Abstract
Sulfonylurea drugs are widely used for the therapy of non-insulin-dependent diabetes mellitus. These drugs improve glucose and lipid levels by stimulating insulin secretion by the pancreatic β-cell comprising two generations with the second one more potent than the first. Glibenclamide is a well-known and potent second-generation sulfonylurea oral hypoglycemic drug which is most widely used in type II diabetes. In this research, five new analogs were synthesized by exchanging the lipophilic phenyl and urea moieties in the first and the end of the molecule with chlorobenzamide and hydrophilic and antidiabetic aminobenzothiazole substituents. Their glucose and lipid-lowering activities were evaluated and compared to the standard drug. Results showed that all of the new compounds exhibited better activities. In addition, in particular aminomethylbenzothiazoles derivatives could have exerted prominent hypoglycemic and a noticeable hypolipidemic effects superior to glibenclamide.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type II diabetes is a chronic metabolic disorder characterized by abnormalities in carbohydrate and lipid metabolism, which lead to postprandial and fasting hyperglycemia, dyslipidemia, and hyperinsulinemia. Insulin resistance is considered to be the significant pathogenic factor in type II diabetes and an obvious target for antidiabetic medication [1].
Blood glucose levels are usually sufficiently controlled by diet therapy and exercise in the early stage of type II diabetes. However, when this control becomes poor, various antidiabetic drugs are used depending on the pathophysiology of each patient, such as α-glucosidase inhibitors for postprandial hyperglycemia, biguanides for enhancing insulin action at the post-receptor level in peripheral tissues such as muscle, thiazolidinediones for enhancing insulin action and promoting glucose utilization in peripheral tissue for insulin resistance therapy, and sulfonylureas for deficiency of insulin secretion from pancreatic β cells [2, 3].
The most commonly used antidiabetic agents are sulfonylureas (SUs). They have been used for type II diabetes therapy over 40 years, helping to counter the detrimental effects of insulin deficiency and progressive loss of pancreatic beta cell responsiveness associated with chronic hyperglycemia [4]. There are two generations of SU antidiabetic drugs (first and second generations) where the latter is more potent.
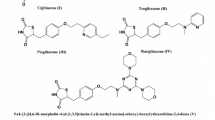
Glibenclamide (5-chloro-N-(4-[N-(cyclohexylcarbamoyl)sulfamoyl]phenethyl)-2-methoxybenzamide, glyburide, 1) is a well-known and potent second-generation drug which is widely used in oral type II antidiabetic drugs clinically [5]. Up to now, many derivatives of 1 have been synthesized and their anti-hyperglycemic and anti-hyperlipidemic activities evaluated [6–9]. According to SAR studies, the search for novel glibenclamide derivatives is originated to influence the in vivo behaviors by adding new structural moieties to its structure while preserving its binding affinity to the receptor (Scheme 1) [9, 10].

In this research, because of our previous finding about significant hypoglycemic and hypolipidemic activities of a chloro derivative of 1 in its phenyl moiety [7], new analogs by keeping this side were synthesized. In addition, cyclohexyl moiety (D moiety in Scheme 1) of 1 was also replaced by substituted benzothiazoles with many pharmacological (such as antidiabetic) activities [11] in these new analogs 8a–8e. Glucose and lipid-lowering activities of 8a–8e were evaluated and compared to 1 and the control groups by known procedures [12, 13].
Results and discussion
Chemistry
Sulfonylureas are derivatives with an arylsulfonyl group to one nitrogen and another group which usually determines the lipophilic properties of the molecule attached to terminal nitrogen of urea viz., alkyl, alicyclic, heterocycles, etc. [14].
The desired products 8a–8e were synthesized from readily available materials. Moreover, a convenient synthesis route was developed (Scheme 2). Initially, 2,4-dichlorobenzoyl chloride (2) was synthesized from 2,4-dichlorobenzoic acid and thionyl chloride in the presence of DMF as a catalyst. Then, it was reacted with 2-phenylethylamine in the presence of potassium hydroxide to obtain 2,4-dichloro-N-phenethylbenzamide (3). Reaction of 3 with chlorosulfonic acid in chloroform and ammonium hydroxide solution yields sulfonyl chloride 4 and sulfonamide 5 intermediates [7].

The sulfonamide compound 5 was converted to carbamate intermediate 6 by refluxing with ethyl chloroformate in dry acetone containing anhydrous K2CO3 according to a known procedure [15]. The resulting carbamate was subsequently condensed with amines 7a–7e in dry toluene to give the aminobenzothiazol-substituted benzenesulfonylureas 8a–8e.
Pharmacology
Blood serum glucose
Four days after alloxan injection, none of derivates have significant effects on blood serum glucose (Fig. 1). However, on days 9–16 after alloxan application, a significant reduction in glucose level was found between the treatment and control animals. On day 9, glibenclamide, 8b, and 8c could identically reduce blood serum glucose to: 38.40, 36.40, and 38.37 %. However, the related values on day 16 changed to 45.17, 40.61, and 41.18 %.
Blood serum triglyceride (TG), cholesterol, LDL, HDL, and VLDL
The mean values of blood serum parameters including triglyceride, cholesterol, LDL, HDL, and HDL/LDL ratio are shown in Fig. 2. As indicated, 8a, 8b, and 8c could markedly diminish TG and VLDL levels. The TG level in the control animal (118.5) reaches 81.4, 82.6, and 82.01 mg/dm3 using 8a, 8b, and 8c. Also, related values for VLDL yielded 16.28, 16.02, and 16.40 in comparison with the control ones (23.7). The animals treated with 8c and 8d showed a significant loss in serum LDL level, 45.14 and 44.9, respectively, which were control animals (64.01). Finally, a high HDL/LDL ratio was found to be good marker for the lipid profile in animals treated with 8b.
The principal action of the sulfonylureas is to stimulate the release of insulin from β cells even when glucose levels are low. They act by affecting the ATP-sensitive potassium channels. These channels are hetero-octameric complexes which are also composed of two subunits: sulfonylurea receptors (SUR1, SUR2A, and SUR2B), and the inwardly rectifying potassium channels KIR6.1 or KIR6.2 [16].
All compounds that block ATP channels stimulate insulin secretion, but only those that interact with the SUR subunit are used therapeutically to treat type II diabetes [17]. They not only bind tightly to SUR1 in β cells but also to SUR2A (as found in cardiac smooth muscle) and SUR2B (as found in brain and smooth muscle). In the first generation of SUs (for example: tolbutamide), it has been reported to bind with high affinity to SUR1 but with more than 100-fold lower affinity to SUR2A and SUR2B [4]. In the second generation (such as glibenclamide), it has been reported that they bind with high affinity to SUR1 (due to lipophilic substitution on its urea group, D moiety in Scheme 1) and with lower affinity to SUR2A and SUR2B (due to the methoxy group as well as halogen atoms on the phenyl ring in the other lipophilic side, the moiety in Scheme 1) [16].
Therefore, the search for novel glibenclamide derivatives originates from the idea to influence the in vivo behavior by adding new chemical moieties to its structure by changing the A–D moieties on the molecule (Scheme 1) with different lipophilic or hydrophilic chemical structures for obtaining more hypoglycemia and hypolipidemia activities while preserving its binding affinity to SUR2 with a higher degree compared to SUR1 or only SUR2-selective drugs targeted to glycaemic control on one hand and to reduction of cardiovascular complications on the other hand [9, 18].
In this paper, because of significant results about addition of chloride instead of methoxy substitutions on the phenyl moiety (A moiety in Scheme 1) of 1, for decreasing the serum glucose and LDL levels [7], this side of 8a–8e was maintained. Also, because an addition of hydrophilic substitutions could result in lead compounds for the development of SUR2-selective drugs with low affinity to SUR1 [9], the cyclohexyl ring with lipophilic property (D moiety in Scheme 1) was replaced by substituted aminobenzothiazoles 7a–7e with hydrophilic and many pharmacological (such as antidiabetic) activities [11, 19].
The results showed that 8a–8e exhibited higher hypoglycemic and hypolipidemic activities compared to the control but only derivatives with aminomethylbenzothiazole substitutions (8b and 8c) could reduce blood glucose and lipid profiles better than glibenclamide (1) too. It seems that exchanging the lipophilic side on urea (D moiety in Scheme 1) of Glibenclamide (which is responsible for SUR1 binding of the molecule) by hydrophilic aminobenzothiazole could improve antidiabetic and antilipidemic activities due to probably high binding to SUR2. But this affinity is higher for aminomethylbenzothiazole derivatives 8b and 8c as compared to chlorine, bromine, or unsubstituted ones (8a, 8d, and 8e).
Also, it seems that these higher activities of methylaminobenzothiazole analogs 8b and 8c may influence the hydrophilic/lipophilic balancing ratio or electron-donating property of methyl compared to halogen groups (with electron-withdrawing activities) in 8d and 8e. Comparing bromine and chlorine substitutions on amino benzothiazole derivatives, 8d and 8e also showed a higher hypoglycemic effect for 8e which may result from the lower electron-withdrawing property of bromine compared to chlorine atoms on the molecules.
The lipid profiles of 8a–8e showed that TG and cholesterol were decreased and the HDL/LDL ratio increased compared to the control and glibenclamide groups.
Conclusion
It can be concluded that changing the lipophilic urea moiety of glibenclamide by hydrophilic and antidiabetic aminobenzothiazole substitutions 7a–7e could produce new antidiabetic drugs 8a–8e. Aminomethylbenzothiazoles derivatives 8b and 8c exhibited higher hypoglycemic and hypolipidemic activities compared to glibenclamide which may result from the higher affinity of these new drugs toward SUR2 more than SUR1.
Experimental
2,4-Dichlorobenzoic acid, thionyl chloride, chlorosulfonic acid, ethyl chloroformate, 2-phenylethyl amine, dimethyl formamide (DMF), substituted benzothiazoles, and all other chemicals were purchased from Merck (Darmstadt, Germany) and Sigma-Aldrich chemical Co. (USA). Melting points were determined with a digital Electrothermal melting point apparatus (model 9100, Electrothermal Engineering Ltd., Essex, UK). The 1H and 13C NMR spectra were recorded with a Bruker AMX 300 MHz spectrometer (Karlsruhe, Germany; internal reference: TMS). The IR spectra were recorded with a Thermo Nicolet FT-IR Nexus-870 spectrometer (Nicolet Instrument Corp, Madison, WI, USA). Mass spectra were recorded with an Agilent Technologies 5973, Mass Selective Detector (MSD) spectrometer (Wilmington, USA). Column chromatographic separations were performed over Acros silica gel (particle size 35–70 μm, Geel, Belgium). Elemental analyses were carried out with a Perkin-Elmer, CHN elemental analyzer model 2400; their results were within ±0.4 % of the theoretical values. The purity of the compounds was determined by TLC using ethyl acetate/n-hexane as the eluent. Compounds 2–5 were prepared according to the procedure described in our previous study [7].
General procedure for the preparation of new compounds 8a–8e
A mixture of 3.73 g sulfonamide intermediate 5 (0.01 mol) and 1.07 g anhydrous potassium carbonate in 100 cm3 anhydrous acetone was refluxed for 15 min. Then, 1.41 g ethyl chloroformate (0.013 mol) was dropwise added and refluxed for 8 days. After monitoring by TLC, acetone was removed by distillation under reduced pressure; the residue was suspended in 100 cm3 water and neutralized with acetic acid. The product separated out was filtered, washed, dried, and recrystallized from ethanol to obtain carbamate intermediate 6 (yield: 51 %, m.p.: 192–196 °C) which was used in the next step without further purification.
Appropriate aminobenzothiazoles 7a–7e (0.0011 mol) were added to a solution of carbamate intermediate 6 (0.001 mol) in 50 cm3 boiling toluene. The mixture was subsequently refluxed for further 10 days and cooled. Benzenesulfonylureas 8a–8e, which precipitated out on cooling, were filtered and recrystallized from ethanol.
2,4-Dichloro-N-[2-[4-[(2-benzothiazolylamino)sulfamoyl]phenyl]ethyl]benzamide (8a, C23H18Cl2N4O4S2)
White solid; yield 63 %; m.p.: 168 °C; IR (KBr): \(\bar{\nu }\) = 3448, 3285, 3067, 2936, 2736, 1645, 1537, 1410, 1310, 1276, 1070, 870, 734, 565 cm−1; 1H NMR: δ = 2.85–2.90 (2H, m), 3.43–3.49 (2H, m), 7.29–7.74 (10H, m), 8.56–8.60 (1H, m) ppm; 13C NMR: δ = 34.5, 40.3, 117.8, 119.4, 125.6, 127.2, 129.1, 129.2, 130.1, 131.1, 134.3, 135.8, 142.1, 143.4, 165.2, 175.3 ppm; MS: m/z (%) = 550 (96), 538 (14), 524 (20), 478 (40), 445 (15), 420 (30), 409 (44), 403 (40), 372 (100), 360 (80).
2,4-Dichloro-N-[2-[4-[(4,6-dimethyl-2-benzothiazolylamino)sulfamoyl]phenyl]ethyl]benzamide (8b, C25H22Cl2N4O4S2)
Orange solid; yield 62 %; m.p.: 178–180 °C; IR (KBr): \(\bar{\nu }\) = 3302, 3257, 2924, 2853, 1645, 1585, 1543, 1457, 1302, 1156, 1095, 835, 697 cm−1; 1H NMR: δ = 2.38 (3H, s), 2.58 (3H, s), 2.81–2.86 (2H, m), 3.46–3.51 (2H, m), 7.25–8.02 (8H, m), 8.43–8.50 (1H, m) ppm; 13C NMR: δ = 16.4, 20.9, 35.0, 42.0, 118.0, 124.3, 126.5, 127.0, 127.9, 128.3, 128.8, 129.1, 129.2, 130.3, 134.1, 135.3, 143.6, 143.8, 165.2, 176.3 ppm; MS: m/z (%) = 576 (70), 507 (45), 455 (27), 430 (49), 403 (31), 387 (100), 359 (49), 343 (31), 333 (30).
2,4-Dichloro-N-[2-[4-[(4-methyl-2-benzothiazolylamino)sulfamoyl]phenyl]ethyl]benzamide (8c, C24H20Cl2N4O4S2)
White solid; yield 68 %; m.p.: 174 °C; IR (KBr): \(\bar{\nu }\) = 3340, 3204, 3060, 1650, 1537, 1450, 1306, 1245, 1147, 1084, 883, 729, 611 cm−1; 1H NMR: δ = 2.49 (3H, s), 2.85–2.94 (2H, m), 3.46–3.57 (2H, m), 7.01–7.81 (9H, m), 8.56–8.58 (1H, m) ppm; 13C NMR: δ = 15.2, 36.9, 45.1, 119.9, 121.3, 125.0, 125.2, 126.0, 127.3, 128.2, 128.4, 130.1, 132.8, 134.3, 136.3, 142.3, 148.3, 165.3, 172.3 ppm; MS: m/z (%) = 562 (54), 536 (29), 479 (45), 460 (27), 435 (72), 423 (45), 398 (100).
2,4-Dichloro-N-[2-[4-[(6-chloro-2-benzothiazolylamino)sulfamoyl]phenyl]ethyl]benzamide (8d, C23H17Cl3N4O4S2)
White solid; yield 71 %; m.p.: 173–176 °C; IR (KBr): \(\bar{\nu }\) = 3402, 3360, 3333, 2922, 1632, 1591, 1491, 1460, 1273, 1156, 1087, 817, 764 cm−1; 1H NMR: δ = 2.82–2.87 (2H, m), 3.45–3.53 (2H, m), 7.27–7.73 (9H, m), 8.69–8.73 (1H, m) ppm; 13C NMR: δ = 35.7, 40.2, 119.6, 121.3, 125.2, 127.4, 129.0, 129.1, 130.1, 131.8, 134.3, 135.3, 142.3, 144.3, 148.2, 165.4, 175.0 ppm; MS: m/z (%) = 582 (70), 560 (30), 547 (50), 514 (100), 487 (30).
2,4-Dichloro-N-[2-[4-[(6-bromo-2-benzothiazolylamino)sulfamoyl]phenyl]ethyl]benzamide (8e, C23H17BrCl2N4O4S2)
Light brown solid; yield 65 %; m.p.: 161 °C; IR (KBr): \(\bar{\nu }\) = 3310, 3256, 2927, 2856, 1645, 1539, 1328, 1156, 1098, 826 cm−1; 1H NMR: δ = 2.74–2.79 (2H, m), 3.62–3.71 (2H, m), 7.1–7.9 (9H, m), 8.70–8.72 (1H, m) ppm; 13C NMR: δ = 34.6, 42.3, 124.4, 124.5, 127.6, 128.1, 128.3, 130.8, 133.2, 135.7, 138.2, 141.2, 142.3, 144.1, 151.7, 169.4, 174.2 ppm; MS: m/z (%) = 627 (68), 613 (10), 590 (8), 528 (12), 503 (10), 496 (25), 478 (15), 466 (24), 459 (100).
Animals
Initially, 84 adult male Wistar rats, weighing 190–220 g (Razi Institute, Iran) with blood glucose under 150 mg/dm3 as non-diabetic animals were randomly selected and housed three to four per cage in a temperature-controlled colony room under a 12 h light/dark cycle. Animals were given free access to water and standard laboratory rat chow (Pars Company, Tehran, Iran). All the experiments were conducted between 11 a.m. and 4 p.m. under the normal room light at 25 °C. This study was carried out in line with the policies provided in the Guide for the Care and Use of Laboratory Animals (NIH) and those in the Research Council at Shahed University of Medical Sciences (Tehran, Iran).
Mortality (number of deaths), morbidity (defined as any abnormal condition or behavior due to a disorder), irritability (a condition of aggressiveness or increased response on handling), and other related abnormal states were observed in experimental animals. However, the motor coordination index (measured by means of Rota-rod apparatus, Harvard, UK) did not indicate any significant differences between treated rats.
Serum parameters analysis
For inducing diabetes, a single dose of alloxan (Sigma, UK) in 150 mg/kg (immediately before usage dissolved in 0.9 % saline) was injected intraperitoneally to all selected animals (with serum glucose under 150 mg/dl) and randomly divided into seven groups; control, glibenclamide, and 8a–8e [12, 13]. These drugs (each one at 0.25 mg/kg, i.p) [20] were injected to the animals on day 3–16 after alloxan injection. Glucose in the serum as the main parameter for efficiency of origin or synthesized antidiabetic drugs was measured due to the alloxan injection on days 4, 9, and 16. Other serum parameters, i.e., triglyceride, total cholesterol, LDL, HDL, and VLDL were calculated on day 16 after alloxan injection. To measure the mentioned serum parameters, blood samples were collected. Serum glucose concentrations, triglyceride, total cholesterol, and HDL cholesterol levels were spectrophotometrically measured with appropriate kits (Zistshimi, Tehran). Only the animals with serum glucose content of higher than 250 mg/dl were selected as diabetics for the following measurements. LDL and a low-density lipoprotein (VLDL) cholesterol levels were calculated using following formulae:
Statistical analysis
Measurement data were tabulated as mean ± SEM. Comparisons were carried out using one-way analysis of variances (ANOVA) followed by post hoc Tukey test and P value <0.05 as the level of significance.
References
Chakrabarti R, Vikramadithyan RK, Mullangi R, Sharma VM, Jagadheshan H, Rao YN, Sairam P, Rajagopalan R (2002) J Ethnopharmacol 81:343
Satoh J, Takahashi K, Takizawa Y, Ishihara H, Hirai M, Katagiri H, Hinokio Y, Suzuki S, Tsuji I, Oka Y (2005) Diabetes Res Clin Pract 70:291
Jawale DV, Pratap UR, Rahuja N, Srivastava AK, Mane RA (2012) Bioorg Med Chem Lett 22:436
McClenaghan NH, Ball AJ, Flatt PR (2001) Biochem Pharmacol 61:527
Pill J, Kühnle HF (1999) Metabolism 48:34
Ahmadi A, Khalili M, Farsadrooh M, Ghiasi M, Nahri-Niknafs B (2013) Drug Res 63:614
Ahmadi A, Khalili M, Khatami K, Farsadrooh M, Nahri-Niknafs B (2014) Mini Rev Med Chem 14:208
Velingkar VS, Dandekar VD, Murugananthan K (2009) Inter J Pharm Pharm Sci 1:149
Schneider S, Ueberberg S, Korobeynikov A, Schechinger W, Schwanstecher C, Schwanstecher M, Klein HH, Schirrmacher E (2007) Regul Pept 139:1
Zaman MK, Arayne MS, Sultana N, Farooq A (2006) Pak J Pharm Sci 19:114
Mariappan G, Prabhat P, Sutharson L, Banerjee J, Patangia U, Nath S (2012) J Korean Chem Soc 56:251
Patel SS, Shah RS, Goyal RK (2009) Indian J Exp Biol 47:564
Latha RC, Daisy P (2011) Chem Biol Interact 189:112
Tyagi S, Kumar S, Kumar A, Singla M (2010) Int J Pharm World Res 1:1
Rathish IG, Javed K, Bano S, Ahmad S, Alam MS, Pillai KK (2009) Eur J Med Chem 44:2673
Meyer M, Chudziak F, Schwanstecher C, Schwanstecher M, Panten U (1999) Br J Pharmacol 128:27
Proks P, Reimann F, Green N, Gribble F, Ashcroft F (2002) Diabetes 51:S368
Calderone V, Rapposelli S, Martelli A, Digiacomo M, Testai L, Torri S, Marchetti P, Breschi MC, Balsamo A (2009) Bioorg Med Chem 17:5426
Meltzer-Mats E, Babai-Shani G, Pasternak L, Uritsky N, Getter T, Viskind O, Eckel J, Cerasi E, Senderowitz H, Sasson S, Gruzman A (2013) J Med Chem 56:5335
Devaki K, Beulah U, Akila G, Narmadha R, Gopalakrishnan VK (2011) J Basic Clin Pharm 2:167
Acknowledgments
This work was a research project at Karaj Branch, Islamic Azad University, Iran and the authors would like to express their gratitude to them. They thank Fariba Ansari for her assistance with the pharmacological tests. They appreciate Mojtaba Chaichi, EFL educator at Safir English Language Academy, for proofreading the initial draft of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmadi, A., Khalili, M., Ghaderi, P. et al. Synthesis and blood glucose and lipid-lowering effects of benzothiazole-substituted benzenesulfonylurea derivatives. Monatsh Chem 146, 2059–2065 (2015). https://doi.org/10.1007/s00706-015-1471-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-015-1471-2