pathways suggests that the movement of small hydrophilic/polar molecules between the endosperm and the embryo cannot be excluded as a means of tissue communication.
The embryo–endosperm boundary is not alone in containing cuticular material. Beeckman et al. report the presence of a continuous layer reacting positively to osmium tetroxide staining, which separates the embryo sac from the inner integument where the two structures are in direct contact (Beeckman et al. 2000). The authors suggest that this is the original cuticle of the inner integument (which is epidermal in origin) and note that it is maintained throughout embryo development. Similarly, in cereals such as wheat, a cuticle separates the testa tissues from the endosperm, where they are in direct contact. In wheat, this cuticular material has been proposed to act as a barrier which helps to channel active nutrient traffic to the embryo, through the endosperm (Zee and O'Brien 1970). No cuticle deposition is observed where integument cells and nucellar cells are juxtaposed in either species, for example, at the chalazal pole of the Arabidopsis embryo sac or between the testa and the nucellar projection found in the “groove” running down wheat seeds. In cereals such as wheat, much of nutrient uptake by the developing endosperm occurs via the nucellar projection (called the pigment strand in rice, (Oparka and Gates 1982). However, in Arabidopsis and legumes, it has been suggested that the highly specialized integument cell layer surrounding most of the embryo sac (the endothelium) is actively involved in trafficking nutrients to the endosperm. The presence of cuticular material between the endothelium and the endosperm has led several authors to challenge this view (Beeckman et al. 2000; Kapil and Tiwari 1978). It is indeed possible that as proposed in wheat, the presence of lipidic material around much of the embryo sac ensures that the majority of active nutrient uptake achieved by the endosperm occurs at its chalazal pole.
The view that integuments may play a relatively minor role in nutrient provision is supported by the fact that, like the suspensor, the endothelium undergoes cell death well before nutrient accumulation by the developing embryo is complete in many species, including Arabidopsis. However, the expression of specific sugar transporters in the integuments, and also in the micropylar endosperm during early seed development in Arabidopsis, suggests that at least some of the uptake of sucrose by the endosperm could involve its movement across this observed cuticular barrier (Baud et al. 2005; Stadler et al. 2005). In addition, studies on Brassica suggest that radio-labelled sucrose fed to intact developing seeds enters the micropylar region of the embryo sac before more chalazal regions (Morley-Smith et al. 2008). Interestingly, some authors have reported the presence of breaks in the cuticle layer between the endothelium and the embryo sac, specifically in the micropylar domain, although these are not usually observed until endosperm cellularisation (Chamberlin et al. 1994; Gunning and Pate 1974; Kapil and Tiwari 1978; Schulz and Jensen 1974). Such breaks have not been reported in Arabidopsis.
Thus, as in endosperm–embryo communication, the movement of small polar molecules across the apoplast separating the testa and endosperm cannot be ruled out and indeed, as will be seen below, almost certainly occurs. It should be noted, however, that the presence of cuticular material at the endothelium/endosperm boundary might considerably restrict the sizes and types of molecules which can transverse this apoplastic domain. The proposed points at which cuticular material could gate movement in the Arabidopsis seed apoplast are shown in Fig. 2b
In summary, the three main compartments of the developing seed (testa, endosperm and embryo) are symplastically isolated from around the moment of fertilization, allowing symplastic communication to be ruled out as a means of co-ordinating seed tissue growth and development. However, no conclusive evidence for boundaries which would definitively preclude the use of apoplastic signalling pathways has been generated to date.
Act3. A little give and take: regulating early endosperm growth
In many species, including species where the mature seed is largely filled with embryonic structures (such as legumes and Arabidopsis), much of the actual growth of the seed occurs very soon after fertilization and largely independently of embryo growth and development (reviewed in Berger 2003; Berger et al. 2006; Olsen 2001). Considerable evidence from several species indicates that this growth is mainly driven by a rapid expansion of the endosperm and co-ordinately regulated expansion of the integument-derived testa tissues. Understanding how the growth of endosperm and testa tissues is achieved and co-ordinated is thus of utmost importance in understanding the control of final seed size in angiosperms.
The endosperm is an intriguing tissue since in many angiosperms, including most of the species which have been studied in any molecular detail, its post-fertilization growth starts with a period of syncytial development, in which nuclear divisions occur in a common cytoplasm (Berger et al. 2006; Boisnard-Lorig et al. 2001; Lid et al. 2005; Olsen 2004). In effect, during its early expansive growth phase, the endosperm is composed of a layer of multinucleate cytoplasm surrounding a large central vacuole. How can this structure drive the early expansion of the developing seed? Much of the recent literature concerning the control of endosperm development and its effects on seed size has concerned the epigenetic basis for parent of origin effects. In most of the systems in which the molecular biology of seed development has been widely studied, the nuclear genetic contribution of the mother to the endosperm is twice that of the father. Excess contribution from the paternal genome to the endosperm has been shown to cause over proliferation and delayed cellularisation of the endosperm in Arabidopsis, leading to increased seed size (Scott et al. 1998). Excess maternal contributions cause reduction in endosperm growth due to reduced proliferation and premature cellularisation and, thus, smaller seed sizes. The fact that the endosperm repartitions nutrients from the mother to the developing embryo has led to the hypothesis that these effects are an example of “parental conflict”. Put simply, in out-crossing species, that it is in the mother’s interest to limit and repartition equally the resources which she provides to her offspring (to each of which she is equally related), in order to maximise her reproductive success. This may explain the selective advantage of the “double” maternal contribution to triploid endosperm (Haig and Westoby 1989; Stewart-Cox et al. 2004). Conversely, it is in the interest of the male to maximise maternal contributions to each of his offspring. The molecular basis for these effects is the subject of intensive research (reviewed recently in Huh et al. 2007; Jullien and Berger 2009; Kinoshita 2007). However, although regulation of both growth and nutrient partitioning are likely to be key targets of parental conflict (Moore and Haig 1991), this work has not directly shed light on mechanisms regulating seed tissue growth co-ordination and will therefore not be considered further in the context of this review.
Sweet secrets in the endosperm: the sugar that drives seed growth
Although the precise mechanism is somewhat unclear, it seems that sugars play a very important regulatory role in driving endosperm growth. Sugar is actively transported into plant “sink” tissues (like seeds) almost exclusively as sucrose, and it is in this form that photo-assimilates are unloaded from the phloem into the sporophytic tissues of developing seeds. From this point, they are actively transported into the developing endosperm across the apoplast. Studies in various species including Sorghum, maize, Brassica and legumes have shown that during early seed development, much of the sucrose taken up by the developing seed into the endosperm is converted to glucose and fructose (Cheng et al. 1996; Hill et al. 2003; Jain et al. 2008; Melkus et al. 2009; Morley-Smith et al. 2008). As a result, a transiently high hexose/sucrose ratio is observed, which persists for the period during which endosperm expansion occurs, and then drops off as embryonic growth accelerates. The conversion of sucrose to hexoses during early seed development appears to be important for endosperm growth and seed expansion, rather than directly for embryo nutrition. In maize miniature1 mutants, which lack an invertase activity in the endosperm basal transfer layer (where most active nutrient uptake occurs into the maize endosperm), increased hexose levels are not observed in the basal endosperm, and seed growth is dramatically reduced (Cheng et al. 1996; Vilhar et al. 2002). Moreover, in Brassica, the use of radioactive tracers has shown that the hexose pool present in the early bulk endosperm is not directly destined for the nutrition of the developing embryo (Morley-Smith et al. 2008).
The exact compartment and mechanism via which sucrose is hydrolysed into hexoses in developing seeds remain controversial and, moreover, is likely to differ between plant groups. Morley Smith et al. propose that in Brassica, the endosperm compartment in which hexoses accumulate during early seed development is likely the large central vacuole, which is derived from the vacuole of the fertilized central cell, rather than the endosperm cytoplasm. The authors suggest that sucrose conversion occurs in this compartment via the action of vacuolar invertases (Morley-Smith et al. 2008). However, in legumes and barley, the invertase activity necessary for increasing hexose concentrations in young seeds is thought to be derived, at least in part, from the maternal tissues (Weber et al. 1997; Weschke et al. 2003). In legumes, acid invertases are produced in the so-called thin walled parenchyma, the inner cell layer of the seed coat (developmental equivalent of the Arabidopsis endothelium), suggesting that hexoses can transverse the apoplastic divide between the integuments and endosperm in this system. In barley, in addition to their presence in endosperm cells, acid invertases have been localized to maternal cells in the nucellar projection. Acid/cell wall invertases produced in maternal cells have been proposed to act on sucrose before it is exported into the endosperm, with glucose and fructose subsequently being taken up into the endosperm cytoplasm by hexose transporters (Weber et al. 1997; Weschke et al. 2003). In Arabidopsis, the apetala2 (ap2) mutant shows a marked increase in seed mass. Recent studies have shown that this is due to an enhanced increase in endosperm proliferation and a delay in endosperm cellularisation, which are, in turn, correlated (but have not been causally linked) to an increased hexose/sucrose ratio during an abnormally prolonged period of early seed development (Ohto et al. 2005). The effects of the ap2 mutation on seed development are maternal sporophytic, indicating that the primary role for AP2 in permitting normal seed growth could reside in the integuments or nucellar tissues. It has been suggested that this effect could involve the regulation of a cell wall invertase expressed in the endothelium, although this remains speculative. Recent in silico data from young developing seeds in Arabidopsis, however, do support the idea that the conversion of sucrose to hexose in seeds could occur, at least partially, in maternal tissues. Notably, two of the six identified genes encoding cell wall invertases identified in Arabidopsis (Sherson et al. 2003), AtcwINV3 and AtcwINV5, show extremely strong expression in the chalazal seed coat during very early seed development. Moreover, several of the STP-class of hexose transporters (Buttner 2007), including STP8, STP12 and STP14, show strong expression in the chalazal and peripheral endosperm during early seed development, consistent with a role in importing hexoses into the endosperm (G. Ingram pers. Obs.).
Why would increasing hexose concentrations drive growth in the endosperm? At a basic level, it has been suggested that sucrose breakdown might simply increase the sink strength of the developing seed (Barratt et al. 2009; Cheng et al. 1996; Jain et al. 2008; Weschke et al. 2003). By decreasing sucrose concentrations in the seed endosperm, transport from the phloem would be accelerated. Some authors have suggested that, in addition, the effects of hexoses and, particularly, the presence of glucose, on developing endosperm, occur at the level of nuclear proliferation since several reports have suggested that high glucose levels can prolong periods of cell division in other tissues (Weschke et al. 2003; reviewed in Borisjuk et al. 2004).
Another attractive model which might partially explain the proposed role of sugar conversion in driving growth relies on the fact that breaking down sucrose into hexose derivatives effectively doubles its osmotic activity. Thus, the action of invertases, particularly within the endosperm vacuole, might cause a transient increase in osmotic potential within the endosperm causing increased water influx into the vacuole and driving expansion of the endosperm and surrounding tissues through increasing turgor pressure (Morley-Smith et al. 2008). A similar mechanism has been proposed to drive root growth in Arabidopsis (Sergeeva et al. 2006) and rapid stem extension in Tulip (Balk and de Boer 1999), where co-expression of invertases with water-channel proteins was reported. Interestingly, several aquaporin molecules have been found to be co-expressed with invertases in pea seed coats (Schuurmans et al. 2003). However, although this is an attractive hypothesis, it is rather difficult to test, partly due to plasticity and redundancy in the pathways involved in carbohydrate metabolism.
Recent studies on maize have provided a further potential mechanism which could also explain some of the observed activity of hexoses in driving seed expansion. LeClere et al. report that maize miniature1 mutant kernels, in addition to showing reduced hexose concentrations and increased sucrose concentrations in the basal endosperm, also show reduced levels of IAA accumulation (LeClere et al. 2010). This is correlated with decreased expression of ZmYUCCA, which encodes an enzyme involved in Tryptophan-dependent auxin biosynthesis. The authors show that expression of this gene in a heterologous system (Arabidopsis) is extremely responsive to glucose concentrations, although how this regulation is mediated is not totally clear. However, since auxins are known to mediate cell expansion, it is possible that expression of ZmYUCCA in response to endosperm glucose accumulation could play a key role in regulating early seed growth, at least in maize. In light of this research, it is interesting to note that a recent transcriptome analysis of proliferating Arabidopsis endosperm actually showed a marked under-representation of auxin-related genes but a significant enrichment for genes involved in cytokinin signalling-associated transcripts (Day et al. 2008). High levels of cytokinins have been reported during early seed development in several species (Yang et al. 2002). Cytokinin signalling and sugar status seem to be linked, although the exact nature of this link appears complex (reviewed in Hartig and Beck 2006). Most reports suggest that cytokinins are mainly synthesized in the developing endosperm. Whether this endosperm localized cytokinin plays a role in co-ordinating endosperm or seed growth is unclear. Interestingly, mutants lacking the function of multiple cytokinin receptors have been reported to produce very large seeds (Hutchison et al. 2006; Riefler et al. 2006). Reciprocal crosses to wild type suggest that the requirement for cytokinin receptors to control seed size could be maternal (Riefler et al. 2006). However, the fact that higher order mutants also show dramatically reduced seed set makes these results difficult to interpret, as infertility can, in itself, lead to increased seed size.
Seed expansion vs embryo growth: a role for endosperm partitioning?
Although the exact form in which sugars are absorbed by the young embryo has not been determined, several lines of evidence, including the expression of transporters in the suspensor (Kawashima and Goldberg 2009), suggest that sucrose may be the major species, at least during early embryogenesis. However, the activity of invertases in the early endosperm would make it difficult for the embryo to actively absorb photo-assimilates in the form of sucrose. This would be a particular problem if all the sucrose entering the developing endosperm passed through regions of high invertase activity. However, there is considerable evidence that the endosperm could be metabolically compartmentalised, at least in legumes, maize and Brassica. Compartmentalization in legumes has mainly been proposed based on morphology of the early endosperm (discussed in Chamberlin et al. 1994). In Brassica, it has been shown that the sugar taken up by the embryo during early seed development is not, in fact, transported through the main endosperm cavity but likely through an entirely independent route (Hill et al. 2003; Morley-Smith et al. 2008). During early endosperm development in most species including Arabidopsis, the plasma-membrane of the un-cellularised endosperm is closely juxtaposed to the cell wall of the developing embryo. In Arabidopsis, the sucrose transporter AtSUC5 is expressed exclusively in the micropylar endosperm where it has been shown to be required to support early embryo growth (Baud et al. 2005). These data support the hypothesis, discussed previously, that some sucrose is imported from the endothelium to the endosperm, at least in micropylar regions of young developing seeds. This sucrose must be transported symplastically through the outer integument after phloem unloading and then actively moved across several symplastic boundaries to reach the apoplastic space surrounding the embryo (Morley-Smith et al. 2008; Stadler et al. 2005). Further evidence for endosperm metabolic compartmentalization also comes from maize, where a putative apoplastic invertase inhibitor, ZM-INVINH1, is specifically expressed in the micropylar endosperm, possibly as a means of preventing sucrose breakdown prior to its uptake by the young embryo (Bate et al. 2004). Interestingly, recently released transcriptome data from developing seed tissues indicate that at least one predicted Arabidopsis invertase inhibitor shows preferential expression in the micropylar endosperm, where it could act to protect sucrose imported via AtSUC5 from breakdown in the apoplastic space surrounding the embryo (G. Ingram pers. obs.).
In summary, repartitioning of sucrose arriving in developing seeds appears to depend upon differential transport in both the apoplast and symplast of maternal tissues, as well as regulated uptake by the endosperm and embryo. How this exquisitely complex process is co-ordinated remains extremely poorly understood and likely involves the integration of factors such as sink strength, the activity of hydrolytic enzymes in the apoplast and vacuole, the gating of the apoplast by cuticular material and the dynamic regulation of multiple transporters in plasma membranes.
Other zygotic pathways regulating early endosperm and seed growth
From the above data, it can be seen that the endosperm plays a seminal role in regulating overall seed growth. Fitting with the role of the endosperm in nutrient repartitioning, this function may involve metabolite sensing, potentially mediated by hormonal pathways. However, genes other than those involved in sugar metabolism have also been shown to play roles in pathways affecting seed size through their zygotic effects on endosperm development. The best characterized of these are a group of genes encoding a WRKY transcription factor called MINISEED3 (MINI3), an LRR-RLK called HAIKU2 (IKU2) and a recently identified VQ-motif containing protein called HAIKU1 (IKU1; Garcia et al. 2003, 2005; Luo et al. 2005; Wang et al. 2010). MINI3, IKU2 and IKU1 are all expressed during early endosperm development, and genetic and transcriptional studies suggest that IKU2 acts downstream of MINI3 in the same pathway (Luo et al. 2005). IKU1 interacts with MINI3 and is likely also to act in this pathway (Wang et al. 2010). Single loss-of-function mutants in all three genes and double mutants between MINI3 and IKU2 give a small seed phenotype caused by reduced proliferation and early cellularisation of the endosperm (Luo et al. 2005; Wang et al. 2010). Both MINI3 and IKU2 are, in turn, targets of SHORT HYPOCOTYL UNDER BLUE1 (SHB1), which associates with their promoters, possibly by interacting with a DNA-binding transcription factor (Zhou and Ni 2010; Zhou et al. 2009). Loss-of-function mutants in shb1 give a small seed phenotype, whilst gain of function mutants, and over-expressing plants produce abnormally large seeds in an IKU2/MINI3-dependent manner. The relationship between IKU1 and SHB1 is, as yet, unclear.
The SHB1-MINI3-IKU2 pathway is enigmatic, and to date, it is unclear how, or even if, these genes link in with other pathways known to affect seed growth. It is, however, intriguing to note that IKU2, a receptor kinase, is expressed in the endosperm, prior to its cellularisation. IKU2 protein is therefore likely to localize to the outer membrane of the embryo sac, from where it would be ideally placed to perceive apoplastic signals derived from the surrounding maternal integument cells. Whether such signals exist and how they regulate growth co-ordination during seed development will be an intriguing direction for future research.
Parental control: the role of the integuments/testa in controlling seed size
A considerable amount of data suggests that zygotically driven growth in the endosperm is both permitted and constrained by regulated expansion of the testa. Unlike the endosperm, in which cell division is exclusively a post-fertilization event, the integuments undergo proliferative growth both prior to and post fertilization, although much of their cell expansion occurs in the later phase. In the context of fully understanding inter-organismal communication in the developing seed, it is therefore important to ascertain whether the phenotype of any given gene/pathway with a maternal sporophytic effects on seed development are due to defects in integument growth per se (i.e. potentially with phenotypes which are already apparent in the developing ovule), whether they are due to inability to perceive or respond to signals which would usually co-ordinate the growth of the testa with that of the underlying endosperm, or indeed, whether they are due to profound changes in the nutrient status in the maternal plant. For example, as mentioned previously, partial sterility can lead to increased seed size due to resource re-allocation.
Several genes that have been reported to act in maternal tissues to regulate seed size have been reported. APETALA2, as previously mentioned, has multiple effects on floral development, including a largely maternal effect on seed size (Ohto et al. 2005). However, the pleiotropic nature of the ap2 phenotype makes it extremely difficult to interpret. More recently, a mutation in the DA1 gene, which encodes a potential ubiquitin receptor, was shown to affect seed size by restricting the period during which integument cells proliferate (Li et al. 2008). A similar affect has been reported for mutants in AUXIN RESPONSE FACTOR2 (ARF2)/MEGAINTEGUMENTA (Hughes et al. 2008; Okushima et al. 2005; Schruff et al. 2006; Vert et al. 2008). The fact that endosperm and, subsequently, embryo size is increased in these mutants indicates that endosperm growth is restricted by the integuments although since both da1 and arf2 mutants also influence the growth of organs other than integuments, effects could be indirect. However, recent work testing the role of KLUH/CYP78A5 specifically in regulating seed growth supports this theory (Adamski et al. 2009). KLUH is usually expressed in the inner integument and, while loss-of-function mutants show reduced seed size, over-expression can dramatically increase seed size. Since KLUH acts non-cell autonomously (Anastasiou et al. 2007; Eriksson et al. 2010), via an as yet unidentified signal, it is not strictly possible to ascertain whether maternal effects of KLUH expression in the testa are solely due to changes in testa cell growth, or whether the KLUH signal might also stimulate endosperm growth non-cell autonomously. To overcome this problem, Adamski et al. expressed KLUH in integuments only during ovule development (i.e. before endosperm initiation) and found that this was sufficient to cause marked increases in seed size, suggesting that the phenotypic effects of KLUH are largely due to changes in cell numbers in integuments (Adamski et al. 2009). This observation is interesting in light of results from Garcia et al. who tested the maternal effects of reducing cell proliferation in integuments by over-expressing KIP RELATED PROTEIN2. They showed that significant decreases in cell numbers could be compensated for by significantly increased cell expansion during seed coat development, when KRP2 over-expressing integuments overlay a genetically wild-type endosperm. They also note that although seed size in Arabidopsis ecotypes may be similar, cell numbers in integuments can vary markedly (Garcia et al. 2005). The fact that compensatory effects in cell expansion were not observed in kluh mutants may indicate hidden complexities in the mechanism of KLUH action. Mutants in the WRKY transcription factor-encoding gene TRANSPARENT TESTA GLABRA2 (TTG2), like kluh mutants, lead to a reduction in seed size. However, in the case of ttg2 mutants, the causal defect seems to be in the ability of testa/integument to elongate, post fertilization (Debeaujon et al. 2003; Garcia et al. 2005). The basis of this defect is unclear, but TTG2 plays broader roles in regulating testa differentiation, suggesting that defects could be due to abnormal cellular properties in the testa.
The interaction between maternal and filial growth regulation has been most carefully examined by Garcia et al. who tested the genetic interactions between the zygotically acting iku mutants and the maternally acting ttg2 mutant (Garcia et al. 2005). Both ttg2 (acting maternally) and iku (acting zygotically) show reduced cell numbers and premature cellularisation of the endosperm, leading the authors to propose that physically restricting endosperm cell growth could trigger cellularisation, possibly by allowing a threshold nucleo/cytoplasmic ratio to be reached prematurely. The additive phenotype of the double mutant, in which cellularisation occurs extremely early, giving tiny seeds which often abort, supports this view. Based on the observation that integument cell elongation is able to compensate for relatively extreme variations in cell division, the authors propose an attractive model in which an IKU-dependent pathway controls the amount of elongation necessary in the integuments to co-ordinate their growth with that of the endosperm (Garcia et al. 2005). This implies that a pressure/tension-sensing mechanism exists which regulates the degree of tension generated and maintained in the developing testa during seed growth by regulating testa expansion. This tension-sensing mechanism almost certainly involves the elusive mechano-sensing pathways which have been proposed to exist in plants and which are currently the subject of intensive research (Hamant et al. 2008; Hamant and Traas 2010; Monshausen and Gilroy 2009; Richter et al. 2009; Seifert and Blaukopf 2010; Uyttewaal et al. 2010)
Act4. Live and let die: the complex interactions of the embryo and endosperm
The ultimate goal of seed development is to produce a well-established seedling when the seed eventually germinates. Endosperm-mediated expansion of the young seed is crucial for supporting the subsequent growth and development of the embryo and for permitting the sequestration of enough reserves to permit successful germination and seedling establishment. As described above, this early seed expansion, which is marked by specific metabolic phenomena such as a massive increase in hexose concentrations in the endosperm, occurs prior to any major growth or storage product accumulation in the embryo. The possible routes of nutrient entry into the developing embryo from the endosperm have been discussed briefly above in the context of these phenomena. However, recent studies have indicated that the cross-talk between the embryo and the endosperm is likely to be much more complex and subtle than a simple one-way flux of nutrients, and it is this cross-talk, during early seed development, which will be addressed in this final section.
Before trying to understand the relationship of the embryo and the endosperm, it is helpful to reflect on the evolutionary origin of the endosperm. Based on recent molecular phylogenies, angiosperms are thought to have evolved from plants with a life history similar to that of gymnosperms, in which seeds are produced but an endosperm sensu stricto is not. In many gymnosperms, instead of being surrounded by the endosperm during its growth, the developing embryos thrust invasively into cellularised nutritive tissues produced by the female gametophyte prior to fertilization. These tissues degenerate as the embryos grow, both releasing nutrients and allowing embryo expansion into the space defined by the integuments. Many gymnosperms also produce several egg-containing archegonia per seed, and more than one may be fertilized, leading to competition between multiple embryos during their early development and the eventual dominance of one individual.
In this context, two potential evolutionary origins for the endosperm have been proposed (reviewed in Baroux et al. 2002; Friedman 2001). The first posits that the central cell of the gametophyte is gametic in origin and would originally, upon fertilization, have given rise to a second embryo which, over the course of evolution, developed the supporting role which angiosperm endosperms fulfil today. Thus, sibling rivalry would have given way to an altruistic interaction. One prediction of this hypothesis would be that basal angiosperms might have a diploid, rather than a triploid endosperm. The second proposal is that the progenitor of part of the proliferating female gametophyte somehow became sexualized, linking the majority of gametophyte proliferation to fertilization. Baroux et al. suggest that such an event would have fitness advantages, since it would prevent wasted resource allocation to unfertilized seeds (Baroux et al. 2002). In this scenario, the nutritive role of the endosperm would have remained essentially unchanged but simply have become linked to a novel fertilization competence. Studies of gametophyte and seed development in basal angiosperms have been used to support both hypotheses, and the question remains formally unresolved. Despite this, the majority of authors favour the second hypothesis, based on several arguments, including the developmental similarities between the angiosperm endosperm and the gymnosperm female gametophyte and the presence of a triploid endosperm in the very basal angiosperm Amborella (Baroux et al. 2002).
Does the embryo talk to the endosperm?
The question of whether the embryo plays a role in regulating endosperm development is complex and has not yet been satisfactorily answered. This is a particularly difficult question to answer in a species with a transient endosperm such as Arabidopsis, where in most cases the development of an intact endosperm in the presence of a seriously defective embryo will lead to effectively the same phenotype (seed collapse and death), as the defective development of both tissues. In Arabidopsis, these mutants have been massed together under the heading embryo lethal (emb; Meinke and Sussex 1979; Tzafrir et al. 2004). However, in maize, where endosperm is persistent, seed lethal mutants have been classified into two main groups, defective kernel (dek) and embryo specific (emb). Mutants of the former class (dek) have defects in endosperm development (in the absence or presence of a defect in embryo development) which lead to seed collapse (Neuffer and Sheridan 1980; Sheridan and Neuffer 1980). Interestingly, however, emb mutants show normal endosperm development, in the near or total absence of an embryo (Clark and Sheridan 1991; Heckel et al. 1999), supporting the hypothesis that in maize, at least, the developing embryo has little influence on the development of the endosperm. This view has been challenged by recent reports of the behaviour of ovules fertilized by pollen carrying mutations in CYCLIN-DEPENDENT KINASE A;1 (CDKA;1) and F-BOX-LIKE PROTEIN 17 (FBL17) in Arabidopsis cdka;1, and fbl17 mutants produce pollen containing a single sperm cell, due to a defect in pollen mitosis II. Intriguingly, this pollen can fertilize ovules but was reported to fuse only with the egg cell, leading to the initiation of embryo development and, surprisingly, a limited but marked proliferation of the endosperm. This pointed to the presence of a signal from the developing embryo, which was capable of causing fertilization-independent development of the endosperm (Gusti et al. 2009; Nowack et al. 2006; Ungru et al. 2008). However, a recent publication has voiced some uncertainty as to whether the endosperm proliferation observed in these mutants is truly fertilization independent (Nowack et al. 2010), leaving the existence of such a signal in some doubt.
In conclusion, to date there is a startling lack of evidence for specific embryo-derived signals which could be involved in regulating the early development of the endosperm. However, if interpreted in the light of the evolutionary origin of the endosperm being a nutritive tissue developing prior to embryo development, this is perhaps less surprising than it might at first appear.
Interestingly and perhaps more surprisingly, several situations, including pollination with cdka;1 pollen, have been described in which endosperm proliferation is reduced, or even absent, but in which embryo development occurs seemingly normally until around the globular stage, suggesting that the early stages of embryo development are, to some extent, autonomous (Ngo et al. 2007; Weijers et al. 2003). These cases have been reviewed by Nowack et al. who suggest that a checkpoint exists at the globular stage in Arabidopsis embryogenesis, which is activated in case of a defect in endosperm development (Nowack et al. 2010). This is consistent with studies of the development of isolated maize zygotes, which were found to arrest at around the transition stage (roughly equivalent to the late globular stage) in the absence of nurse cells (Leduc et al. 1996). However, whether these arrests are due to a nutritional block or due to the absence of developmental signals is unclear. It is interesting to note, however, that studies characterizing the cellular identities found in complex embryogenic culture systems have regularly noted the presence of cells expressing endosperm markers, in addition to embryonic cells, leading to the suggestion that endosperm functions are, indeed, necessary for normal embryo development in culture (Magnard et al. 2000; Massonneau et al. 2005; Van Hengel et al. 2002). Since in these systems nutrients are generally derived from the culture media, it is tempting to speculate that endosperm cells could be providing developmental signals, and a potential role for Arabinogalactan proteins has been suggested in this context (Letarte et al. 2006; Tang et al. 2006; Van Hengel et al. 2002).
The enigma of the embryo surrounding region
The region of the endosperm surrounding the embryo was first described as the “Embryo Surrounding Region” (ESR) in studies carried out in maize (Opsahl-Ferstad et al. 1997). The term has since passed into common usage in both cereals and other species and formalizes observations that this particular group of cells differ from other cells in the developing endosperm in terms of both cytological characteristics and gene expression profiles, in most species studied (reviewed in Cossegal et al. 2007). Part of the reason for this difference may lie in the specialized role played by these cells in transferring nutrients to the embryo during early development. As mentioned earlier, genes involved in sucrose transport and metabolism are specifically expressed in these cells, and the ESR may represent a relatively isolated metabolic compartment in at least some species. During early embryo development at least, the role of the ESR in transferring nutrients to the embryo, probably via the suspensor, is likely to be extremely important for embryo development. However, the view that the ESR is solely involved in the nutrition of the embryo has long been disputed, and several lines of evidence converge to suggest that this region of the embryo may have evolved specific signalling functions which are required for the normal development of embryo. This section will first address the role of endosperm breakdown in the ESR in embryonic development and will then address some of the signalling pathways which have been proposed to exist between the embryo and endosperm.
The invasive growth of the angiosperm embryo into the endosperm has been likened to that of developing gymnosperm embryo into the nutritive tissues of the female gametophyte. In both cases, cell breakdown takes place around the embryo, and this probably serves two purposes: firstly to release nutrients from the developing endosperm and secondly to give the embryo space to grow. In species such as Arabidopsis, this cell degeneration is almost complete so that by the completion of seed development, only a single layer of endosperm cells remains surrounding the mature embryo. However, in species with persistent endosperm, such as maize, cell degeneration in the ESR must be relatively restricted. It is important to note here that cell death in the ESR should not be confused with the cell death observed in the starchy endosperm of cereals. The two events are spatially and temporally separated and serve completely different purposes, the former to release nutrients and space and the later to maximise and stabilise nutrient storage. As a result, the molecular control of the two events is likely to be extremely different.
Very little is known about the control of cell death in the ESR of angiosperms. The recent isolation of an endosperm-specific basic helix-loop-helix containing transcription factor ZHOUPI (ZOU)/RETARDED GROWTH OF EMBRYO1 (Kondou et al. 2008; Yang et al. 2008), in which degeneration of the endosperm does not occur (Yang et al. 2008), is the first instance of a reported defect in this process. In zou mutants, the endosperm is persistent, and embryos are severely stunted. To what extent stunting is due to reduced passage of nutrients to the embryo and to what extent it is due to physical impedance of embryo expansion is, as yet, unclear. However, although ZOU is expressed in the ESR, zou mutants do not appear to affect ESR specification per se, since genes like AtSUC5 are still expressed normally in mutant endosperms and seed abortion is very rarely observed. It therefore seems likely that nutrient transport pathways function normally, at least during the early development of zou seeds. Consistent with this, developmental defects are not observed until around the mid heart stage of embryo development. Thus, at least some of the stunting observed in zou embryos is likely due to the growth of the embryo being constrained by the presence of an abnormally persistent endosperm. Could physical cues, such as compression, activate the expression of ZOU and subsequent cell death in the ESR during normal development? Alternatively, is some other embryo-derived signal involved? Neither of these possibilities seems very likely for two main reasons. Firstly, the ZOU promoter is activated immediately after fertilization, before the embryo has started to develop (Yang et al. 2008). Secondly, ZOU is expressed apparently normally in mutants where embryo development is arrested at extremely early developmental stages (G. Ingram, unpublished results). More indirectly, in maize endosperm, degeneration in the ESR occurs normally, even in the absence of normal embryo development (for example, in emb mutants; Clark and Sheridan 1991), indicating that ESR cell degeneration, which could be regulated by a ZOU-like gene, occurs independently of embryogenesis in this system. Interestingly, however, in rice mutants showing defects in embryo development (reduced embryo mutants and giant embryo mutants), the endosperm cavity appears to adapt its size to that of the embryo, suggesting that ESR degeneration could be regulated differently in rice (Cossegal et al. 2007; Hong et al. 1996).
Although AtSUC5 expression is not affected in zou mutants, expression of other ESR-specific genes, such as the subtilisin protease encoding ABNORMAL LEAF SHAPE1 (ALE1), is lost. ALE1 has been reported to play a role in the normal formation of the epidermal surface in developing embryos, but not in endosperm degradation (Tanaka et al. 2001). Consistent with the observed loss of ALE1 expression in zou mutants, zou mutant embryos show serious defects in surface development, including cuticular abnormalities, and extensive adhesion to the endosperm (Yang et al. 2008). It is therefore possible that ZOU regulates two different processes: cell degradation in the endosperm and an ALE1-mediated pathway required for normal surface formation in the developing embryo. Consistent with this, recent research suggests that ZOU-regulated genes fall into two major groups, one of which includes ALE1 and has no obvious links to cell death pathways (J. Goodrich, Q. Xing and G. Ingram unpublished results). ALE1 has been shown to regulate protoderm-specific gene expression in developing embryos, despite being expressed exclusively in the developing endosperm (Tanaka et al. 2007). The ALE1 protein is predicted to be exported into the apoplast, and other related proteins have been proposed to play roles in processing peptide ligands implicated in cell–cell signalling during, for example, stomatal patterning in the leaf epidermis (Srivastava et al. 2008; Von Groll et al. 2002). It is therefore possible that ALE1, under the control of ZOU, processes a peptide ligand present in the extra-embryonic space which is necessary for the normal development of the embryonic surface. Small secreted peptides are known to be expressed in the ESR of maize (Bonello et al. 2000, 2002; Magnard et al. 2000; Opsahl-Ferstad et al. 1997), and although obvious homologues of these maize proteins do not exist in dicotyledonous species, in silico data show that some small secreted peptides are expressed in the developing endosperm of Arabidopsis (G. Ingram unpublished results). Moreover, a range of receptor-like kinases, expressed in the developing embryo, has been shown to be involved in embryonic surface formation, including ARABIDOPSIS CRINKLY4 (ACR4; Gifford et al. 2003; Tanaka et al. 2004; Watanabe et al. 2004), ABNORMAL LEAF SHAPE2 (ALE2; Tanaka et al. 2007) and GASSHO1 and GASSHO2 (Tsuwamoto et al. 2008). Although ALE1 and ZOU have been shown to act in a parallel pathway to ACR4 and ALE2 (Tanaka et al. 2007; Yang et al. 2008), their genetic relationship to the GASSHO genes remains to be determined.
In summary, from recent research, it appears that the developing embryo has little influence on the development of the endosperm, at least in Arabidopsis and maize. However, the endosperm does appear to have an important developmental influence on the embryo. This may not be restricted to the provision of nutrients but may extend to more fundamental developmental roles, such as the regulation of surface formation and growth. The proposed developmental roles of the endosperm are intriguing in the context of its evolutionary origin, as some may have been acquired relatively recently, after the appearance of the first angiosperms, or even after the split between the monocotyledonous and dicotyledonous lineages. This could explain why, for example, some of the genes expressed in the embryo surrounding region in cereals have no immediate homologues in dicotyledonous plants. It may also explain differences in the behaviour of the endosperm in terms of ESR cavity formation between rice and maize. Investigation of the divergence of the role of endosperm in its interactions with the developing embryo could provide an exciting future field of research in the “Evo devo” field.
Conclusions
This review has focussed on the potential pathways and mechanisms through which the three components of the young developing seed, the embryo, the endosperm, and the seed coat, communicate to co-ordinate their growth and development. In discussing these issues, several interesting questions have emerged. For example, the question of how barriers to communication could be involved in co-ordinating developmental programmes, the question of how the multiple cell death events which occur in various maternal and zygotic tissues during seed development are triggered and controlled and the question of how physical cues such as stretching and squashing are perceived by different seed tissues during development. These questions, for the main part, remain unanswered but will provide intellectually stimulating subjects for future research, with potential long-term benefits in terms of both understanding the basics of plant development and the identification of lucrative targets for biotechnology and crop improvement.
Abbreviations
- AP2:
-
APETALA2
- ALE1:
-
ABNORMAL LEAF SHAPE1
- ALE2:
-
ABNORMAL LEAF SHAPE2
- ACR4:
-
ARABIDOPSIS CRINKLY4
- AGP:
-
Arabinogalactan protein
- AGO9:
-
ARGONAUTE 9
- ATO:
-
ATROPOS
- ARF2:
-
AUXIN RESPONSE FACTOR2
- CLO:
-
CLOTHO
- C/M:
-
Chalazal/micropylar
- CDKA;1:
-
CYCLIN-DEPENDENT KINASE A;1
- DEK:
-
DEFECTIVE KERNEL
- EMB:
-
EMBRYO SPECIFIC
- ESR:
-
Embryo surrounding region
- FBL17:
-
F-BOX-LIKE PROTEIN 17
- FM:
-
Functional megaspore
- IKU1:
-
HAIKU1
- IKU2:
-
HAIKU2
- IAA:
-
Indole-3-acetic acid
- IPT:
-
ISOPRENYL TRANSFERASE
- KPR2:
-
KIP RELATED PROTEIN2
- LIS:
-
LACHENSIS
- LRR-RLK:
-
Leucine-rich repeat receptor-like kinase
- MMC:
-
Megaspore mother cell
- MINI3:
-
MINISEED3
- MSP1:
-
MULTIPLE SPOROCYTE1
- RNA:
-
Ribonucleic acid
- SHB1:
-
SHORT HYPOCOTYL UNDER BLUE1
- siRNAs:
-
Small interfering RNAs
- SPL:
-
SPOROCYTELESS
- SAP:
-
STERILE APETALA
- TTG2:
-
TRANSPARENT TESTA GLABRA2
- ZOU:
-
ZHOUPI
References
Adamski NM, Anastasiou E, Eriksson S, O'Neill CM, Lenhard M (2009) Local maternal control of seed size by KLUH/CYP78A5-dependent growth signaling. Proc Natl Acad Sci USA 106:20115–20120
Anastasiou E, Kenz S, Gerstung M, MacLean D, Timmer J, Fleck C, Lenhard M (2007) Control of plant organ size by KLUH/CYP78A5-dependent intercellular signaling. Dev Cell 13:843–856
Balk PA, de Boer AD (1999) Rapid stalk elongation in tulip (Tulipa gesneriana L. cv. Apeldoorn) and the combined action of cold-induced invertase and the water-channel protein gammaTIP. Planta 209:346–354
Baroux, C., Spillane, C. and Grossniklaus, U. (2002). Evolutionary origins of the endosperm in flowering plants. Genome Biol 3(9): reviews1026.
Barratt DH, Derbyshire P, Findlay K, Pike M, Wellner N, Lunn J, Feil R, Simpson C, Maule AJ, Smith AM (2009) Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc Natl Acad Sci USA 106:13124–13129
Bate NJ, Niu X, Wang Y, Reimann KS, Helentjaris TG (2004) An invertase inhibitor from maize localizes to the embryo surrounding region during early kernel development. Plant Physiol 134:246–254
Baud S, Wuilleme S, Lemoine R, Kronenberger J, Caboche M, Lepiniec L, Rochat C (2005) The AtSUC5 sucrose transporter specifically expressed in the endosperm is involved in early seed development in Arabidopsis. Plant J 43:824–836
Beeckman T, De Rycke R, Viane R, Inze D (2000) Histological study of seed coat development in Arabidopsis thaliana. J Plant Res 113:139–148
Berger F (2003) Endosperm: the crossroad of seed development. Curr Opin Plant Biol 6:42–50
Berger F (2009) Reproductive biology: receptor-like kinases orchestrate love songs in plants. Curr Biol 19:R647–R649
Berger F, Grini PE, Schnittger A (2006) Endosperm: an integrator of seed growth and development. Curr Opin Plant Biol 9:664–670
Boisnard-Lorig C, Colon-Carmona A, Bauch M, Hodge S, Doerner P, Bancharel E, Dumas C, Haseloff J, Berger F (2001) Dynamic analyses of the expression of the HISTONE::YFP fusion protein in Arabidopsis show that syncytial endosperm is divided in mitotic domains. Plant Cell 13:495–509
Boisson-Dernier A, Frietsch S, Kim TH, Dizon MB, Schroeder JI (2008) The peroxin loss-of-function mutation abstinence by mutual consent disrupts male–female gametophyte recognition. Curr Biol 18:63–68
Boisson-Dernier A, Roy S, Kritsas K, Grobei MA, Jaciubek M, Schroeder JI, Grossniklaus U (2009) Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development 136:3279–3288
Bonello JF, Opsahl-Ferstad HG, Perez P, Dumas C, Rogowsky PM (2000) Esr genes show different levels of expression in the same region of maize endosperm. Gene 246:219–227
Bonello JF, Sevilla-Lecoq S, Berne A, Risueno MC, Dumas C, Rogowsky PM (2002) Esr proteins are secreted by the cells of the embryo surrounding region. J Exp Bot 53:1559–1568
Borisjuk L, Rolletschek H, Radchuk R, Weschke W, Wobus U, Weber H (2004) Seed development and differentiation: a role for metabolic regulation. Plant Biol (Stuttg) 6:375–386
Bruck DK, Walker DB (1985) Cell determination during embryogenesis in Citrus jambhiri. I. Ontogeny of the epidermis. Bot Gaz 146:188–195
Buttner M (2007) The monosaccharide transporter(-like) gene family in Arabidopsis. FEBS Lett 581:2318–2324
Byzova MV, Franken J, Aarts MG, de Almeida-Engler J, Engler G, Mariani C, Van Lookeren Campagne MM, Angenent GC (1999) Arabidopsis STERILE APETALA, a multifunctional gene regulating inflorescence, flower, and ovule development. Genes Dev 13:1002–1014
Chamberlin MA, Horner HT, Palmer RG (1994) Early endosperm, embryo and ovule development in Glycine max (L.) Merr. Int J Plant Sci 155:421–436
Chapman LA, Goring DR (2010) Pollen-pistil interactions regulating successful fertilization in the Brassicaceae. J Exp Bot 61:1987–1999
Chen YH, Li HJ, Shi DQ, Yuan L, Liu J, Sreenivasan R, Baskar R, Grossniklaus U, Yang WC (2007) The central cell plays a critical role in pollen tube guidance in Arabidopsis. Plant Cell 19:3563–3577
Cheng WH, Taliercio EW, Chourey PS (1996) The Miniature1 seed locus of maize encodes a cell wall invertase required for normal development of endosperm and maternal cells in the pedicel. Plant Cell 8:971–983
Clark JK, Sheridan WF (1991) Isolation and characterization of 51 embryo-specific mutations of maize. Plant Cell 3:935–951
Cossegal M, Vernoud V, Rogowsky PM (2007) The embryo surrounding region. In: Olsen O-A (ed) Endosperm: developmental and molecular biology. Springer, Berlin, pp 57–71
Day RC, Herridge RP, Ambrose BA, Macknight RC (2008) Transcriptome analysis of proliferating Arabidopsis endosperm reveals biological implications for the control of syncytial division, cytokinin signaling, and gene expression regulation. Plant Physiol 148:1964–1984
Debeaujon I, Nesi N, Perez P, Devic M, Grandjean O, Caboche M, Lepiniec L (2003) Proanthocyanidin-accumulating cells in Arabidopsis testa: regulation of differentiation and role in seed development. Plant Cell 15:2514–2531
Deng Y, Dong H, Mu J, Ren B, Zheng B, Ji Z, Yang WC, Liang Y, Zuo J (2010) Arabidopsis histidine kinase CKI1 acts upstream of histidine phosphotransfer proteins to regulate female gametophyte development and vegetative growth. Plant Cell 22:1232–1248
Diboll AG, Larson DA (1966) An electron microscopic study of the mature megagametophyte in Zea mays. Am J Bot 53:391–402
Dominguez F, Cejudo FJ (2006) Identification of a nuclear-localized nuclease from wheat cells undergoing programmed cell death that is able to trigger DNA fragmentation and apoptotic morphology on nuclei from human cells. Biochem J 397:529–536
Dominguez F, Moreno J, Cejudo FJ (2001) The nucellus degenerates by a process of programmed cell death during the early stages of wheat grain development. Planta 213:352–360
Dresselhaus T, Marton ML (2009) Micropylar pollen tube guidance and burst: adapted from defense mechanisms? Curr Opin Plant Biol 12:773–780
Drews GN, Yadegari R (2002) Development and function of the angiosperm female gametophyte. Annu Rev Genet 36:99–124
Drews GN, Lee D, Christensen CA (1998) Genetic analysis of female gametophyte development and function. Plant Cell 10:5–17
Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQ, Gerentes D, Perez P, Smyth DR (1996) AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 8:155–168
Engell K (1994) Embryology of barley. IV. Ultrastructure of the antipodal cells of Hordeum valgare L. cv. Bomi before and after fertilization of the egg cell. Sex Plant Reprod 4:333–346
Eriksson S, Stransfeld L, Adamski NM, Breuninger H, Lenhard M (2010) KLUH/CYP78A5-dependent growth signaling coordinates floral organ growth in Arabidopsis. Curr Biol 20:527–532
Friedman WE (2001) Developmental and evolutionary hypotheses for the origin of double fertilization and endosperm. C R Acad Sci III 324:559–567
Garcia D, Saingery V, Chambrier P, Mayer U, Jurgens G, Berger F (2003) Arabidopsis haiku mutants reveal new controls of seed size by endosperm. Plant Physiol 131:1661–1670
Garcia D, Fitz Gerald JN, Berger F (2005) Maternal control of integument cell elongation and zygotic control of endosperm growth are coordinated to determine seed size in Arabidopsis. Plant Cell 17:52–60
Gifford ML, Dean S, Ingram GC (2003) The Arabidopsis ACR4 gene plays a role in cell layer organisation during ovule integument and sepal margin development. Development 130:4249–4258
Greenwood JS, Helm M, Gietl C (2005) Ricinosomes and endosperm transfer cell structure in programmed cell death of the nucellus during Ricinus seed development. Proc Natl Acad Sci USA 102:2238–2243
Gross-Hardt R, Kagi C, Baumann N, Moore JM, Baskar R, Gagliano WB, Jurgens G, Grossniklaus U (2007) LACHESIS restricts gametic cell fate in the female gametophyte of Arabidopsis. PLoS Biol 5:e47
Gunning BES, Pate JS (1974) Transfer Cells. In: Robards AE (ed) Dynamic aspects of plant ultrastructure. McGraw-Hill Book Company, New York, pp 441–480
Gusti A, Baumberger N, Nowack M, Pusch S, Eisler H, Potuschak T, De Veylder L, Schnittger A, Genschik P (2009) The Arabidopsis thaliana F-box protein FBL17 is essential for progression through the second mitosis during pollen development. PLoS ONE 4:e4780
Haig D (1990) New perspectives on the angiosperm female gametophyte. Bot Rev 56:236–274
Haig D, Westoby M (1989) Parent-specific expression and the triploid endosperm. Am Nat 134:147–155
Hamant O, Traas J (2010) The mechanics behind plant development. New Phytol 185:369–385
Hamant O, Heisler MG, Jonsson H, Krupinski P, Uyttewaal M, Bokov P, Corson F, Sahlin P, Boudaoud A, Meyerowitz EM et al (2008) Developmental patterning by mechanical signals in Arabidopsis. Science 322:1650–1655
Han YZ, Huang BQ, Zee SY, Yuan M (2000) Symplastic communication between the central cell and the egg apparatus cells in the embryo sac of Torenia fournieri Lind. before and during fertilization. Planta 211:158–162
Hartig K, Beck E (2006) Crosstalk between auxin, cytokinins, and sugars in the plant cell cycle. Plant Biol (Stuttg) 8:389–396
Heckel T, Werner K, Sheridan WF, Dumas C, Rogowsky PM (1999) Novel phenotypes and developmental arrest in early embryo specific mutants of maize. Planta 210:1–8
Hejatko J, Pernisova M, Eneva T, Palme K, Brzobohaty B (2003) The putative sensor histidine kinase CKI1 is involved in female gametophyte development in Arabidopsis. Mol Genet Genomics 269:443–453
Higashiyama T, Yabe S, Sasaki N, Nishimura Y, Miyagishima S, Kuroiwa H, Kuroiwa T (2001) Pollen tube attraction by the synergid cell. Science 293:1480–1483
Hill LM, Morley-Smith ER, Rawsthorne S (2003) Metabolism of sugars in the endosperm of developing seeds of oilseed rape. Plant Physiol 131:228–236
Hiratsuka R, Yamada Y, Terasaka O (2002) Programmed cell death of Pinus nucellus in response to pollen tube penetration. J Plant Res 115:141–148
Hong SK, Kitano H, Satoh H, Nagato Y (1996) How is embryo size genetically regulated in rice? Development 122:2051–2058
Hughes R, Spielman M, Schruff MC, Larson TR, Graham IA, Scott RJ (2008) Yield assessment of integument-led seed growth following targeted repair of auxin response factor 2. Plant Biotechnol J 6:758–769
Huh JH, Bauer MJ, Hsieh TF, Fischer R (2007) Endosperm gene imprinting and seed development. Curr Opin Genet Dev 17:480–485
Hutchison CE, Li J, Argueso C, Gonzalez M, Lee E, Lewis MW, Maxwell BB, Perdue TD, Schaller GE, Alonso JM et al (2006) The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell 18:3073–3087
Jain M, Chourey PS, Li QB, Pring DR (2008) Expression of cell wall invertase and several other genes of sugar metabolism in relation to seed development in sorghum (Sorghum bicolor). J Plant Physiol 165:331–344
Jullien PE, Berger F (2009) Gamete-specific epigenetic mechanisms shape genomic imprinting. Curr Opin Plant Biol 12:637–642
Kapil RN, Tiwari SC (1978) The integumentary tapetum. Bot Rev 44:457–490
Kawashima T, Goldberg RB (2009) The suspensor: not just suspending the embryo. Trends Plant Sci 15:23–30
Kennell JC, Horner HT (1985) Influence of the soybean male- sterile gene (msl) on the development of the female gametophyte. Can J Genet Cytol 27:200–209
Kinoshita T (2007) Reproductive barrier and genomic imprinting in the endosperm of flowering plants. Genes Genet Syst 82:177–186
Klucher KM, Chow H, Reiser L, Fischer RL (1996) The AINTEGUMENTA gene of Arabidopsis required for ovule and female gametophyte development is related to the floral homeotic gene APETALA2. Plant Cell 8:137–153
Kondou Y, Nakazawa M, Kawashima M, Ichikawa T, Yoshizumi T, Suzuki K, Ishikawa A, Koshi T, Matsui R, Muto S et al (2008) RETARDED GROWTH OF EMBRYO1, a new basic helix-loop-helix protein, expresses in endosperm to control embryo growth. Plant Physiol 147:1924–1935
LeClere S, Schmelz EA, Chourey PS (2010) Sugar levels regulate tryptophan-dependent auxin biosynthesis in developing maize kernels. Plant Physiol 153:306–318
Leduc N, Matthys-Rochon E, Rougier M, Mogensen L, Holm P, Magnard JL, Dumas C (1996) Isolated maize zygotes mimic in vivo embryonic development and express microinjected genes when cultured in vitro. Dev Biol 177:190–203
Letarte J, Simion E, Miner M, Kasha KJ (2006) Arabinogalactans and arabinogalactan-proteins induce embryogenesis in wheat (Triticum aestivum L.) microspore culture. Plant Cell Rep 24:691–698
Li Y, Zheng L, Corke F, Smith C, Bevan MW (2008) Control of final seed and organ size by the DA1 gene family in Arabidopsis thaliana. Genes Dev 22:1331–1336
Lid SE, Olsen L, Nestestog R, Aukerman M, Brown RC, Lemmon B, Mucha M, Opsahl-Sorteberg HG, Olsen OA (2005) Mutation in the Arabidopisis thaliana DEK1 calpain gene perturbs endosperm and embryo development while over-expression affects organ development globally. Planta 221:339–351
Linkies A, Graeber K, Knight C, Leubner-Metzger G (2010) The evolution of seeds. New Phytol 186:817–831
Lombardi L, Casani S, Ceccarelli N, Galleschi L, Picciarelli P, Lorenzi R (2007) Programmed cell death of the nucellus during Sechium edule Sw. seed development is associated with activation of caspase-like proteases. J Exp Bot 58:2949–2958
Lombardi L, Ceccarelli N, Picciarelli P, Sorce C, Lorenzi R (2010) Nitric oxide and hydrogen peroxide involvement during programmed cell death of Sechium edule nucellus. Physiol Plant doi:10.1111/j.1399-3054.2010.01381.x
Luo M, Dennis ES, Berger F, Peacock WJ, Chaudhury A (2005) MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proc Natl Acad Sci USA 102:17531–17536
Magnard JL, Le Deunff E, Domenech J, Rogowsky PM, Testillano PS, Rougier M, Risueno MC, Vergne P, Dumas C (2000) Genes normally expressed in the endosperm are expressed at early stages of microspore embryogenesis in maize. Plant Mol Biol 44:559–574
Mansfield SG, Briarty LG, Erni S (1991) Early embryogenesis in Arabidopsis thaliana. I. The mature embryo sac. Can J Bot 69:447–460
Marton ML, Cordts S, Broadhvest J, Dresselhaus T (2005) Micropylar pollen tube guidance by egg apparatus 1 of maize. Science 307:573–576
Massonneau A, Coronado MJ, Audran A, Bagniewska A, Mol R, Testillano PS, Goralski G, Dumas C, Risueno MC, Matthys-Rochon E (2005) Multicellular structures developing during maize microspore culture express endosperm and embryo-specific genes and show different embryogenic potentialities. Eur J Cell Biol 84:663–675
Meinke DW, Sussex IM (1979) Embryo-lethal mutants of Arabidopsis thaliana. A model system for genetic analysis of plant embryo development. Dev Biol 72:50–61
Melkus G, Rolletschek H, Radchuk R, Fuchs J, Rutten T, Wobus U, Altmann T, Jakob P, Borisjuk L (2009) The metabolic role of the legume endosperm: a noninvasive imaging study. Plant Physiol 151:1139–1154
Miyawaki K, Matsumoto-Kitano M, Kakimoto T (2004) Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J 37:128–138
Moll C, von Lyncker L, Zimmermann S, Kagi C, Baumann N, Twell D, Grossniklaus U, Gross-Hardt R (2008) CLO/GFA1 and ATO are novel regulators of gametic cell fate in plants. Plant J 56:913–921
Monshausen GB, Gilroy S (2009) The exploring root–root growth responses to local environmental conditions. Curr Opin Plant Biol 12:766–772
Moore T, Haig D (1991) Genomic imprinting in mammalian development: a parental tug-of-war. Trends Genet 7:45–49
Morley-Smith ER, Pike MJ, Findlay K, Kockenberger W, Hill LM, Smith AM, Rawsthorne S (2008) The transport of sugars to developing embryos is not via the bulk endosperm in oilseed rape seeds. Plant Physiol 147:2121–2130
Murgia M, Huang B-Q, Tucker SC, Musgrave ME (1993) Embryo sac lacking antipodal cells in Arabidopsis thaliana (Brassicaceae). Am J Bot 80:824–838
Neuffer MG, Sheridan WF (1980) Defective kernel mutants of maize. I. Genetic and lethality studies. Genetics 95:929–944
Ngo QA, Moore JM, Baskar R, Grossniklaus U, Sundaresan V (2007) Arabidopsis GLAUCE promotes fertilization-independent endosperm development and expression of paternally inherited alleles. Development 134:4107–4117
Nonomura K, Miyoshi K, Eiguchi M, Suzuki T, Miyao A, Hirochika H, Kurata N (2003) The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice. Plant Cell 15:1728–1739
Nowack MK, Grini PE, Jakoby MJ, Lafos M, Koncz C, Schnittger A (2006) A positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nat Genet 38:63–67
Nowack MK, Ungru A, Bjerkan KN, Grini PE, Schnittger A (2010) Reproductive cross-talk: seed development in flowering plants. Biochem Soc Trans 38:604–612
Ohto MA, Fischer RL, Goldberg RB, Nakamura K, Harada JJ (2005) Control of seed mass by APETALA2. Proc Natl Acad Sci USA 102:3123–3128
Okuda S, Tsutsui H, Shiina K, Sprunck S, Takeuchi H, Yui R, Kasahara RD, Hamamura Y, Mizukami A, Susaki D et al (2009) Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458:357–361
Okushima Y, Mitina I, Quach HL, Theologis A (2005) AUXIN RESPONSE FACTOR 2 (ARF2): a pleiotropic developmental regulator. Plant J 43:29–46
Olmedo-Monfil V, Duran-Figueroa N, Arteaga-Vazquez M, Demesa-Arevalo E, Autran D, Grimanelli D, Slotkin RK, Martienssen RA, Vielle-Calzada JP (2010) Control of female gamete formation by a small RNA pathway in Arabidopsis. Nature 464:628–632
Olsen OA (2001) Endosperm development: cellularization and cell fate specification. Annu Rev Plant Physiol Plant Mol Biol 52:233–267
Olsen OA (2004) Nuclear endosperm development in cereals and Arabidopsis thaliana. Plant Cell 16 Suppl:S214–S227
Oparka KJ, Gates PJ (1982) Ultrasructure of the developing pigment strand of rice (Oryza sativa L.) in relation to its role in solute transport. Protoplasma 113:33–43
Opsahl-Ferstad HG, Le Deunff E, Dumas C, Rogowsky PM (1997) ZmEsr, a novel endosperm-specific gene expressed in a restricted region around the maize embryo. Plant J 12:235–246
Pagnussat GC, Alandete-Saez M, Bowman JL, Sundaresan V (2009) Auxin-dependent patterning and gamete specification in the Arabidopsis female gametophyte. Science 324:1684–1689
Palanivelu R, Brass L, Edlund AF, Preuss D (2003) Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114:47–59
Pischke MS, Jones LG, Otsuga D, Fernandez DE, Drews GN, Sussman MR (2002) An Arabidopsis histidine kinase is essential for megagametogenesis. Proc Natl Acad Sci USA 99:15800–15805
Reiser L, Modrusan Z, Margossian L, Samach A, Ohad N, Haughn GW, Fischer RL (1995) The BELL1 gene encodes a homeodomain protein involved in pattern formation in the Arabidopsis ovule primordium. Cell 83:735–742
Richter GL, Monshausen GB, Krol A, Gilroy S (2009) Mechanical stimuli modulate lateral root organogenesis. Plant Physiol 151:1855–1866
Riefler M, Novak O, Strnad M, Schmulling T (2006) Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18:40–54
Robinson-Beers K, Pruitt RE, Gasser CS (1992) Ovule development in wild-type Arabidopsis and two female-sterile mutants. Plant Cell 4:1237–1249
Rotman N, Gourgues M, Guitton AE, Faure JE, Berger F (2008) A dialogue between the SIRENE pathway in synergids and the fertilization independent seed pathway in the central cell controls male gamete release during double fertilization in Arabidopsis. Mol Plant 1:659–666
Russell SD (1979) Fine structure of the megagametophyte of Zea mays. Can J Bot 57:1093–1110
Schreiber L (2005) Polar paths of diffusion across plant cuticles: new evidence for an old hypothesis. Ann Bot 95:1069–1073
Schruff MC, Spielman M, Tiwari S, Adams S, Fenby N, Scott RJ (2006) The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs. Development 133:251–261
Schulz P, Jensen WA (1971) Capsella embryogenesis: the chalazal proliferating tissue. J Cell Sci 8:201–227
Schulz P, Jensen WA (1973) Capsella embryogenesis: the central cell. J Cell Sci 12:741–763
Schulz P, Jensen WA (1974) Capsella embryogenesis: the development of the free nuclear endosperm. Protoplasma 80:183–205
Schuurmans JA, van Dongen JT, Rutjens BP, Boonman A, Pieterse CM, Borstlap AC (2003) Members of the aquaporin family in the developing pea seed coat include representatives of the PIP, TIP, and NIP subfamilies. Plant Mol Biol 53:633–645
Scott RJ, Spielman M, Bailey J, Dickinson HG (1998) Parent-of-origin effects on seed development in Arabidopsis thaliana. Development 125:3329–3341
Seifert GJ, Blaukopf C (2010) Irritable walls: the plant extracellular matrix and signaling. Plant Physiol 153:467–478
Sergeeva LI, Keurentjes JJ, Bentsink L, Vonk J, van der Plas LH, Koornneef M, Vreugdenhil D (2006) Vacuolar invertase regulates elongation of Arabidopsis thaliana roots as revealed by QTL and mutant analysis. Proc Natl Acad Sci USA 103:2994–2999
Sheridan WF, Neuffer MG (1980) Defective kernel mutants of maize II. Morphological and embryo culture studies. Genetics 95:945–960
Sherson SM, Alford HL, Forbes SM, Wallace G, Smith SM (2003) Roles of cell-wall invertases and monosaccharide transporters in the growth and development of Arabidopsis. J Exp Bot 54:525–531
Sprunck S (2010) Let's get physical: gamete interaction in flowering plants. Biochem Soc Trans 38:635–640
Srivastava R, Liu JX, Howell SH (2008) Proteolytic processing of a precursor protein for a growth-promoting peptide by a subtilisin serine protease in Arabidopsis. Plant J 56:219–227
Stadler R, Lauterbach C, Sauer N (2005) Cell-to-cell movement of green fluorescent protein reveals post-phloem transport in the outer integument and identifies symplastic domains in Arabidopsis seeds and embryos. Plant Physiol 139:701–712
Stewart-Cox JA, Britton NF, Mogie M (2004) Endosperm triploidy has a selective advantage during ongoing parental conflict by imprinting. Proc Biol Sci 271:1737–1743
Tanaka H, Onouchi H, Kondo M, Hara-Nishimura I, Nishimura M, Machida C, Machida Y (2001) A subtilisin-like serine protease is required for epidermal surface formation in Arabidopsis embryos and juvenile plants. Development 128:4681–4689
Tanaka T, Tanaka H, Machida C, Watanabe M, Machida Y (2004) A new method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. Plant J 37:139–146
Tanaka H, Watanabe M, Sasabe M, Hiroe T, Tanaka T, Tsukaya H, Ikezaki M, Machida C, Machida Y (2007) Novel receptor-like kinase ALE2 controls shoot development by specifying epidermis in Arabidopsis. Development 134:1643–1652
Tang XC, He YQ, Wang Y, Sun MX (2006) The role of arabinogalactan proteins binding to Yariv reagents in the initiation, cell developmental fate, and maintenance of microspore embryogenesis in Brassica napus L. cv. Topas. J Exp Bot 57:2639–2650
Tsuwamoto R, Fukuoka H, Takahata Y (2008) GASSHO1 and GASSHO2 encoding a putative leucine-rich repeat transmembrane-type receptor kinase are essential for the normal development of the epidermal surface in Arabidopsis embryos. Plant J 54:30–42
Tzafrir I, Pena-Muralla R, Dickerman A, Berg M, Rogers R, Hutchens S, Sweeney TC, McElver J, Aux G, Patton D et al (2004) Identification of genes required for embryo development in Arabidopsis. Plant Physiol 135:1206–1220
Ungru A, Nowack MK, Reymond M, Shirzadi R, Kumar M, Biewers S, Grini PE, Schnittger A (2008) Natural variation in the degree of autonomous endosperm formation reveals independence and constraints of embryo growth during seed development in Arabidopsis thaliana. Genetics 179:829–841
Uyttewaal M, Traas J, Hamant O (2010) Integrating physical stress, growth, and development. Curr Opin Plant Biol 13:46–52
Van Hengel AJ, Van Kammen A, De Vries SC (2002) A relationship between seed development, Arabinogalactan-proteins (AGPs) and the AGP mediated promotion of somatic embryogenesis. Physiol Plant 114:637–644
Vert G, Walcher CL, Chory J, Nemhauser JL (2008) Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proc Natl Acad Sci USA 105:9829–9834
Vilhar B, Kladnik A, Blejec A, Chourey PS, Dermastia M (2002) Cytometrical evidence that the loss of seed weight in the miniature1 seed mutant of maize is associated with reduced mitotic activity in the developing endosperm. Plant Physiol 129:23–30
von Besser K, Frank AC, Johnson MA, Preuss D (2006) Arabidopsis HAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development 133:4761–4769
Von Groll U, Berger D, Altmann T (2002) The subtilisin-like serine protease SDD1 mediates cell-to-cell signaling during Arabidopsis stomatal development. Plant Cell 14:1527–1539
Wang A, Garcia D, Zhang H, Feng K, Chaudhury A, Berger F, James Peacock W, Dennis ES, Luo M (2010) The VQ motif protein IKU1 regulates endosperm growth and seed size in Arabidopsis. Plant J. doi:10.1111/j.1365-313X.2010.04271.x
Watanabe M, Tanaka H, Watanabe D, Machida C, Machida Y (2004) The ACR4 receptor-like kinase is required for surface formation of epidermis-related tissues in Arabidopsis thaliana. Plant J 39:298–308
Weber H, Borisjuk L, Heim U, Sauer N, Wobus U (1997) A role for sugar transporters during seed development: molecular characterization of a hexose and a sucrose carrier in fava bean seeds. Plant Cell 9:895–908
Weijers D, Van Hamburg JP, Van Rijn E, Hooykaas PJ, Offringa R (2003) Diphtheria toxin-mediated cell ablation reveals interregional communication during Arabidopsis seed development. Plant Physiol 133:1882–1892
Weschke W, Panitz R, Gubatz S, Wang Q, Radchuk R, Weber H, Wobus U (2003) The role of invertases and hexose transporters in controlling sugar ratios in maternal and filial tissues of barley caryopses during early development. Plant J 33:395–411
Yadegari R, Drews GN (2004) Female gametophyte development. Plant Cell 16 Suppl:S133–S141
Yang WC, Ye D, Xu J, Sundaresan V (1999) The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev 13:2108–2117
Yang J, Zhang J, Huang Z, Wang Z, Zhu Q, Liu L (2002) Correlation of cytokinin levels in the endosperms and roots with cell number and cell division activity during endosperm development in rice. Ann Bot 90:369–377
Yang S, Johnston N, Talideh E, Mitchell S, Jeffree C, Goodrich J, Ingram G (2008) The endosperm-specific ZHOUPI gene of Arabidopsis thaliana regulates endosperm breakdown and embryonic epidermal development. Development 135:3501–3509
Zee SY, O'Brien TP (1970) Studies on the ontogeny of the pigment strand in the caryopsis of wheat. Aust J Biol 23:1153–1171
Zhao X, de Palma J, Oane R, Gamuyao R, Luo M, Chaudhury A, Herve P, Xue Q, Bennett J (2008) OsTDL1A binds to the LRR domain of rice receptor kinase MSP1, and is required to limit sporocyte numbers. Plant J 54:375–387
Zhou Y, Ni M (2010) SHORT HYPOCOTYL UNDER BLUE1 truncations and mutations alter its association with a signaling protein complex in Arabidopsis. Plant Cell 22:703–715
Zhou Y, Zhang X, Kang X, Zhao X, Ni M (2009) SHORT HYPOCOTYL UNDER BLUE1 associates with MINISEED3 and HAIKU2 promoters in vivo to regulate Arabidopsis seed development. Plant Cell 21:106–117
Acknowledgements
I would like to thank Professor Alison Smith (John Innes Centre, Norwich), Dr Peter Rogowsky (RDP, ENS-Lyon) and Dr Justin Goodrich, Jessica Fitzgibbon and Andrew Waters (University of Edinburgh) for discussions and helpful comments on the manuscript. GI was supported by an RCUK Fellowship.
Conflict of interest
The author declares that she has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: David Robinson
Rights and permissions
About this article
Cite this article
Ingram, G.C. Family life at close quarters: communication and constraint in angiosperm seed development. Protoplasma 247, 195–214 (2010). https://doi.org/10.1007/s00709-010-0184-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-010-0184-y