Abstract
Trollius chinensis Bunge is widely used in China. Total flavonoids of Trollius chinensis Bunge contain large amounts of orientin. To ascertain whether this major fraction has a pronounced anticancer effect, the cytotoxic effect of orientin and its effect on apoptosis of HeLa cells were evaluated. The work showed orientin inhibited proliferation of HeLa cells in a dose-dependent manner. Cell growth arrest was confirmed by flow cytometry. Apoptotic signaling induced by orientin was characterized by an increased Bax/Bcl-2 ratio and up-regulation of Bax and down-regulation of Bcl-2. Orientin treatment also initiated proteolytic activation of caspase-3 and caspase-9. These results indicate that orientin may have applications in the treatment of human cervical carcinoma, and further investigation is warranted.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the World Health Organization (WHO) cancer is one of the leading causes of death; in 2008 it accounted for 7.6 million deaths worldwide (approximately 13 % of all deaths). The WHO has also estimated that worldwide cancer deaths are likely to rise to over 11 million in 2030 [1]. One of the most prevalent types of cancer in females is cervical cancer, which is caused by the human papilloma virus (HPV), which forms warts in the throat and genital area. Although it is the most prevalent cancer in females, it is also, by screening and treatment of precancerous lesions and use of vaccines, the most preventable cancer. Natural products are being tested for treatment of cancer because conventional cancer treatments, for example chemotherapy, destroy cancerous and healthy cells.
Because many Chinese citizens rely on traditional medicines for their primary-health care needs, demand for traditional medicines is high in rural parts in China. Traditional Chinese medicine, in which non-toxic substances are used to interfere with tumors is an important area of research. In China, use of plants for the treatment of cervical cancer is common. Many plant and their extracts are undergoing trials and some have been used for clinical treatment of cervical cancer [2–8].
China has a remarkable flora of many species, most of which are endemic to specific regions. Many of these species are used for traditional medicinal purposes. The plant Trollius chinensis Bunge is dry flower and bud of Trollius chinensis Bunge and T. ledebouri Reichbfor of Ranunculaceae. There are approximately 32 kinds of Trollius species, mainly distributed in northern temperate and cold temperate mountainous regions. In China there are approximately 16 kinds of Trollius species, mainly distributed in the provinces of Hebei, Shanxi, Inner Mongolia, and Northeast regions. Trollius chinensis Bunge was recorded long ago in “Bencao Gangmu Shiyi”, written by Zhao Xuemin of the Qing dynasty. The character of Trollius chinensis Bunge is bitter, cold, and non-toxic; it has been widely used in folk medicine for thousands of years as a drug with the function of heat-cleaning and detoxifying. Scientific research indicates that bioactive components of Trollius chinensis Bunge, for example total flavonoids can reduce the risk of cancer as a result of antimicrobial, antioxidant, and antitumorigenic activity, and are able to directly suppress carcinogen bioactivation [9]. Total flavonoids of Trollius chinensis Bunge contain a large amount of crientin, which has antitumor potential against human esophageal cancer cell lines and dose-dependently inhibits cell proliferation and induces cell apoptosis [10]. Although much work has been conducted on the bioactive components of Trollius chinensis Bunge, no reports are available about the cytotoxic activity and mechanisms of action against cervical cancer (HeLa) cells of orientin in Trollius chinensis Bunge. Research on how orientin blocks the proliferation of tumor cells will benefit the search for a potential antitumor agent.
Apoptosis is a gene-regulated phenomenon induced by many chemotherapeutic agents in cancer treatment [11]. Induction of apoptosis in tumor cells is regarded as very useful in the management and prevention of cancer. Apoptosis has been characterized as a fundamental cellular activity which maintains the physiological balance of the organism. It is involved in the defense mechanisms necessary to protect against carcinogenesis by eliminating damaged or abnormal excess cells which have proliferated owing to induction by a variety of chemical agents [12]. Morphologically, apoptosis is characterized by shrinkage of the cell, dramatic reorganization of the nucleus, active membrane blebbing, and fragmentation of the cell into membrane-enclosed vesicles (apoptotic bodies) [13]. In the early stage of apoptosis, the membrane phospholipid phosphatidylserine (PS) is translocated from the inner to the outer leaflet. Annexin V has high affinity for PS, which can be used to identify apoptosis at this early stage [14, 15]. A significant body of evidence has accumulated revealing the importance of mitochondria as critical regulators of the intrinsic apoptotic pathway in response to DNA damage. Upon apoptotic stimulation, several important events occur in mitochondria, including release of cytochrome c into the cytoplasm [16–18]. Cytochrome c binds to apoptotic protease activating factor 1 (Apaf-1), which then recruits procaspase-9 to form an apoptosome. This complex activates caspase-9 which in turn cleaves and activates effector procaspases to active effector caspases, for example caspase-3. These effector caspases cleave several substrates, resulting in the morphological and biochemical changes of apoptosis. Activation of mitochondria and release of their contents are under regulatory control of several Bcl-2 family proteins [19]. Some of these members, including Bcl-2, prevent the release of cytochrome c and therefore serve as anti-apoptotic proteins, whereas others, for example Bax, promote the release of cytochrome c and serve as pro-apoptotic molecules. The objective of this study was to investigate the anticancer potential of orientin, discover its cytotoxic effect, and further investigate its mechanisms of action on apoptosis.
Results and discussion
Cell viability assay
The effects of orientin on the proliferation of HeLa cells were examined in an MTT-based assay. Treatment with orientin (5, 10, 20, 40, or 80 mol dm−3) significantly inhibited the growth of HeLa cells in a dose-dependent manner (Table 1).
Orientin-induced changes in cellular morphology
To obtain better insight into the mechanism of cytotoxicity induced by orientin, its effect on apoptosis was evaluated. Apoptosis, a major process of programmed cell death, is important in maintaining cellular homeostasis [11, 20–22]. So, induction of apoptosis in cancer cells is a useful strategy for anticancer drug development [23, 24]. Morphological change of the cell is an important aspect of apoptosis [25]. The cytotoxic effects are apparent in HeLa cells after treatment with orientin (10, 20, or 40 mol dm−3) for 48 h. Marked morphological changes indicative of cell apoptosis were clearly observed. Under an inverted microscope, cell shape and its changes can be observed clearly. As shown in Fig. 1, cells in the control group had regular polygonal shapes, cell antennas were short, and there were very few round cells. Obvious morphological changes were observed for cells treated with orientin, including the loss of adhesion, rounding, reduced cell volume, and sporadic distribution, and the tendency was clearly dose-dependent.
Orientin induced apoptosis in HeLa cells
To evaluate whether orientin induces cell death via apoptosis or necrosis, the annexin V-FITC/PI double staining assay was performed [26]. HeLa cells were treated with orientin (10, 20, or 40 mol dm−3) then marked with annexin V-FITC and PI, followed by analysis by flow cytometry. This double-staining method enables live non-apoptotic cells (annexin V/PI) to be distinguished from early apoptotic cells (annexin V/PI) and late apoptotic cells (annexin V/PI) [27, 28]. As illustrated in Fig. 2, treatment with orientin resulted in a change in cell distribution compared with control cells. Orientin (10, 20, and 40 mol dm−3) significantly increased the percentage of early apoptotic cells. In addition, the percentage of late apoptotic cells (or necrotic cells) was also increased by treatment with orientin. By using methods for detection of apoptosis we determined that orientin treatment significantly enhanced apoptosis over control values.
Orientin-induced disruption of the cell cycle
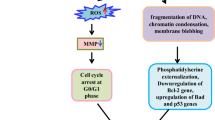
Because activation and execution of apoptosis are regulated by complex molecular mechanisms, there are numerous points of interaction between the regulatory pathway of the cell cycle and apoptosis [29]. The effect of orientin on cell cycle progression was analyzed by flow cytometry. The percentage of cells in the sub-G1 phase after orientin treatment was measured as the apoptotic index. HeLa cells were treated with orientin, and the percentage of cells in each phase of the cell cycle was quantified. This treatment increased the percentage of cells in the sub-G1 phase from 6.12 ± 2.55 to 53.07 ± 2.63 mol dm−3, in a dose-dependent manner (Table 2). The blockage effect of orientin occurred at the G1/S transition, and effect associated with inhibition of DNA synthesis. Therefore, we conclude that orientin induces DNA-strand break and cell cycle arrest before early apoptosis has occurred. It was thus suggested that orientin causes DNA damage leading to G1 cell cycle arrest and prevention of DNA synthesis. However, because the DNA damage caused by orientin was too severe to be repaired, HeLa cells underwent apoptosis via caspase-dependent and mitochondria-dependent pathways.
Western blot analysis
Western blot analysis was performed to further investigate the potential apoptotic pathway in HeLa cells treated with orientin. In orientin-treated HeLa cells, expression of the anti-apoptotic protein Bcl-2 was reduced and that of Bax was increased in a concentration-dependent manner. Orientin induced the activation of caspases-3 and caspases-9 in a dose-dependent manner (Fig. 3). These results suggested that the apoptotic effects of orientin on HeLa cells are associated with an increase in the Bax/Bcl-2 ratio and caspase activation.
The Bcl-2 family proteins Bax and Bcl-2 are important in initiating the mitochondria-mediated apoptotic pathway [30]. Pro-apoptotic protein Bax translocates to the mitochondria where it is integrated into the outer mitochondrial membrane and where it promotes release of cytochrome c into the cytosol. In contrast, anti-apoptotic protein Bcl-2 prevents this process by preserving mitochondrial integrity. Thus, the ratio of Bax to Bcl-2 is crucial to sustenance of drug-induced apoptosis in the mitochondria-mediated apoptotic pathway [31]. This study showed that orientin up-regulated expression of Bax and down-regulated expression of Bcl-2, eventually leading to an increase in the Bax/Bcl-2 ratio. The release of mitochondrial cytochrome c facilitates formation of the apoptosome complex consisting of Apaf-1 and caspase-9, which subsequently activates such effector caspases as caspase-3 and leads to apoptosis [17]. In this study, release of cytochrome c and the activation of caspase-9 and caspase-3 were detected (Fig. 3). These results suggest that orientin-induced apoptosis is via a caspase-dependent and mitochondria-dependent pathway.
In conclusion, our study provides experimental evidence that orientin induces HeLa cell apoptosis via the mitochondria-mediated and caspase-dependent pathway. Collectively, these findings provide important clues for further evaluation of the potential of orientin for use in cancer therapy.
Experimental
Orientin (98 %) was obtained from Chengdu Biopurify Phytochemicals. Orientin was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 80 mol dm−3 and diluted with phosphate-buffered saline (PBS; pH 7.4) to the desired final concentrations. The propidium iodide (PI)/RNAse staining buffer and annexin-FITC kit for apoptosis were from BD Biosciences Pharmingen (San Diego, CA, USA). DMSO and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Amresco (USA). Dulbecco’s modified Eagle’s medium (DMEM), trypsin/EDTA, fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Invitrogen Life Technologies (USA). Anti-caspase-9, anti-caspase-3, anti-Bcl-2, anti-Bax, and anti-β-actin antibodies were purchased from Cell Signaling Technology (USA). BCA protein assay kits and poly(vinylidene fluoride) (PVDF) membranes were purchased from Thermo Scientific (USA) and Millipore (USA). Other chemicals and reagents used were of analytical grade.
Cell culture
HeLa obtained from the Shanghai Institute of Biochemistry and Cell Biology were cultured in DMEM containing 10 % (v/v) heat-inactivated FBS, 100 U cm−3 penicillin, and 100 μg cm−3 streptomycin in a humidified incubator at 37 °C under 5 % CO2 atmosphere.
Cell viability assay
The effects of orientin on HeLa cell viability were determined by use of a colorimetric MTT assay which measures mitochondria-dependent reduction of MTT to formazan. HeLa cells were cultured in DMEM until the mid-exponential phase, and then seeded in a 96-well plate at a density of 1 × 104 cells per well in 0.1 cm3 medium. After incubation for 24 h, the cells were exposed to orientin (5, 10, 20, 40, or 80 mol dm−3) or PBS (control) for 72 h. After treatment, 0.01 cm3 5 mg cm−3 MTT was added and the cells were incubated for 4 h at 37 °C. The supernatant was then discarded, and 0.1 cm3 DMSO was added to each well. The mixture was shaken at room temperature for 10 min on a mini shaker to dissolve the formazan crystals, and the spectrophotometric absorbance at 570 nm was measured by use of an ELISA reader (Thermo Scientific, Marietta OH, USA).
Morphological observation
HeLa cells were seeded at a density of 1 × 104 cells/well into 6-well plates. After 8 h adherence, the cells were treated with orientin (10, 20, or 40 mol dm−3), and the cells were examined after 48 h adherence under an inverted microscope.
Annexin V-FITC/PI apoptosis assay
Annexin V/PI was used to detect cell death progression. The cells were incubated at a density of 1 × 104 cells/well in a 12-well plates. After 48 h of exposure to orientin (10, 20, or 40 mol dm−3) cells were collected by trypsinization, washed with cold PBS, and resuspended in PBS containing annexin V-FITC (1 mg cm−3) and PI and finally analyzed by flow cytometry (Becton–Dickinson Bioscience, USA).
Protein extraction and Western blot analysis
After treatment with orientin (10, 20, or 40 mol dm−3) for 48 h, HeLa cells were collected and washed twice with cold PBS. The cells were lysed in lysis buffer, kept on ice for 30 min, and centrifuged at 12,000 rpm at 4 °C for 30 min. Supernatant was stored at −70 °C before use. Protein concentrations were quantified by use of BCA protein-detection assay kits. Western blotting was performed as described elsewhere [32]. Briefly, equal amounts of protein (90–100 μg) were separated by 10–15 % SDS-PAGE and transferred to a PVDF membrane by use of glycine transfer buffer. After blocking with 5 % non-fat skimmed milk in Tween 20-Tris-buffered saline (T-TBS), the membrane was incubated overnight with primary antibodies, and then for 1 h with secondary antibodies diluted in TBS and 0.1 % Tween 20. The primary polyclonal antibodies used were: Bcl-2, caspase-9, β-actin (1:1,000; Cell Signaling Technology, Danvers, USA), caspase-3 (1:200; Santa Cruz Biotechnology, Santa Cruz, USA), and Bax (1:1,000; BD PharMingen, San Diego, CA, USA). The membrane was then exposed to X-ray film. Protein bands were detected by use of a Western Blot Detection System (Bio-Rad).
Statistical analysis
All experiments were performed in triplicate. The results were analyzed by use of SPSS 13.0 for Windows (SPSS, USA). Statistical analysis was performed by one-way analysis of variance (ANOVA), and differences from the respective controls for each experimental test condition were determined by use of t tests. The criterion for significance was set at p < 0.05. Data were collected and expressed as mean ± standard deviation (SD).
References
Danielle B, Namrita L (2012) Evid-Based Compl Alt 1:11
Ye CP, Zhang HL (2012) Prog Obstet Gynecol 21:760
Zhang Q (2012) Med Inf 4:126
Ji W, Wang EJ, Song J (2012) Chin J Fam Plann 20:164
Zheng QF, Xu SG, Xu CJ (2011) Anti-Tumor Pharm 1:33
Zhou Y, Gu JH, Wang LH (2011) Med Chin PLA 36:225
Sai D, Wang CM, Xu XL (2011) J Chin Med Mater 1:95
Wang YX, Xing JQ, Zhang XB (2011) Chin J Microecol 23:516
Li XQ, Huo TG, Qin F (2007) Chromatogr B 853:221
Zhu DX, Fang AN, Wang SH (2012) Chin Tradit Pat Med 34:2055
Hengartner MO (2000) Nature 407:770
Brown JM, Wouters BG (1999) Cancer Res 59:1391
Earnshaw WC (1995) Curr Opin Cell Biol 7:337
Zhang X, Kim DK (2011) Int J Oncol 39:1609
Reed JC, Green DR (2002) Mol Cell 9:1
Wang C, Youle RJ (2009) Ann Rev Genet 43:95
Ghobrial IM, Witzig TE, Adjei AA (2005) CA Cancer J Clin 55:178
Gupta S (2003) Int J Oncol 22:15
Marzo I, Brenner C, Zamzami N (1998) J Exp Med 187:1261
Thompson CB (1995) Science 267:1456
Green DR, Reed JC (1998) Science 281:1308
Kaufmann SH, Hengartner MO (2001) Trends Cell Biol 11:526
Hu W, Kavanagh J (2003) Lancet Oncol 4:721
Camero A (2002) Brit J Cancer 87:129
Zhang J, Xu M (2000) Cell Res 10:205
Sarveswaran S, Myers CE (2010) Cancer Lett 291:167
Vermes I, Haanen C, Steffens NH, Reutellingsperger C (1995) J Immunol Methods 184:39
Del BG, Darzynkiewicz Z, Degraef C, Mosselmans R, Fokan GP (1999) Cell Prolif 32:25
Evan GI, Vousden KH (2001) Nature 411:342
Gross A, McDonnell JM, Korsmeyer SJ (1999) Genes Dev 13:1899
Qi F, Inagaki Y, Gao B (2011) Cancer Sci 102:951
Moon JY, Kim H, Cho M, Choi HK, Kim YS, Ashik M (2011) Food Chem 125:369
Acknowledgments
This research was supported by the College Innovation Project of Jiangsu Province (1201270046, 1203000514) and the Scientific Research Promotion Fund for Jiangsu University (11JDGO74).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, Q., Tian, X., Yang, A. et al. Orientin in Trollius chinensis Bunge inhibits proliferation of HeLa human cervical carcinoma cells by induction of apoptosis. Monatsh Chem 145, 229–233 (2014). https://doi.org/10.1007/s00706-013-1011-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-013-1011-x