Abstract
The presence of an attached organ to somatic embryos of angiosperms connecting the embryo to the supporting tissue has been a subject of controversy. This study shows that 67% of the morphologically normal somatic embryos of Feijoa sellowiana possess this type of organ and that its formation was not affected by culture media composition. Histological and ultrastructural analysis indicated that the attached structures of somatic embryos displayed a great morphological diversity ranging from a few cells to massive and columnar structures. This contrast with the simple suspensors observed in zygotic embryos which were only formed by five cells. As well as the suspensor of zygotic embryos, somatic embryo attached structures undergo a process of degeneration in later stages of embryo development. Other characteristic shared by zygotic suspensors and somatic embryo attached structures was the presence of thick cell walls surrounding the cells. Elongated thin filaments were often associated with the structures attached to somatic embryos, whereas in other cases, tubular cells containing starch grains connected the embryo to the supporting tissue. These characteristics associated with the presence of plasmodesmata in the cells of the attached structures seem to indicate a role on embryo nutrition. However, cell proliferation in the attached structures resulting into new somatic embryos may also suggest a more complex relationship between the embryo and the structures connecting it to the supporting tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In higher plants, the emergence of a new plant is preceded by embryo formation and development inside the ovule (Raghavan 1997). It is in the ovule that the processes of macrosporogenesis and megagametogenesis lead to the formation of the embryo sac where the female cells involved in double fertilization are present. From this double fertilization results a nutritive tissue, the endosperm, and the zygote, whose differentiation originates the embryo, through the process of embryogenesis (Raghavan 2006). Histological, genetic, and molecular studies carried out in model species such as Arabidopsis thaliana deeply increased our understanding of the mechanisms underlying zygotic embryo formation and development (Ikeda and Kamada 2005; Raghavan 2006; Park and Harada 2008; Capron et al. 2009). In most of the angiosperms, embryo formation proceeds through a very ordered pattern of cell divisions in which the first zygotic asymmetric division produces two cells with distinct fates (Heidstra 2007): an apical cell of smaller dimensions which gives origin to the embryo proper throughout well-characterized developmental stages (globular, heart-shaped, torpedo, cotyledonary in the case of dicots), and a basal cell of larger dimensions and more vacuolated, which undergoes less divisions and, in most of the cases, originates the suspensor (Souter and Lindsey 2000; Czapik and Izmailow 2001). In A. thaliana, the suspensor is a linear structure with a reduced number of cells which reaches its full development by the globular stage and gradually degenerates by a process of programmed cell death (Schwartz et al. 1997; Berleth 1998; Filonova et al. 2000; Bozhkov et al. 2005). Thus, it is an ephemeral organ, essential mainly in the early stages of embryo development during which it appears to play an important role in providing nutritive substances and hormonal factors from the mother plant or from the suspensor itself to the embryo proper (Westhoff et al. 1998; Umehara and Kamada 2005; Raghavan 2006). Among angiosperms, the suspensor is a variable structure that may display morphological modifications within the same species and even in the same plant (Czapik and Izmailow 2001). Besides, there are species that do not form or present a much reduced suspensor (Yeung and Meinke 1993; Raghavan 2006).
The zygote is not the only cell able to develop into an embryo. Several examples of nonzygotic embryogenesis have been reported in different species (Sharma and Thorpe 1995). Somatic embryogenesis is a type of nonzygotic embryogenesis in which cells cultured in vitro are induced to form embryos in the presence of an auxin or other stimulus (Thorpe and Stasolla 2001; Yang and Zhang 2010). Since somatic embryos are not physically conditioned by ovular tissues, they tend to display a more variable ontogeny and morphology than their zygotic counterparts although the characteristic phases of embryo development could normally be seen (Lindsey and Topping 1993; Dodeman et al. 1997). One particular aspect of somatic embryo development that has originated more controversy is the presence, or not, of a suspensor-like organ connecting the embryo and the mother tissue. In gymnosperms, somatic embryos usually form well-developed suspensor-like structures quite similar to those formed during zygotic embryo development (Ciavatta et al. 2001; von Arnold 2008; von Arnold and Clapham 2008). However, in angiosperms, the question is more complex and, while some authors have sustained that a suspensor is not present during somatic embryo development (Lindsey and Topping 1993; Mandal and Gupta 2003), in other situations, a structure connecting the embryo to the mother tissue and usually referred as “suspensor-like structure” has been reported (Rugkhla and Jones 1998; Canhoto et al. 1999; Jayasankar et al. 2003). Even when suspensor-like organs have been reported in somatic embryos of different species, no detailed studies were made about their frequencies of formation, morphology, origin, and function on somatic embryo development. According to some authors, the presence of a suspensor could be related with the origin of the somatic embryos, with embryos of unicellular origin showing a suspensor, whereas embryos of multicellular origin have no suspensor and present a large area of contact with the maternal tissue (Williams and Maheswaran 1986; Quiroz-Figueroa et al. 2006). Considering this conflicting interpretations, we decided to study the structures connecting somatic embryos to the mother tissue during somatic embryogenesis induction in pineapple guava (Feijoa sellowiana), a myrtaceous plant also known as feijoa. This species is a subtropical plant native to South America (Brazil, Paraguay, and Uruguay) that spread all over the world as an ornamental tree (Canhoto and Cruz 1996a). Their edible fruits are rich in vitamin C and iodine and New Zealand is now the main producer and exporter of this crop. The conditions for somatic embryo formation and development in this species are well understood (Cruz et al. 1990; Canhoto and Cruz 1994; Reis et al. 2008) and the histological and ultrastructural modifications occurring in the explants during somatic embryogenesis induction have been characterized (Canhoto and Cruz 1996b; Canhoto et al. 1996; Cangahuala-Inocente et al. 2004; Reis et al. 2008), making this species a good model to characterize the structures attached to the somatic embryos. Since these structures have not yet been characterized at the molecular level and its role on embryo development remains largely unknown, it was decided to refer to these structures as “attached structures” and not as “suspensor-like structures.” Thus, the objective of the reported work was to quantify the frequencies of attached structures present in somatic embryos of pineapple guava, to characterize them by cytological, histological, and electron microscopy analysis, and to compare these structures with the suspensor formed during zygotic embryo development.
Material and methods
Plant material
For somatic embryogenesis induction, mature fruits were collected from two pineapple guava trees growing at the Botanical Garden of the University of Coimbra, Coimbra (Portugal) during the months of September and October. Seeds were excised and surface sterilized for 20 min in a calcium hypochlorite solution containing two to three drops of Tween 20. For zygotic embryo analysis, immature fruits at different stages of development (see Fig. 1a) and collected 3–4 months after pollination were used.
Analysis of the suspensor in zygotic embryos of feijoa. a Fruits at different developmental stages. b Isolated and cleared heart-shaped embryo showing the small suspensor (arrow). c Higher magnification of a suspensor. Note the five cells and their thick cell walls (arrows). d Basal region of a zygotic embryo showing a three-cellular suspensor (arrows). e SEM image of a heart-shaped embryo from stage 3 fruits. f Late torpedo embryo from stage 3 fruits. The arrow indicates the suspensor. g Cotyledonary embryo. h Cleared cotyledonary embryo showing a rudimentary suspensor (arrow in the boxed zone). i Amplification of the boxed zone in g analyzed by SEM. Note the degenerating suspensor (ds). b basal cell of the suspensor, cc cells of the suspensor in contact with the embryo, ds degenerating suspensor, e embryo, i intermediate cells of the suspensor, s starch grains
Zygotic embryo analysis
Zygotic embryos were carefully isolated from developing seeds at different stages of development (see Fig. 1a) under a dissecting microscope with the help of thin needles. The isolated embryos were treated for a few seconds in commercial bleach, washed in water, and then mounted in microscope slides in a drop of water or in a drop of a glycerol solution (40% v/v). Zygotic embryos were also stained with acetocarmine or with the iodine-KI reagent to increase contrast and to better observe the specimens under the light microscope. Following a protocol identical to that used with somatic embryos, zygotic embryos were also prepared for histological and scanning electron microscopy (SEM) analysis.
Somatic embryogenesis induction
Cotyledonary zygotic embryos were isolated intact from seeds and inoculated in two different media which consisted of MS (Murashige and Skoog 1962) nutrients containing 4.5 µM 2,4-dichlorophenoxyacetic acid (2,4-D) and 0.09 M (FS1) or 0.27 M (FS2) sucrose. The media were jellified with agar (7 g L−1) and the pH adjusted to 5.7 prior to autoclaving (121°C, 120 kPa, 20 min). The cultures were kept at 25 ± 1°C in the dark for 10 weeks. A more detailed description of this methodology can be seen elsewhere (Cruz et al. 1990; Canhoto and Cruz 1994). After 10 weeks in the induction media, the explants were observed under a dissecting microscope and the presence/absence of an attached structure was evaluated. The morphology of the embryos and their developmental stage were also determined. Since embryos in the early stages of development (globular/heart-shaped) were almost transparent, a treatment with 1% (w/v) osmium tetroxide in 0.1 M phosphate buffer (pH 7.0) was applied to give the embryos a dark coloration that makes them easier to analyze under the dissecting microscope. In other situations, they were treated with acetocarmine or the iodine-KI reagent. For somatic embryogenesis induction, each treatment consisted of at least four replicates of 30 to 40 somatic explants (zygotic embryos). For statistical analysis, all quantitative data expressed as percentages were submitted to arcsine transformation and the means corrected for the bias before a new conversion of the means and standard error (SE) back into percentages (Zar 1996). Statistical analysis (Statistica 7.0) was performed by analysis of variance and the significantly different means were identified by using the Tukey test (p = 0.05).
Histological and ultrastructural studies
For histological studies, isolated somatic or zygotic embryos at different developmental stages or embryogenic explants cultured for 10 weeks in the induction media were fixed for 1–1.5 h at room temperature in a 2.5% (v/v) glutaraldehyde solution prepared with 0.1 M phosphate buffer, pH 7.0, and postfixed in 1% (w/v) osmium tetroxide prepared with the same buffer. The samples were further dehydrated in an ascending ethanol series (20%, 40%, 60%, 80%, 95%, and 100% v/v) and embedded in Spurr’s resin (Spurr 1967). Polymerization of the resin blocks, with the samples properly orientated, was made at 60°C overnight. Sections (1–3 µm) were cut with glass knives on a ultramicrotome (LKB Ultratome III 8801A) and stained with 0.2% (w/v) toluidine blue for 1 h at room temperature. Microscope slides were then washed in running water and observed in a Nikon Eclipse E400 microscope equipped with a Nikon digital camera (model Sight DS-U1) using the Act-2U software.
Material for SEM and transmission electron microscopy observations was fixed as described for the histological studies. Ultrathin sections (50–60 nm) were cut with a diamond knife on a LKB ultramicrotome and collected on uncoated copper grids. Contrast of the grids was carried out with uranyl acetate (10 min) and lead citrate (5–10 min) and the observations were conducted on a transmission electron microscope (Siemens Elmiskop-101) at 80 kV. For SEM studies, the samples were critical point dried in a Balzers CPD 020 apparatus using carbon dioxide as the transition fluid (3 × 10 min, 40°C, 80 atm) and coated with gold. The specimens were mounted in aluminum stubs and examined in a JEOL JSM-T330 scanning electron microscope operating at 20 kV. For more details, see Canhoto et al. (1996).
Results
Suspensor of zygotic embryos
Zygotic embryogenesis in feijoa is an asynchronous process. The more developed suspensors were observed at the transition between the globular and heart-shaped phase (Fig. 1b). At this stage, the suspensor was formed by five cells (Fig. 1c): two cells in contact with the embryo, two intermediate cells, and a large basal cell. All of them were surrounded by a translucent sheath of gelatinous material. The possibility that the number of suspensor cells could be higher must not be ruled out since other cells may be in a different plan. At earlier stages, embryos showing suspensors with only three cells (Fig. 1d) were seen, indicating that the cells closer to the embryo undergo a further anticlinal division giving origin to the five cells observed in later stages (Fig. 1c). Due to their small dimensions and translucent aspect, globular embryos were hard to find among the liquid endosperm. Even when these embryos were isolated with success, we were unable to visualize the correspondent suspensor in most of the embryos. Analysis of developing seeds showed that, for fruits of the same age and at the same developmental stage, embryos were at different developmental stages. For example, in stage 3 fruits (Fig. 1a), seeds containing heart-shaped (Fig. 1e) or torpedo (Fig. 1f) embryos were observed. Moreover, fruits of different lengths have embryos at the same developmental stage. In more advanced stages of embryo development (Fig. 1g, h), the suspensor appears as an unorganized structure as a result of its degeneration (Fig. 1i).
Somatic embryogenesis induction and analysis of the attached structures
Somatic embryo formation (Fig. 2a) was achieved in both culture media and the rates of embryos formed per cultured explant did not differ significantly: 8.9 ± 2.1 embryos on the FS1 medium and 9.8 ± 5.4 in the FS2 medium. The number of embryos per induced explant considerably varied, ranging from only a few to more than 100. Somatic embryo formation was highly asynchronous with embryos at the different stages of development being observed in the same explant. By the end of the culture period (10 weeks), the explants showed somatic embryos in all phases of development: globular, heart-shaped, torpedo, and cotyledonary. The morphology of the embryos was also highly variable (Fig. 2b) and embryos morphologically normal and abnormal occurred in the same explant. However, explants cultured in the presence of higher amounts of sucrose (FS2 medium) often showed fused embryo axis and appeared in clusters, whereas somatic embryos formed on the FS1 medium usually appeared scattered on the mother tissue and were easily detachable. The number of normal embryos (Fig. 2c), which were morphologically identical to their zygotic counterparts, was identical in both media (Table 1). The anomalous somatic embryos were divided into two groups according to their morphological appearance. One group consisted of white embryos displaying some kind of morphological abnormality such as more than two cotyledons, fused embryos, cup-shaped cotyledons, only one cotyledon, or enlarged embryos (Fig. 2d). The other group was characterized by somatic embryos that germinated precociously, showed poorly differentiated cotyledons, usually without hypocotyls, and had very elongated roots where root caps and hairy roots were normally present (Fig. 2e–g). The results showed that high sucrose concentrations (FS2 medium) significantly decreased (p < 0.05) the number of precociously germinated embryos which, in the FS2 medium, were residual. In fact, from a total of 2,256 embryos analyzed, <60 showed this phenotype (Table 1). The presence of a structure connecting the embryo to the mother tissue was analyzed in normal and white abnormal somatic embryos. The results indicated that 67% (FS2 medium) of the normal embryos showed such attached structure (Fig. 2h, i) with no statistical differences (p ≥ 0.05) found between the two media used. By contrast, the attached structure was only found in 5% (FS1) or less (FS2) of the morphologically abnormal white somatic embryos. Embryos without an attached structure (Fig. 2j, k) were mainly formed in a quite direct way practically without the appearance of a pronounced callus (Fig. 2k).
Somatic embryo morphology in embryogenic cultures of F. sellowiana. a Explant with several somatic embryos after 10 weeks of culture. b Detail of an embryogenic explant with somatic embryos showing a great diversity in morphology. c Morphologically normal somatic embryo with two identical cotyledons (arrow). d Anomalous white somatic embryos. e–g Aspects of precociously germinated somatic embryos. Note the incipient cotyledons, the elongated roots, and the absence of hypocotyls in most of the embryos. The arrows in g indicate the root hairs. h Heart-shaped somatic embryo possessing a small attached structure (arrow). i Somatic embryo connected to the callus through an attached structure (arrow). j Isolated heart-shaped somatic embryo with no attached structure. k SEM image of two somatic embryos without attached structure. The arrows indicate the zone of connection to the mother tissue. c cotyledon, ca callus, e embryo, h hypocotyls, r root hairs, rc root cap
Morphology of the attached structures
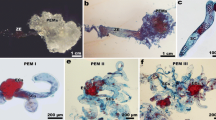
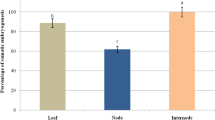
The morphology of the somatic embryo attached structures, like that of the somatic embryos, was quite variable. Usually, the attached structure was a prominent columnar mass of cells easily distinguishable from the embryo proper and the maternal tissues (Fig. 3a, b). At the globular (Fig. 3b) and heart-shaped (Fig. 3c) phases, the structures connecting the somatic embryos to the mother tissues reached their maximum development. Cytological studies seem to indicate that the attached structure and the embryo proper may have its origin from an asymmetric division resulting in a smaller apical and a larger vacuolated cell (Fig. 3d). Further divisions on these putative bicellular proembryos gave origin to the embryo proper consisting of dense cytoplasm-rich cells and the attached structures showing less stainable and vacuolated cells (Fig. 3e, f). In some of the somatic embryos, the attached structure was constituted by a reduced number of cells (Fig. 3g), showing some analogy with the suspensor found in zygotic embryos (see Fig. 1c). When the presence of the attached structure was analyzed in embryos at different developmental stages, it was found that the percentage of cotyledonary embryos showing such entity was significantly lower (p < 0.05) than in the other stages independently of the culture media used (Table 2). In some embryos, the attached structure is a mass of large disorganized cells where thin elongated filaments were often present (Fig. 3h–j). These elongated filaments displayed an irregular surface and their exact origin could not be determined. Tubular cells extending outward from the root pole of the embryos (Fig. 3k) or from the attached structures (Fig. 3l) and growing through the surrounding tissues were common. Cytological analysis of these structures showed that they represent extremely elongated cells containing starch grains and possessing large vacuoles (Fig. 3m). Occasionally, it was also observed that some of the attached structures proliferated in an anomalous way and gave origin to a secondary structure resembling a somatic embryo (Fig. 3n). An attached structure was also observed in later stages of somatic embryo development (Fig. 3o), although at these stages, it was in general a more reduced organ, as a consequence of its senescence
Morphology of the somatic embryo attached structures. a Two somatic embryos (stained with iodine-KI) at the globular stage. The attached structure of one of the embryos is seen (arrow). b SEM image of a globular embryo and its columnar attached structure. c Heart-shaped embryo with a massive attached structure. d Putative bicellular proembryo showing a small apical cell (a) and a larger basal cell (b). e Multicellular embryo showing the embryo proper zone (e) and the cells of the attached structure (at). f Early globular embryo (e) and the attached structure (at) surrounded by the cotyledonary mother tissue (mt). g Detail of a heart-shaped attached structure formed by a reduced number of cells. h Torpedo embryo showing a disorganized attached structure. i Higher magnification of the encircled zone in h. Note the thin elongated filaments (arrows) associated with the cells of the attached structure. j Detail of a thin elongated filament. k Somatic embryo showing tubular cells (arrows). l Higher magnification of the embryo showed in k. The arrow signals one of the tubular cells. m Apical zone of a tubular cell showing several starch grains. n Globular somatic embryo in which the attached structure proliferated into a secondary embryo. o Cotyledonary embryo showing a residual small attached structure (arrow). a apical cell, at attached structure, b basal cell, ca callus, e embryo, s starch grains, v vacuole
Histological and ultrastructural studies
Longitudinal sections of the globular to heart-shaped somatic embryos clearly showed the attached structures connecting the embryo proper and the mother tissue (Fig. 4a). These embryos displayed a slightly apical–basal polarity presenting cytoplasm-rich cells in the apical region and more vacuolated cells in the basal region, attached structure included as observed in earlier stages (see Fig. 3e, f). Cells of the apical region where more or less isodiametric, showing a large nucleus and a prominent nucleolus (Fig. 4b). These cells were characterized by the presence of numerous mitochondria and plastids, profiles of rough endoplasmic reticulum and small vacuoles. Starch grains were occasionally found in the plastids and the cells were connected by plasmodesmata. At the cut end of the attached structure, phenolic-rich cells were often observed (Fig. 4c), whereas cells of the basal region of the embryo (Fig. 4d) presented larger vacuoles than those of the apical region. Electron-dense compounds, probably of phenolic origin, accumulated in the vacuoles of these cells (Fig. 4d). Mitochondria and elongated plastids, normally possessing a few starch grains, were characteristic of these cells. In the transition zone between the embryo and the attached structure (see Fig. 4a), cell walls were often associated with translucent zones. Furthermore, in some of these cells, well-defined plasmodesmata were observed (Fig. 4e). The presence of numerous vacuoles, empty vesicles, and mitochondria and plastids showing signals of degeneration were other particularities of these cells. In more advanced stages of embryo development (heart-shaped embryos), the cells of the attached structure were practically reduced to the cell wall and to small deposits of electron-dense materials (Fig. 4f). At this stage, most of the cells were empty and no particular organelles could be found. The only exception were some cells located in the middle region of the attached structure in which a cytoplasm in degeneration was still visible and droplets of electron-dense unknown material were spread in the vacuolated cytoplasm (Fig. 4g). A particular feature of these degenerated cells, in particular those situated at the periphery and at the basal cut end, was the presence of a thick cell wall that isolated the embryo from the mother tissue (Fig. 4h). Torpedo and cotyledonary embryos showed only residual attached structures formed by degenerated cells.
Histological and ultrastructural analysis of the attached structures. a Longitudinal section through a somatic embryo showing the attached structure (arrow). A well-defined protoderm can also be observed. b Cell of the apical part of the embryo showing a large nucleus and a conspicuous nucleolus. c Basal part of an attached structure where phenolic-rich cells are visible (asterisks). d Section of a basal cell of an embryo showing a large vacuole where phenolic compounds were present. Several mitochondria and a plastid can be seen. e Section through the transition zone between the embryo and the attached structure (boxed zone in a). A thick cell wall associated with translucent zones (arrowheads) and large plasmodesmata (arrows) can be observed in some of the cells. Empty vacuoles and mitochondria and plastids in degeneration were common features. f Section through an attached structure of a heart-shaped embryo. Most of the cells were empty, whereas others showed signals of degeneration. Thick cell walls can be observed at the periphery of the attached structure and in some cells of the central zone (arrows). g Detail of an attached structure cell of a heart-shaped embryo showing the accumulation of osmiophilic droplets (arrows) of unknown composition and origin. Nuclei in degeneration can also be seen. h Detail of the thick cell walls present in cells of an attached structure. ca callus, cw cell wall, m mitochondria, n nucleus, nu nucleolus, p plastid, ph phenolic compounds, pr protoderm, v vacuole
Discussion
The formation of a suspensor by somatic embryos of angiosperms has been questioned by several authors (Ho and Vasil 1983; Emons 1994; Arruda et al. 2000; Ciavatta et al. 2001) probably due to the lack of detailed studies concerning this embryonic organ. By contrast, in gymnosperms, in particular among conifers, the presence of a suspensor during somatic embryo development is well characterized (von Arnold et al. 2002; Stasolla and Yeung 2003; von Arnold 2008) and has been used as a model system to understand the complex interactions occurring between the embryo proper and the suspensor (Ciavatta et al. 2001; Umehara and Kamada 2005).
With a few exceptions, the suspensor is usually present during the initial stages of zygotic embryo development of angiosperms (Yeung and Meinke 1993; Souter and Lindsey 2000) and its crucial role on embryo nutrition and on influencing embryo development is now recognized (Schwartz et al. 1994; Zhang and Somerville 1997; Raghavan 2006). However and in spite of an extensive literature published about somatic and pollen embryogenesis, it is surprising that only a reduced number of reports focused on the analysis of the suspensor in somatic or pollen embryos (Jayasankar et al. 2003; Chanana et al. 2005). It seems likely that the small dimensions of the suspensor, the fact that somatic embryos are usually surrounded by non-embryogenic cells, and the belief that, during somatic embryogenesis, the embryo is nurtured by callus cells or by the mother tissue (Yeung 1995) may explain the reduced interest on the study of somatic embryo attached structures.
The present results clearly demonstrate that feijoa somatic embryos have an attached structure resembling a suspensor connecting them to the mother tissue and that the culture media do not affect the rate of embryos displaying such structure. However, the number of precociously germinated somatic embryos was significantly higher on the low sucrose-containing medium (FS1). The role of high sucrose levels on preventing precocious germination was also referred by other authors (Levi and Sink 1990). In Vitis vinifera, it was suggested that precocious germination was related with the presence of a persistent suspensor in somatic embryos (Jayasankar et al. 2003). This does not seem to be the case in feijoa since the rate of attached structure formation was not significantly different in the two used media, although, as stated above, more somatic embryos germinated precociously on the medium with lower levels of sucrose (FS1).
The formation of attached structures in somatic embryos of angiosperms has been reported in species such as Acacia mangium (Xie and Hong 2001), Carica papaya (Fernando et al. 2001), Ceratonia siliqua (Canhoto et al. 2006), Dactylis glomerata (Trigiano et al. 1989), Myrtus communis (Canhoto et al. 1999), and V. vinifera (Jayasankar et al. 2003) among many others. In most of these studies, the structures were not analyzed in detail and only slight allusions to their presence or to the rate at which they were formed have been made.
Our data also showed that, besides the embryos that form an attached structure, there are a considerable number (reaching about 40% in the FS1 medium and 33% in the FS2 medium) of the morphologically normal somatic embryos that did not show this organ. As pointed out by Williams and Maheswaran (1986), the formation, or not, of a suspensor-like structure may be the result of different somatic embryo origins. Those of unicellular origin usually form a suspensor, whereas embryos arising from a group of cells are connected to the mother tissue by a broad region and do not display such structure. Observations made in Santalum album and Santalum spicatum support this hypothesis (Rugkhla and Jones 1998). In previous reports about the ontogeny of feijoa somatic embryos, it was also found that embryos can have a unicellular or multicellular origin (Canhoto and Cruz 1996b; Canhoto et al. 1996). However, in this species, more detailed studies are necessary to relate the presence/absence of an attached structure with a unicellular/multicellular origin of the embryos.
Analysis of the attached structures found in feijoa somatic embryos showed a high degree of variability, ranging from those formed by a group of just a few cells to those organized in massive or columnar structures. By contrast, the suspensors observed in zygotic embryos appeared to be very simple, being formed by a maximum of five cells. Rudimentary suspensors have been observed in other members of the Myrtaceae family such as Campomanesia pubescens (Strassburg 2004), Darwinia micropetala and Darwinia fascicularis (Prakash 1969), and Eugenia hookeri (Johnson 1936). Another difference between zygotic suspensors and the attached structures found in somatic embryos was their persistence. Thus, it was found that about 30% of the somatic embryos at the cotyledonary stage still showed an attached structure. This may reflect an alteration occurring in vitro in the mechanisms of programmed cell death involved in suspensor senescence during zygotic embryogenesis (Filonova et al. 2000; Raghavan 2006).
The variability of the morphology of attached structures found in feijoa has been also referred in somatic embryos of other species. For example, in V. vinifera, two types of structures were observed, depending if the embryos formed in a solid or in a liquid medium (Jayasankar et al. 2003). The diversity detected in somatic embryo attached structures should not be unexpected since for some species, feijoa included, somatic embryos also display a wide degree of morphological variation (Williams and Maheswaran 1986; Gray 1995). The differences found between zygotic suspensors and somatic embryo attached structures of feijoa may be the result of cell proliferation occurring in vitro in the presence of the auxin 2,4-D. The origin of the suspensor is another factor that can influences its morphology. During zygotic embryogenesis, the suspensor is usually formed from the basal cell after the first asymmetric division of the zygote following a predictable pattern of cell divisions (Souter and Lindsey 2000; Capron et al. 2009). This regular pattern of development does not necessarily occur during somatic embryo formation (Williams and Maheswaran 1986; Canhoto and Cruz 1996b; Canhoto et al. 1996; Krikorian 2000) and the attached structures may be the result of cell proliferation in the supporting tissue as pointed out in S. album and S. spicatum by Rugkhla and Jones (1998). This does not seem to be the case in feijoa since the cytological analysis showed that the embryo and the attached structure seem to have its origin in the same cell that by an asymmetric division produces an apical and a basal cell involved on embryo and attached structure formation, respectively.
A striking feature of some of the embryos observed in this work was the presence of thin elongated filaments and tubular cells. These formations have been found during zygotic embryogenesis of some species (see Yeung and Meinke 1993; Raghavan 2006) where they seem to be involved in the nutrition of the embryo. A similar role may be assigned to these formations during somatic embryogenesis, but further experiments are necessary to clarify its origin and function. The presence of well-defined plasmodesmata in the transition zone between the attached structure and the embryo also supports this perspective.
Suspensor cells of zygotic embryos usually possess cell wall ingrowths, a characteristic of transfer cells, and a particular type of plastids containing spherical bodies (Yeung and Meinke 1993; Raghavan 1997; Raghavan 2006). None of these characteristics were observed in our study. According to Yeung and Meinke (1993), lack of these specialized structures is incompatible with a role on embryo nutrition. Cells of the attached structure in somatic embryos of feijoa were highly vacuolated even in the earlier stages of embryo differentiation, contrasting with the cytoplasm-rich cells of the embryo proper. In V. vinifera, the cytoplasm of the attached structure cells was variable, with some structures showing cells with dense cytoplasm, whereas in others, the cells were highly vacuolated (Jayasankar et al. 2003). These authors suggested that embryos possessing attached structures with vacuolated cells were unable to develop a normal shoot apical meristem and, as a consequence, to form a complete plant. It is possible that premature vacuolation in attached structures of feijoa could be also related with poor development into plantlets. However, it seems likely that this is rather due to deficiencies in storage compound (lipids and proteins) accumulation in the developing embryo than to shoot apical meristem malformation. The low rates of somatic embryo conversion obtained in feijoa (Canhoto and Cruz 1994) together with precocious vacuolation and abortion of the embryos (Canhoto and Cruz 1996b; Canhoto et al. 1996) may sustain this hypothesis. Thick cell walls mainly found in the cells in contact with the supporting tissue and at the periphery of the attached structure were common and may contribute to embryo isolation. The lack of plasmodesmata in these cell walls tends to support this assumption. The presence of phenolic compounds in the basal cells of the attached structure was also commonly detected. The role of phenolic compounds on somatic embryo formation and development in feijoa was recently evaluated (Reis et al. 2008).
This work is a first attempt to analyze the attached structures observed in somatic embryos of feijoa. If these structures present on feijoa (or any other species) somatic embryos have any role during embryo development needs further investigation. However, the fact that in anomalous white somatic embryos these structures are often absent can be more than a coincidence. It is possible that the absence of this structure during the early stages of development may lead to deficiencies in embryo nutrition that can be related to the morphological abnormalities and further embryo conversion into plantlets. The reduced rates of plant conversion in somatic embryos of feijoa (Canhoto and Cruz 1994) and the low levels of protein and lipid storage observed in some somatic embryos (Canhoto et al. 2009) seem to support this hypothesis in spite of other factors that can also interfere with normal somatic embryo development. The exact role of the attached structures during somatic embryo induction and development in angiosperms is far from being understood. Is it a passive structure without a specific role or is it involved on somatic embryo nutrition? Does it have a role in influencing the development of the embryo itself through more complex interactions as has been demonstrated in zygotic embryos of A. thaliana (Schwartz et al. 1994; Vernon and Meinke 1994; Haecker et al. 2004; Lukowitz et al. 2004; Raghavan 2006)? The observation that some attached structures of feijoa somatic embryos are able to proliferate in vitro into secondary embryo-like structures seems to indicate that a dual role (nutrition and control of embryo development) for attached structures of somatic embryos is likely to occur. Recently, Supena et al. (2008) developed a system for high yield production of microspore-derived embryos in Brassica napus showing long suspensor-like filaments by manipulating the culture conditions. Moreover, they have shown that in microspore-derived embryos possessing no or rudimentary suspensor-like structures development was strongly delayed. This seems to indicate a pivotal role for the suspensor also during pollen embryogenesis. Once established that a structure similar to the zygotic suspensor also occurs during somatic embryogenesis of feijoa, it will be now necessary to understand how the embryo and the attached structure influence each other in an artificial environment that is considerably different from the conditions occurring inside the ovule.
References
Arruda SCC, Souza GM, Almeida M, Gonçalves AN (2000) Anatomical and biochemical characterization of the calcium effect on Eucalyptus urophylla callus morphogenesis in vitro. Plant Cell Tissue Organ Cult 63:143–154
Berleth T (1998) Experimental approaches to Arabidopsis embryogenesis. Plant Physiol Biochem 36:69–82
Bozhkov PV, Filonova LH, Suarez MF (2005) Programmed cell death in plant embryogenesis. Curr Top Dev Biol 67:135–179
Cangahuala-Inocente GC, Steiner N, Santos M, Guerra MP (2004) Morphohistological analysis and histochemistry of Feijoa sellowiana somatic embryogenesis. Protoplasma 224:33–40
Canhoto JM, Cruz GS (1994) Improvement of somatic embryogenesis in Feijoa sellowiana Berger (Myrtaceae) by manipulation of the induction and regeneration media. In Vitro Cell Dev Biol 30:21–25
Canhoto JM, Cruz GS (1996a) Feijoa sellowiana Berg (pineapple guava). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol. 35, trees IV. Springer, Berlin, pp 155–171
Canhoto JM, Cruz GS (1996b) Histodifferentiation of somatic embryos in cotyledons of pineapple guava (Feijoa sellowiana Berg). Protoplasma 191:34–35
Canhoto JM, Cruz GS, Mesquita JF (1996) Ultrastructural changes in cotyledons of pineapple guava (Myrtaceae) during somatic embryogenesis. Ann Bot 78:513–521
Canhoto JM, Lopes L, Cruz GS (1999) Somatic embryogenesis and plant regeneration in myrtle (Myrtaceae). Plant Cell Tissue Organ Cult 57:13–21
Canhoto JM, Rama S, Cruz GS (2006) Somatic embryogenesis and plant regeneration in carob (Ceratonia siliqua L.). In Vitro Cell Dev Biol 42:514–519
Canhoto JM, Correia SI, Marques CI (2009) Factors affecting somatic embryogenesis induction and development in Feijoa sellowiana Berg. Acta Hortic 839:149–156
Capron A, Chatfield S, Provart N, Berleth T (2009). Embryogenesis: pattern formation from a single cell. In: The Arabidopsis book. American Society of Plant Biologists, Rockville. doi:10.1199/tab.0051. Available at DIALOG, http://www.aspb.org/publications/arabidopsis/of subordinate document. Accessed 16 February 2010
Chanana NP, Dhawan V, Bhojwani SS (2005) Morphogenesis in isolated microspore cultures of Brassica juncea. Plant Cell Tissue Organ Cult 83:169–177
Ciavatta VT, Morillon R, Pullman GS, Chrispeels MJ, Cairney J (2001) An aquaglyceroporin is abundantly expressed early in the development of the suspensor and the embryo proper of loblolly pine. Plant Physiol 127:1556–1567
Cruz GS, Canhoto J, Abreu MA (1990) Somatic embryogenesis and plant regeneration from zygotic embryos of Feijoa sellowiana Berg. Plant Sci 66:263–270
Czapik R, Izmailow R (2001) Zygotic embryogenesis—structural aspects. In: Bhojwani SS, Soh WY (eds) Current trends in the embryology of angiosperms. Kluwer Academic, Dordrecht, pp 197–222
Dodeman VL, Ducreux G, Kreis M (1997) Zygotic embryogenesis versus somatic embryogenesis. J Exp Bot 48:1493–1509
Emons AMC (1994) Somatic embryogenesis: cell biological aspects. Acta Bot Neerl 43:1–14
Fernando AJ, Melo M, Soares MKM, Appezzato-da-Glória B (2001) Anatomy of somatic embryogenesis in Carica papaya L. Braz Arch Biol Technol 44:247–255
Filonova LH, Bozhkov PV, Brukhin VB, Daniel G, Zhivotovsky B, von Arnold S (2000) Two waves of programmed cell death occur during formation and development of somatic embryos in the gymnosperm, Norway Spruce. J Cell Sci 113:4399–4411
Gray DJ (1995) Somatic embryogenesis in grape. In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants, vol 2. Kluwer Academic, Dordrecht, pp 191–218
Haecker A, Groβ-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T (2004) Expression dynamics of WOX genes mark cell fate decisions during early embryogenic patterning in Arabidopsis thaliana. Development 131:657–668
Heidstra R (2007) Asymmetric cell division in plant development. In: Macieira-Coelho A (ed) Asymmetric cell division. Springer, Berlin, pp 1–38
Ho WJ, Vasil JK (1983) Somatic embryogenesis in sugarcane (Saccharum officinarum L.). I. The morphology and physiology of callus formation and the ontogeny of somatic embryos. Protoplasma 118:169–180
Ikeda M, Kamada H (2005) Comparison of molecular mechanisms of somatic and zygotic embryogenesis. In: Mujib A, Šamaj J (eds) Somatic embryogenesis. Springer, Heidelberg
Jayasankar S, Bondada BR, Li Z, Gray DJ (2003) Comparative anatomy and morphology of Vitis vinifera (Vitaceae) somatic embryos from solid and liquid-culture-derived proembryogeneic masses. Am J Bot 90:973–979
Johnson AM (1936) Polyembryony in Eugenia hookeri. Am J Bot 23:83–88
Krikorian AD (2000) Historical insights into some contemporary problems in somatic embryogenesis. In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants, vol 6. Kluwer Academic, Dordrecht, pp 17–49
Levi A, Sink KC (1990) Differential effects of sucrose, glucose, glucose and fructose during somatic embryogenesis in Asparagus. J Plant Physiol 137:184–189
Lindsey K, Topping JF (1993) Embryogenesis: a question of pattern. J Exp Bot 44:359–374
Lukowitz W, Roeder A, Parmenter D, Somerville C (2004) A MAPKK kinase gene regulates extra-embryonic cell fate in Arabidopsis. Cell 116:109–119
Mandal AKA, Gupta D (2003) Somatic embryogenesis in safflower: influence of auxins and ontogeny of somatic embryos. Plant Cell Tissue Organ Cult 72:27–31
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Park S, Harada JJ (2008) Arabidopsis embryogenesis. In: Suárez MF, Bozhkov PV (eds) Methods in molecular biology, vol 427, Plant embryogenesis. Humana, Totowa, pp 191–218
Prakash N (1969) Reproductive development in two species of Darwinia Rugde (Myrtaceae). Aust J Bot 17:215–227
Quiroz-Figueroa FR, Rojas-Herrera R, Galaz-Avalos RM, Loyola-Vargas VM (2006) Embryo production trough somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell Tissue Organ Cult 86:285–301
Raghavan V (1997) Molecular embryology of flowering plants. Cambridge University Press, Cambridge
Raghavan V (2006) Double fertilization—embryo and endosperm development in flowering plants. Springer, Berlin
Reis E, Batista MT, Canhoto JM (2008) Effect and analysis of phenolic compounds during somatic embryogenesis induction in Feijoa sellowiana Berg. Protoplasma 232:193–202
Rugkhla A, Jones MGK (1998) Somatic embryogenesis and plantlet formation in Santalum album and S. spicatum. J Exp Bot 49:563–571
Schwartz BW, Yeung EC, Meinke DW (1994) Disruption of morphogenesis and transformation of the suspensor in abnormal suspensor mutants of Arabidopsis. Development 120:3235–3245
Schwartz BW, Vernon DM, Meinke DW (1997) Development of the suspensor: differentiation, communication and programmed cell death during plant embryogenesis. In: Larkins BA, Vasil IK (eds) Cellular and molecular biology of plant seed development, vol 2. Kluwer Academic, Dordrecht, pp 53–72
Sharma KK, Thorpe TA (1995) Asexual embryogenesis in vascular plants in nature. In: Thorpe TA (ed) In vitro embryogenesis in plants. Kluwer Academic, Dordrecht, pp 17–72
Souter M, Lindsey K (2000) Polarity and signalling in plant embryogenesis. J Exp Bot 51:971–983
Spurr AR (1967) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Stasolla C, Yeung EC (2003) Recent advances in conifer somatic embryogenesis: improving somatic embryo quality. Plant Cell Tissue Organ Cult 74:15–35
Strassburg RC (2004) Estrutura e origem da semente e embrião em espécies de Myrtaceae da Serra do Cipó-MG. Dissertation, Instituto de Biociências, Universidade de São Paulo, Brazil
Supena EDJ, Winarto B, Riksen T, Dubas E, van Lammeren A, Offringa R, Boutilier K, Custers J (2008) Regeneration of zygotic-like microspore-derived embryos suggests an important role for the suspensor in early embryo patterning. J Exp Bot 59:803–814
Thorpe M, Stasolla H (2001) Somatic embryogenesis. In: Bhojwani SS, Soh WY (eds) Current trends in the embryology of angiosperms. Kluwer Academic, Dordrecht, pp 279–336
Trigiano RN, Gray DJ, Conger BV, McDaniel JK (1989) Origin of direct somatic embryos from cultured leaf segments of Dactylis glomerata. Bot Gaz 150:72–77
Umehara M, Kamada H (2005) Development of the embryo proper and the suspensor during plant embryogenesis. Plant Biotechnol 22:253–260
Vernon DM, Meinke DN (1994) Embryogenic transformation of a suspensor in twin, a polyembryonic mutant of Arabidopsis. Dev Biol 165:566–573
von Arnold S (2008) Somatic embryogenesis. In: George EF, Hall MA, De Klerk D (eds) Plant propagation by tissue culture: volume 1. The background, 3rd edn. Springer, Dordrecht, pp 335–354
von Arnold S, Clapham D (2008) Spruce embryogenesis. In: Suárez MF, Bozhkov PV (eds) Methods in molecular biology, vol 427, Plant embryogenesis. Humana, Totowa, pp 31–47
von Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult 69:233–249
Westhoff P, Jeske H, Jurgens G, Kloppstech K, Link G (1998) Molecular plant development—from gene to plant. Oxford University Press, Oxford
Williams EG, Maheswaran G (1986) Somatic embryogenesis: factors influencing coordinated behaviour of cells as an embryogenic group. Ann Bot 57:443–462
Xie DY, Hong Y (2001) Regeneration of Acacia mangium through somatic embryogenesis. Plant Cell Rep 20:34–40
Yang X, Zhang X (2010) Regulation of somatic embryogenesis in higher plants. Crit Rev Plant Sci 29:36–57
Yeung EC (1995) Structural and developmental patterns in somatic embryogenesis. In: Thorpe TA (ed) In vitro embryogenesis in plants. Kluwer Academic, Dordrecht, pp 205–247
Yeung EC, Meinke DW (1993) Embryogenesis in angiosperms: development of the suspensor. Plant Cell 5:1371–1381
Zar JH (1996) Biostatistical analysis, 3rd edn. Prentice-Hall, Upper Saddle River
Zhang JZ, Somerville CR (1997) Suspensor-derived polyembryony caused by altered expression of valyl-tRNA synthetase in the twn2 mutant of Arabidopsis. Proc Natl Acad Sci USA 94:7349–7355
Acknowledgments
This work was supported by the Fundação para a Ciência e Tecnologia.
Conflict of interest
The authors declare that they have no conflict of interest with the organization that sponsored the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Correia, S.M., Canhoto, J.M. Characterization of somatic embryo attached structures in Feijoa sellowiana Berg. (Myrtaceae). Protoplasma 242, 95–107 (2010). https://doi.org/10.1007/s00709-010-0130-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-010-0130-z