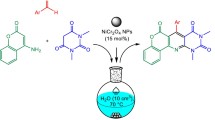
Abstract
An efficient one-pot, three-component condensation reaction between 4-hydroxycoumarin, aryl glyoxals, and malononitrile for the synthesis of new dihydropyrano[c]chromenes is reported. The reactions were efficiently catalyzed by magnetically recyclable Fe3O4 nanoparticles to afford the desired products in good to excellent yields. The method is simple and completely in accordance with green chemistry principles.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The continual insistence on the development of facile, convenient, and nonpolluting synthetic procedures drives chemists to update methods and increase their potency with respect to green chemistry factors [1–3]. Multicomponent reactions (MCR) of three or more substrates are one approach to address this challenge, because they offer the greatest possibilities for molecular diversity in one step with minimum synthetic time and effort; many attempts have been made to extend new MCRs in the past decade [4–6]. Other promising areas of green chemistry are the design of organic reactions in nontoxic and inexpensive solvents and using safe and recyclable catalysts [7–9].

The design and synthesis of biologically active compounds is one of the most important aspects of medicinal chemistry. Pyranochromenes represent an interesting template for medicinal chemistry and play an essential role as biologically active molecules, e.g., antifungal, insecticidal, anticancer, anti-HIV, anti-inflammatory, and antibacterial agents [10–12]. Figure 1 shows some drugs with dihydropyran substructures and a 4-hydroxycoumarin moiety. Among pyranochromenes, the dihydropyrano[c]chromenes are the most synthetically feasible.
To our knowledge, a number of methods are available for the synthesis of dihydropyrano[c]chromenes via domino Knoevenagel condensation and Michael addition reaction of aromatic aldehyde with malononitrile and 4-hydroxycoumarin, but there is no report on using aryl glyoxal instead of aromatic aldehydes [13].
Recently, iron oxide nanoparticles (Fe3O4 NPs) were extensively applied as a powerful catalyst for some organic transformations [14, 15]. Fe3O4 NPs have special features that make them advantageous over some common catalysts and suitable for many organic reactions. For instance, they are safe, inexpensive, stable, and recyclable materials. In this context, we report the use of Fe3O4 NPs as an eco-friendly and efficient catalyst for the synthesis of new dihydropyrano[c]chromenes containing an aryloyl group.
Results and discussion
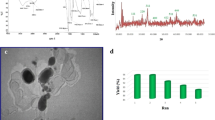
The Fe3O4 NPs, homogeneous in size and composition, were prepared according to previous methods [16] by modification and characterized by Fourier transform infrared (FT-IR) and transmission electron microscopy (TEM). The FT-IR spectrum of Fe3O4 NPs is shown in Fig. 2. The absorbance band at 583.3 cm−1 can be ascribed to Fe2+–O2−, which is consistent with the reported IR spectra for Fe3O4 NPs [17]. The morphology and microstructure of the NPs were further investigated by TEM analysis. The TEM images in Fig. 3 reveal spherical Fe3O4 NPs with an average size of 10–15 nm.
In continuation of our interest in developing convenient methodologies for new heterocyclic synthesis using green catalysts [18–22], we found that treatment of 4-hydroxycoumarin (1), aryl glyoxals 2, and malononitrile (3) via a one-pot, three-component reaction in the presence of catalytic amounts of Fe3O4 NPs provided the dihydropyrano[c]chromenes 4.
The synthesis of pyrano[c]chromenes 4 from the aryl glyoxals was unknown. In order to optimize the reaction conditions, we studied the model reaction between 4-hydroxycoumarin, phenyl glyoxal, and malononitrile. The reaction was initially conducted in EtOH/H2O (1:1) without using catalysts, but no product formation was observed after 6 h at rt or reflux conditions. Addition of a small amount of Fe3O4 NPs (2 mol%) to the reaction mixture afforded the desired product after 180 min. Increasing the catalyst to 3 mol% increased the reaction rate. Further increase did not improve the reaction rate or product yield.
A few additional experiments were performed to investigate whether this transformation took place when other common catalysts (p-TSA, Na2CO3, FeCl3, AcOH, and ZnCl2) were used in place of the Fe3O4 NPs, but no reaction was observed in all cases. Next, some experiments were performed to investigate the solvent effect. Solvent-free reactions produced a mixture of undesired and nonisolable products. Among solvents screened, EtOH/H2O (1:1) was found to be the best in terms of the product yield and completion time in comparison with common organic solvents. The results of these studies are summarized in Table 1. This reaction was efficiently completed using Fe3O4 NPs (3 mol%) under controlled temperature (see “Experimental” section) in EtOH/H2O (1:1) after about 100 min (entry 3).
Having established the optimized reaction conditions, we concentrated on the scope of this reaction with a variety of aryl glyoxals to check the viability of this protocol in obtaining a library of dihydropyrano[c]chromenes (Table 2). It is worth noting that the experimental procedure is very efficient, convenient, and rapid and has the ability to tolerate a variety of functional groups, such as methoxy, nitro, and halides under optimized reaction conditions.
We also examined the use of alkyl cyanoactates to get the corresponding products but there was no product formation even after 12 h under the optimized reaction conditions.
The structures of compounds 4 were assigned on the basis of comprehensive spectroscopic analyses such as IR, 1H and 13C NMR spectroscopy, and CHN analysis. The 1H NMR spectrum of 4a exhibited two sharp singlets identified as methine (δ = 5.42 ppm) and NH2 (7.69 ppm) protons. Also, the signals at 7.53–8.17 ppm corresponded to aromatic protons. The proton-decoupled 13C NMR spectrum of 4a showed 18 distinct resonances in agreement with the proposed structure. The IR spectrum of 4a showed characteristic bands at 3402–3292 cm−1 (NH2), 2201 cm−1 (CN), and at 1708–1678 cm−1 (C=O).
The Fe3O4 NPs are magnetically separable and could be recovered easily and reused for subsequent runs. As it is shown in Fig. 4, no obvious decrease was observed on the recovered catalyst. After three reuses, the Fe3O4 NPs maintained their high catalytic efficiency in the model reaction.
In summary, an efficient, economical, and environmentally benign multicomponent protocol for the construction of a new class of dihydropyrano[c]chromenes under Fe3O4 NP-catalyzed conditions has been achieved. The use of controlled heating allows the tuning of reaction conditions to access products in good to excellent yield. The promising points of the presented methodology are the avoidance of toxic and expensive solvents, use of safe and recyclable catalyst, and simplicity of operation. It is also noteworthy that the presence of transformable functionalities in the products makes them potentially valuable for further synthetic manipulations.
Experimental
All chemicals were purchased from Merck and Aldrich. Aryl glyoxals were synthesized according to our previous method [23]. The reactions were monitored by thin-layer chromatography (TLC; silica-gel 60 F254, n-hexane/ethyl acetate). IR spectra were recorded on an FT-IR JASCO-680 and the 1H NMR spectra were obtained on a Bruker-Instrument DPX-400 and 300 MHz Avance 2 model. The varioEl CHNS system at Isfahan Industrial University was used for elemental analysis. Transmission electron microscopy (TEM) images were taken with a Philips CM-10 TEM microscope operated at 100 kV. The structures and purity of the obtained products were deduced from their IR, elemental analysis, and NMR spectral data.
Preparation of Fe3O4 NPs
FeCl3·6H2O (6.1 g, 0.02 mol) and 2.35 g FeCl2·4H2O (0.01 mol) were dissolved in 100 cm3 de-ionized H2O under magnetic stirring for 10 min. The solution was then heated to 90 °C under nitrogen atmosphere. Subsequently, 10 cm3 ammonium hydroxide solution (25 %) was added dropwise to the reaction mixture which was then stirred for about 1 h. The reaction mixture was cooled to room temperature and the black precipitate was separated from the reaction mixture using a magnetic field and then washed with de-ionized H2O several times to remove the impurities.
General procedure for synthesis of 4
To a stirred solution of 4-hydroxycoumarin (1 mmol), aryl glyoxal (1 mmol), and malononitrile (1.2 mmol) in 10 cm3 EtOH/H2O (1:1) was added Fe3O4 NPs (0.03 mmol). The mixture was stirred under reflux for 40 min. The reaction progress was monitored by TLC (hexane/AcOEt, 1:1). After completion of the reaction, the precipitate was filtered, dried, and dissolved in hot EtOH/THF (3:1) to separate the catalyst using a magnet. Pure 4 was obtained after recrystallization from EtOH/THF (3:1).
2-Amino-4-benzoyl-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (4a, C20H12N2O4)
IR (KBr): \( \bar{\nu } \) = 3,402, 3,292, 2,201, 1,708, 1,678, 1,606, 1,373, 1,064 cm−1; 1H NMR (DMSO-d 6, 300 MHz): δ = 8.16 (d, 2H, J = 7.2 Hz), 7.90 (dd, 1H, J = 8.2, 1.6 Hz), 7.81–7.73 (m, 2H), 7.69 (s, 2H), 7.62 (t, 2H, J = 8.2 Hz), 7.57–7.53 (m, 2H), 5.42 (s, 1H) ppm; 13C NMR (DMSO-d 6, 75 MHz): δ = 198.12, 160.08, 159.55, 154.72, 152.11, 135.34, 134.13, 133.35, 129.13, 128.89, 125.03, 122.15, 118.54, 116.83, 112.58, 101.91, 51.91, 37.14 ppm.
2-Amino-4-(4-fluorobenzoyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (4b, C20H11FN2O4)
IR (KBr): \( \bar{\nu } \) = 3,474, 3,404, 2,205, 1,712, 1,677, 1,595, 1,371, 1,218, 1,061 cm−1; 1H NMR (DMSO-d 6, 300 MHz): δ = 8.27 (m, 2H), 7.90 (dd, 1H, J = 8.2, 1.8 Hz), 7.82–7.76 (m, 1H), 7.70 (s, 2H), 7.58–7.53 (m, 2H), 7.46 (t, 2H, J = 8.8 Hz), 5.44 (s, 1H) ppm; 13C NMR (DMSO-d 6, 75 MHz): δ = 196.77, 160.08, 159.54, 154.74, 152.13, 133.37, 132.32, 132.19, 125.04, 122.17, 118.50, 116.84, 116.15, 115.86, 112.58, 101.76, 51.85, 37.19 ppm.
2-Amino-4-(4-chlorobenzoyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (4c, C20H11ClN2O4)
IR (KBr): \( \bar{\nu } \) = 3,319, 3,186, 3,027, 2,871, 2,205, 1,713, 1,673, 1,587, 1,375, 1,058, 759 cm−1; 1H NMR (DMSO-d 6, 300 MHz): δ = 8.20 (d, 2H, J = 8.4 Hz), 7.89 (dd, 1H, J = 8.2, 1.4 Hz), 7.81–7.69 (m, 5H), 7.57–7.52 (m, 2H), 5.43 (s, 1H) ppm; 13C NMR (DMSO-d 6, 75 MHz): δ = 197.28, 160.08, 159.54, 154.75, 152.13, 139.22, 134.13, 133.39, 130.99, 129.07, 125.04, 122.18, 118.49, 116.83, 112.55, 101.64, 51.70, 37.26 ppm.
2-Amino-4-(4-bromobenzoyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (4d, C20H11BrN2O4)
IR (KBr): \( \bar{\nu } \) = 3,316, 3,186, 3,027, 2,871, 2,203, 1,715, 1,673, 1,582, 1,374, 1,057, 620 cm−1; 1H NMR (DMSO-d 6, 300 MHz): δ = 8.10 (d, 2H, J = 8.6 Hz), 7.91–7.76 (m, 4H), 7.71 (s, 2H), 7.58–7.53 (m, 2H), 5.42 (s, 1H) ppm; 13C NMR (DMSO-d 6, 75 MHz): δ = 197.51, 159.54, 154.75, 152.14, 134.45, 133.40, 132.04, 131.05, 125.05, 122.19, 116.84, 112.55, 101.64, 51.70, 37.25 ppm.
2-Amino-4-(3-nitrobenzoyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (4e, C20H11N3O6)
IR (KBr): \( \bar{\nu } \) = 3,415, 3,086, 2,928, 2,197, 1,712, 1,673, 1,609, 1,525, 1,385, 1,352, 1,063 cm−1; 1H NMR (DMSO-d 6, 300 MHz): δ = 8.83 (t, 1H, J = 1.8 Hz), 8.65 (d, 1H, J = 7.8 Hz), 8.61–8.57 (m, 1H), 7.98–7.89 (m, 2H), 7.82–7.77 (m, 3H), 7.59–7.53 (m, 2H), 5.57 (s, 1H) ppm; 13C NMR (DMSO-d 6, 75 MHz): δ = 197.01, 160.15, 159.57, 154.81, 152.16, 148.17, 136.61, 135.29, 133.51, 130.91, 128.39, 125.11, 123.17, 122.22, 118.51, 116.89, 112.50, 101.33, 51.24, 37.65 ppm.
2-Amino-4-(4-nitrobenzoyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (4f, C20H11N3O6)
IR (KBr): \( \bar{\nu } \) = 3,461, 3,336, 2,192, 1,720, 1,681, 1,613, 1,517, 1,383, 1,326, 1,070, 1,064 cm−1; 1H NMR (DMSO-d 6, 300 MHz): δ = 8.46–8.38 (m, 4H), 7.90 (dd, 1H, J = 8.2, 1.4 Hz), 7.82–7.77 (m, 3H), 7.59–7.54 (m, 2H), 5.51 (s, 1H) ppm; 13C NMR (DMSO-d 6, 75 MHz): δ = 197.79, 160.15, 159.56, 154.81, 152.17, 150.49, 140.22, 133.50, 130.43, 125.10, 124.01, 122.24, 116.88, 112.51, 101.33, 51.24, 37.98 ppm.
2-Amino-4-(3-methoxybenzoyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (4g, C21H14N2O5)
IR (KBr): \( \bar{\nu } \) = 3,477, 3,344, 3,072, 2,934, 2,196, 1,721, 1,674, 1,577, 1,386, 1,065 cm−1; 1H NMR (DMSO-d 6, 300 MHz): δ = 7.90 (dd, 1H, J = 8.2, 1.8 Hz), 7.81–7.75 (m, 2H), 7.70 (s, 2H), 7.62 (t, 1H, J = 2.4 Hz), 7.57–7.52 (m, 3H), 7.32 (dd, 1H, J = 7.8, 2.1 Hz), 5.40 (s, 1H), 3.87 (s, 3H) ppm; 13C NMR (DMSO-d 6, 75 MHz): δ = 197.77, 160.04, 159.61, 159.44, 154.73, 152.13, 136.69, 133.34, 130.02, 125.02, 122.16, 121.68, 120.22, 118.57, 116.82, 113.52, 112.60, 101.96, 55.39, 52.02, 37.41 ppm.
2-Amino-4-(4-methoxybenzoyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (4h, C21H14N2O5)
IR (KBr): \( \bar{\nu } \) = 3,426, 3,320, 2,926, 2,200, 1,714, 1,673, 1,597, 1,383, 1,062 cm−1; 1H NMR (DMSO-d 6, 300 MHz): δ = 8.15 (d, 2H, J = 9.0 Hz), 7.89 (dd, 1H, J = 8.2, 1.4 Hz), 7.80–7.74 (m, 1H), 7.65 (s, 2H), 7.57–7.52 (m, 2H), 7.14 (d, 2H, J = 9.0 Hz), 5.36 (s, 1H), 3.90 (s, 3H) ppm; 13C NMR (DMSO-d 6, 75 MHz): δ = 196.24, 163.92, 160.04, 159.50, 154.67, 152.08, 133.26, 131.65, 128.10, 124.99, 122.12, 118.62, 116.79, 114.12, 112.62, 102.10, 55.65, 52.28, 36.72 ppm.
2-Amino-4-(1-naphthoyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (4i, C24H14N2O4)
IR (KBr): \( \bar{\nu } \) = 3,477, 3,327, 3,050, 2,923, 2,191, 1,728, 1,675, 1,574, 1,382, 1,177 cm−1; 1H NMR (DMSO-d 6, 300 MHz): δ = 8.37 (m, 2H), 8.24 (d, 1H, J = 8.4 Hz), 8.09–8.05 (m, 1H), 7.92 (dd, 1H, J = 8.2, 1.8 Hz), 7.83–7.70 (m, 4H), 7.66–7.54 (m, 4H), 5.43 (s, 1H) ppm; 13C NMR (DMSO-d 6, 75 MHz): δ = 200.33, 160.26, 159.65, 154.68, 152.21, 134.20, 133.46, 133.39, 133.29, 129.87, 129.08, 128.56, 128.03, 126.59, 125.10, 125.05, 124.76, 122.20, 118.33, 116.86, 112.63, 101.71, 51.45, 38.65 ppm.
2-Amino-4-(2-naphthoyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (4j, C24H14N2O4)
IR (KBr): \( \bar{\nu } \) = 3,428, 3,321, 2,938, 2,199, 1,714, 1,674, 1,631, 1,597, 1,569, 1,382, 1,172 cm−1; 1H NMR (DMSO-d 6, 300 MHz): δ = 8.44 (s, 1H), 7.98–7.95 (m, 2H), 7.83 (d, 1H, J = 8.4 Hz), 7.76–7.72 (m, 1H), 7.62–7.52 (m, 3H), 7.49–7.42 (m, 2H), 7.39 (s, 2H), 7.33 (d, 1H, J = 7.2 Hz), 5.47 (s, 1H) ppm; 13C NMR (DMSO-d 6, 75 MHz): δ = 199.66, 159.56, 157.85, 153.82, 152.05, 133.27, 132.93, 130.93, 128.47, 127.43, 126.18, 126.13, 126.02, 125.85, 125.75, 124.74, 123.43, 122.45, 119.15, 116.61, 112.96, 104.65, 53.62, 37.27 ppm.
References
Gu Y (2012) Green Chem 14:2091
Khalafi-Nezhad A, Panahi F (2011) Green Chem 13:2408
Li B, Devaraj K, Darcel C, Dixneuf PH (2012) Green Chem 14:2706
Banerjee S, Horn A, Khatri H, Sereda G (2011) Tetrahedron Lett 52:1878
Yavari I, Aminkhani A, Skoulika S (2013) J Iran Chem Soc 10:107
Shiri M (2012) Chem Rev 112:3508
Khodabakhshi S, Baghernejad M (2013) Iran J Catal 3:67
Rajpara V, Banerjee S, Sereda G (2010) Synthesis 2835
Wilson K, Clark JH (2000) Pure Appl Chem 72:1313
Liu Y, Zhu J, Qian J, Jiang B, Xu Z (2011) J Org Chem 76:9096
Karami B, Khodabakhshi S, Eskandari K (2012) Tetrahedron Lett 53:1445
Shen X, Chen G, Zhu G, Fong W-F (2006) Bioorg Med Chem 14:7138
Ziarani GM, Hajiabbasi P (2013) Heterocycles 87:1415
Karami B, Hoseini SJ, Nikoseresht S, Khodabakhshi S (2012) Chin Chem Lett 23:173
Karami B, Nikoseresht S, Khodabakhshi S (2012) Chin J Catal 33:298
Liu JF, Zhao ZS, Jiang GB (2008) Environ Sci Technol 42:6949
Hoseini SJ, Nasrabadi H, Azizi M, Salimi-Beni A, Khalifeh R (2013) Synth Commun 43:1683
Karami B, Eskandari K, Khodabakhshi S, Hoseini SJ, Hashemian F (2013) RSC Adv 3:23335
Khodabakhshi S, Karami B (2012) Catal Sci Technol 2:1940
Karami B, Khodabakhshi S, Hashemi F (2013) Tetrahedron Lett 54:3583
Kamaei V, Karami B, Khodabakhshi S (2014) Polycyclic Aromat Compd 34:1
Khodabakhshi S, Karami B, Eskandari K (2014) C R Chimie 17:35
Karami B, Khodabakhshi S, Nikrooz N (2011) Polycyclic Aromat Compd 31:97
Acknowledgments
Financial support for this work by the Young Researchers and Elite Club (Iran) and the Iranian Nanotechnology Initiative Council is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khodabakhshi, S., Karami, B. & Baghernejad, M. Iron(II,III) oxide nanoparticle-catalyzed selective synthesis of unknown dihydropyrano[c]chromenes under green conditions. Monatsh Chem 145, 1839–1843 (2014). https://doi.org/10.1007/s00706-014-1240-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-014-1240-7