Abstract
Dengue disease is characterized by a marked decrease in platelet count, which is life threatening. In the present study, we investigated the antiviral activity of an aqueous extract of Carica papaya leaves (PLE) against dengue virus (DENV) and its effect on platelet augmentation. The anti-dengue activity of PLE in DENV-infected THP-1 cells was examined by immunoblotting and flow cytometry. The effect of PLE on erythrocyte damage was investigated using hemolytic and anti-hemolytic assays. Virus-infected THP-1 cells were assayed for IFN-α secretion. The effect of PLE on platelet augmentation in rats with cyclophosphamide-induced thrombocytopenia was also investigated. The platelet count of blood from the retro-orbital plexus of rats was determined on the 1st, 4th, 7th, 11th and 14th day of study. On the 14th day, the rats were sacrificed for histopathological examination of the liver, kidney and spleen. Plasma of thrombocytopenic rats was tested for thrombopoietin (TPO) and IL-6 secretion. The data suggest that PLE significantly decreases the expression of the envelope and NS1 proteins in DENV-infected THP-1 cells. A marked decrease in intracellular viral load upon PLE treatment confirmed its antiviral activity. This also resulted in a significant decrease in erythrocyte damage and hydrogen-peroxide-induced lipid peroxidation. A significant increase in the number of platelets was found in thrombocytopenic rats treated with PLE, along with an increase in IL-6 and TPO levels. These findings suggest that PLE can potentially be used as an antiviral agent, as it helps in platelet augmentation and exhibits antiviral activity against DENV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dengue is an arboviral disease caused by dengue virus (DENV), a member of the family Flaviviridae. It is endemic throughout tropical and subtropical regions of the world, causing around 390 million new infections annually [7]. It is asymptomatic in the majority of people but can cause non-severe dengue with or without warning signs in some cases, but if untreated, it can lead to severe dengue, with the hallmark symptom of thrombocytopenia [13, 15, 34]. Studies suggest that a low platelet count is one of the major causes of bleeding in dengue patients. The platelet count drops below the normal level (150,000–450,000 platelets/μL or mm3) and may reach as low as < 40,000 platelets/mm3 during the critical phase (day 3–7) of fever in many patients. Thrombocytopenia has always been one of the most decisive criteria used by the World Health Organization (WHO) guidelines as a potential indicator of clinical severity [33]. According to WHO guidelines, thrombocytopenia is defined as a rapid decline in platelet count or a platelet count less than 150,000 per microliter of blood [34]. There are several potential reasons for thrombocytopenia in dengue disease, including bone marrow suppression, increased platelet destruction, platelet dysfunction, and imbalance between coagulation, fibrinolysis and anti-coagulant pathways, [5, 17, 19]. Recently, Ojha et al. reported that platelet activation determines the severity of thrombocytopenia in dengue infection [26]. There is no approved anti-dengue drug or vaccine available on the market, although the Dengvaxia vaccine produced by Sanofi Pasteur has shown promising results in clinical trials but is still not considered safe for children [11]. Recent studies have shown that it is also not safe for use in dengue-seronegative individuals [2, 14]
Natural products have been the main source of test materials in the development of antiviral drugs based on traditional medical practices [18]. These products are considered to be effective and non-toxic. Currently, not a single natural product has actually been approved as an antiviral drug against DENV, although a few have been reported to have antiviral activity [21, 24, 29, 36].
Carica papaya, commonly known as papaya, is a tree-like herbaceous plant belonging to the family Caricaceae. It is cultivated globally, mainly for its fruit [30], but other parts of the plant are used for various purposes, and some have anti-bacterial properties [10]. Papaya leaves have been reported to reduce symptoms of asthma, worm infestations and dysentery [27] and to have anti-cancer properties [25].
Carica papaya leaf extracts are prescribed as a tonic for the heart and also for the treatment of fever, pyrexia, diabetes, gonorrhea, syphilis, and inflammation and for dressing infected wounds [3, 16, 23]. Previous phytochemical analysis [6] has shown the presence of flavonoids, alkaloids, carbohydrates, saponins, glycosides, phytosterols, phenolics, terpenoids and tannins in the leaves of Carica papaya. A few studies have reported an increase in platelet count after administration of PLE to dengue patients [31, 35], but the effect of PLE on dengue virus infection in vitro has not been studied so far. Therefore, the objective of the present study was to investigate the possible anti-dengue effects of papaya leaf extract and its role in platelet augmentation. The present study provides scientific support for the potential use of PLE as a therapeutic drug against dengue virus infection.
Materials and methods
Plant material
Fresh middle-stage-age Carica papaya leaves were purchased from a nursery in Delhi, India. Leaves were thoroughly washed with running tap water and then dried in the shade. They were then finely powdered using a grinder. Seventeen grams of papaya leaf powder was mixed in 100 ml of autoclaved Milli-Q water, and this mixture was then kept on a rotary shaker for three days to ensure mixing. After three days, the mixture was centrifuged and filtered using a 0.2-µm Whatman filter. It was found that 3.6 gm of papaya leaf powder is soluble in 80 ml of Milli-Q water. A 45 mg/ml stock solution of PLE was stored in a sterile glass bottle at 4 °C for use in further experiments.
Cells
The cell line THP-1, maintained in Rosewell Park Memorial Institute (RPMI) medium (Sigma) was used as an infection model for DENV, and the cell line C6/36, maintained in minimum essential medium (MEM) (Sigma) was used to propagate DENV. RPMI was supplemented with 10% fetal bovine serum (FBS) (Sigma) and 100 U of penicillin (Sigma), and 100 µg of streptomycin (Sigma), per ml at 37 °C in a 5% CO2 atmosphere in an incubator (Sanyo, Japan).
Animals
Male Sprague Dawley (SD) rats weighing about 180-200 g and aged 8-9 weeks were used for a cyclophosphamide-induced thrombocytopenia study. Two experimental trials were done using 18 animal in the first study and 30 in the second. The first experiment was a pilot study to standardize the cyclophosphamide dose, its duration, and minimum effective dose of papaya. The second experiment provided the results presented here. Animals were housed under standard conditions of temperature and light in the animal house of DIPAS, DRDO. Ethical approval was received from the animal ethical review committee of DIPAS, DRDO Delhi (IAEC/DIPAS/2015-25, 18/10/2015). The animals were given standard rat foods and allowed to drink water. Proper cleaning measures were taken regularly.
Development of a virus infection model
The DENV serotype 2 New Guinea C strain was propagated in C6/36 cells to produce virus stock for infection of THP-1 cells. The titer of the virus stock was determined by plaque assay to be 109 plaque-forming units (PFU) per ml. THP-1 cells were infected with DENV as described previously [29]. Briefly, 2 × 106 THP-1 cells per ml were infected with DENV serotype 2 at a multiplicity of infection (MOI) of 3 in a serum-free medium at 37 °C for 2 h. Cell culture plates were gently agitated for optimum virus and cell contact, and unadsorbed virus was removed by washing with incomplete medium. The mock-infected and DENV-infected cells were then provided with fresh RPMI medium containing 2% fetal bovine serum (FBS) and incubated for 48 h at 37 °C. After infection, the cells were harvested, and the cell-free supernatant was stored at – 80 °C until assayed for cytokine profiling.
LC-MS analysis
The PLE extract was dried under a gentle stream of nitrogen and reconstituted in methanol. The compounds present in the extract were analyzed by liquid chromatography mass spectrometry (LC-MS/MS). An ultra performance liquid chromatography (UPLC) system was coupled to a hybrid quadrupole, orthogonal time-of-flight (Q-TOF) tandem mass spectrometer (SYNAPT G2 HDMS, Waters, Manchester, U.K.) equipped with ESI. Chromatographic separation was performed on an Acquity UPLC BEH C18 column (3.0 mm × 150 mm, 1.7 μm, waters, Ireland) at a temperature of 40 °C. The mobile phases consisted of eluent A (0.1% formic acid in water, v/v) and eluent B (0.1% formic acid in acetonitrile, v/v). These eluents were delivered at a flow rate of 0.4 mL/min with a linear gradient program as follows: 20–80% B from 0 to 15 min, 80–95% B from 15.0 to 15.5 min, hold at 95% B from 15.5 to 18.0 min, 95-20% B from 18.0 to 19.0 min, and hold at 25% B from 19.0 to 20.0 min. The operating parameters were as follows: capillary voltage, 3 kV (ESI+); sample cone voltage, 35 V; extraction cone voltage, 4 V; source temperature, 100 °C; desolvation temperature, 300 °C; cone gas flow, 50 L/h; desolvation gas flow, 800 L/h. In MSE mode, the trap collision energy for the low-energy function was set at 5 eV, while the ramp trap collision energy for the high-energy function was set at 20–50 eV. Argon was used as the collision gas for collision-induced dissociation (CID) in MSE mode. To ensure mass accuracy and reproducibility, the mass spectrometer was calibrated over a range of 50–1500 Da using a solution of sodium formate.
Cell viability assay
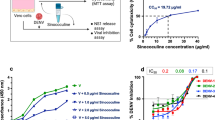
The cytotoxicity of papaya in THP-1 cells was determined by MTT assay. THP-1 cells were treated with PLE at 50, 75, 100, 200 and 300 µg/ml for 24 h, 48 h and 72 h. The cytotoxicity of PLE in THP-1 cells was determined using 3-(4, 5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazoliumbromide (MTT) (Sigma-Aldrich, USA) dye . Ten µl of MTT stock was added to the cells, which were incubated for 4 h at 37 °C. MTT, a yellow tetrazole, was reduced to form purple formazan crystals by the NADPH-dependent enzyme oxidoreductase present in viable THP-1 cells. The crystals were dissolved by addition of DMSO and the optical density was measured at 570 nm using a spectrophotometer (Biotek Instruments, USA). All experiments were repeated three times in triplicate at 24 h, 48 h and 72 h.
Assessment of antiviral activity of PLE
Mock-infected and virus-infected THP-1 cells were treated with or without PLE at a dose of 100 µg/ml or 200 µg/ml, and after two hours of virus infection, the cells were incubated for 48 h and a whole-cell lysate was prepared. The concentration of protein present in whole-cell lysate was estimated by the Bradford method, and 40 µg of protein of each sample was loaded on a 10% sodium dodecyl sulfate (SDS) polyacrylamide gel and separated by electrophoresis. The proteins were then transferred to a polyvinylidene difluoride (PVDF) membrane. Nonspecific binding of antibodies was blocked using 3% bovine serum albumin (BSA) in TBS buffer (0.1 M Tris-HCl, pH 7.4, 0.15 M NaCl), followed by washing with TBST20 (0.1% Tween-20 in TBS). The membrane was then incubated with a primary rabbit antibody against the DENV envelope protein (cat. no. PA5-32246, Thermo Scientific USA) and the NS1 protein (cat. no. SAB2700022 SIGMA). The membrane was again washed three times with TBST20 and incubated with biotinylated goat anti-rabbit immunoglobulin (IgG) (cat. no. B8895 Sigma) and streptavidin peroxidase (cat. no. S2438 Sigma). The proteins were detected by chemiluminescence.
Intracellular staining of DENV
DENV-infected THP-1 cells at a concentration of 2 × 106 cells/ml were treated with 100 or 200 µg of PLE per ml and incubated for 48 h. Cells were harvested and then washed twice with 0.01 M PBS, fixed, and permeabilised with cytofix/cytoperm buffer (BD Biosciences, USA) for 20 min, followed by incubation with anti-DENV FITC-conjugated polyclonal IgG antibody (cat. no. orb15512, Biorbyt, UK) at a 1:200 dilution for 60 min. Unbound antibodies were removed by washing again with PBS. The cells were then suspended in 0.5 ml PBS (0.01 M) and analyzed using a flow cytometer (BD FACSCalibur) with Cell Quest Pro software.
Measurement of IFN-α by ELISA
DENV-infected THP-1 cells were treated with PLE and incubated for 48 h in IRPMI medium supplemented with 2% FBS. The supernatant was collected and kept at – 80 °C. IFN-α was detected using a human IFN-α ELISA kit (cat. no. 201-12-0077, Sunred Biological Technology, China).
Haemolytic activity
Seven ml of fresh venous blood was collected from three healthy human volunteers in 15-ml Falcon tubes containing heparin and washed three times with sterile pyrogen-free saline solution (0.15 M NaCl) by centrifugation at 1500 rpm for 5 min. The pellet was resuspended in 0.5% saline solution. A volume of 0.5 ml of the cell suspension was mixed with 0.5 ml of saline solution containing (40, 20, 10, 5, or 2.5 mg of PLE per ml). The mixtures were incubated at 37 °C for 30 min and centrifuged at 2000 rpm for 10 min. The free haemoglobin content in the supernatants was measured spectrophotometrically at 540 nm. Saline and distilled water were used as negative and positive haemolytic controls.
Anti-haemolytic activity
Human red blood cells (RBCs) were diluted in PBS buffer to obtain a 4% suspension. The plant extracts were prepared in PBS buffer at five concentrations: 40, 20, 10, 5, and 2.5 mg mL−1. To 2 mL of RBC suspension, we added 1 mL of plant extract (in the above-mentioned concentrations) and 2 mL of PBS (0.01 M) to reach the final volume of 5 mL. After 5 min of incubation at room temperature, 0.5 mL of 0.1 M H2O2 was added to induce oxidative degradation of membrane lipids, and the mixture was shaken at 37 °C for 240 min. The samples were then centrifuged at 1500 g for 10 min, and the resulting supernatant was removed and used to measure haemolysis using a spectrophotometer (UV–Visible EZ201, Perkin Elmer, Norwalk, CA, USA) at an absorbance wavelength of 540 nm. The amount of RBC lysis in the presence of H2O2 and the absence of a plant extract was considered 100% haemolytic activity. Haemolysis in the presence of extracts was calculated relative to this control. Haemolysis inhibition was calculated as follows:
where Ao is the absorbance of the control (H2O2 + RBC, without extract) and A1 is the absorbance in the presence of the extract or vitamin C as the reference antioxidant, used in the same concentrations as the extracts (2.5-40 mg mL−1). Each set of experiments was performed in triplicate, and the inhibitory activity was expressed as a percentage.
Establishment of thrombocytopenia in a rat model
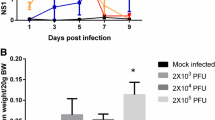
Thrombocytopenia was established in SD rats using cyclophosphamide (cat. no. PHR 1404, Sigma Aldrich). A cyclophosphamide working solution was prepared fresh at the time of injection of animals (0.4 M using saline). Animals were divided into five groups of six animals each: group 1 was a control in which rats were given saline orally for fourteen days. Group 2 was the cyclophosphamide-treated group in which rats were given cyclophosphamide (50 mg/kg body weight or 100 µl of 0.4 M cyclophosphamide) subcutaneously for two consecutive days. Group 3 was the PLE-treated group, in which rats were given PLE (200 mg/kg body weight or 40 mg/ml PLE for a rat weighing 200 g) orally from day one to day six. Group 4 consisted of cyclophosphamide-treated rats and PLE was given prophylactically for six days. In group 5, cyclophosphamide-treated rats were given PLE therapeutically, i.e., after establishment of thrombocytopenia on day seven, for six days. (Fig. 1).
Blood collection
Blood was collected from each of the rats from the retro-orbital plexus of the eye on the 1st, 4th, 7th 11th and 14th day of study. Blood was collected from each animal of each group before any treatment with cyclophosphamide or PLE on day 1, and cyclophosphamide treatment was then given to second, fourth, and fifth group of rats on day 1 after blood collection. Similarly, PLE treatment was given to the third and fourth group of rats after blood collection on day 1 and then for six consecutive days. Analysis of haematological parameters of rat blood was done using a haematological analyzer.
Histopathological analysis
Histopathologic analysis was performed by light microscopy. Liver, kidney and spleen tissue sections were fixed in 10% buffered formalin. After fixation, the sample were washed with running water and processed to obtain 5-μm-thick paraffin sections. All sections were stained with hematoxylin and eosin (HE).
Estimation of TPO and IL-6 in rat plasma of thrombocytopenic rats by ELISA
Blood plasma from thrombocytopenic rats was collected at the same time points, i.e., the first, fourth, seventh, eleventh and fourteenth days. It was stored at – 80 °C for cytokine analysis. TPO and IL-6 levels were determined by ELISA (My Biosource, MBS262061 and eBioscience) as per the manufacturer’s protocol.
Statistical analysis
Data were analyzed using a commercially available statistics software package (SPSS for Windows, version 14.0, Chicago, USA). One-way analysis of variance (ANOVA) was performed. Data are presented as the mean ± standard error (S.E.M), p-values less than 0.05 were regarded as statistically significant.
Results
LC-MS analysis
A papaya leaf aqueous extract (PLE) was prepared, and LC-MS analysis followed by integrated library research showed that it contained 14 constituents in high abundance. Carpaine, with a molecular mass of 479.384 was found to be the major constituent of the PLE (Fig. 2). In addition to carpaine, many other phytoconstituents were also found, including amino acids, organic acids, vitamins, flavanoids, alkaloids, phenolic compounds (Table 1).
Analysis of the chemical constituents of aqueous papaya leaf extract (PLE). (A) Chromatogram of PLE diluted in methanol containing 0.1% formic acid. Diluted extract was directly infused into the electrospray source and mass analyzer at a flow rate of 10 ml/min. (B) The area of spectrum peaks 2 and 3 from m/z 100 to 1400: peak 2, m/z 479.3883; fragment ions, m/z 240.1902, m/z 222.1968; peak 3, m/z 479.3883, fragment ions, m/z 240.1902, m/z 222.1968. The main component, carpaine, has a theoretical monoisotopic mass [M+H]+ of 479.3848 and known MS/MS fragments ions [M+H]+ of 222 and 240. (C) Structure of the carpaine molecule
Cell viability assay
The cytotoxicity of PLE in THP-1 cells was tested by MTT assay. Treatment of cells with PLE at concentrations of 50, 75, 100 and 200 µg/ml did not result in any cytotoxicity in THP-1 cells, even after 72 h of incubation. Lower doses of PLE up to 75 µg/ml, in fact, caused slight cell proliferation. Cell viability at 300 µg/ml decreased slightly to 94.22 ± 0.57% at 72 h of incubation in THP-1 cells (Fig. 3). Therefore, PLE concentrations of 100 and 200 µg/ml were chosen to determine their antiviral activity.
Determination of antiviral activity of PLE
The effect of PLE on DENV infection was determined by measuring DENV envelope protein expression by western blotting. DENV-infected and PLE (100 µg/ml)-treated THP-1 cells showed a marked decrease of more than fourfold in envelope protein expression in comparison to the DENV-infected cells without PLE (Fig. 4A and B) (***, p < 0.001). PLE at a concentration of 200 µg/ml completely inhibited envelope protein expression. Similarly, NS1 protein expression in DENV infected cells was also decreased by more than fourfold by treatment with 100 µg PLE per ml (Fig. 4C and D) (***, p < 0.001), demonstrating the antiviral activity of PLE.
Effect of PLE on dengue virus infected THP-1 cells. (A) Immunoblot of the DENV envelope protein and beta actin in PLE-treated (100 µg/ml and 200 µg/ml) and DENV-infected THP-1 cells (B) Bar graph showing the density ratio of the envelope protein versus beta actin. ***, p < 0.001 vs. virus. (C) Immunoblot of dengue NS1 protein and beta actin in PLE-treated (100 µg/ml and 200 µg/ml) and DENV-infected THP-1 cells. (D) Bar graph showing the density ratio of the NS1 protein versus beta actin. ***, p < 0.001 vs. virus. The data shown are representative of three independent experiments
Determination of the intracellular viral load
The intracellular viral load in DENV-infected and PLE-treated THP-1 cells was determined by FACS analysis. PLE at 100 µg/ml decreased the mean fluorescence intensity (MFI) of FITC-labelled DENV-2-specific antibody by 1.5-fold from 76.6 ± 10.5 to 49.15 ± 9.3 and a 200 µg/ml dose decreased it to 47.25 ± 6.7 (Fig. 5A and B) in PLE-treated THP-1 cells in comparison to virus-infected cells, thus confirming the antiviral activity of PLE against DENV.
Intracellular viral load in DENV-infected and PLE-treated THP-1 cells. (A) Histogram showing the intracellular viral load in DENV-infected and PLE-treated THP-1 cells (B). Bar graph showing the mean fluorescence intensity of DENV-infected and PLE-treated (100 µg/ml and 200 µg/ml) THP-1 cells. The data shown are representative of three independent experiments
Measurement of IFN-α by ELISA
Changes in the expression of IFN-α cytokines during DENV infection and PLE treatment were investigated by ELISA. The expression of IFN-α was found to be almost 1.6-fold higher in DENV-infected THP-1-cells treated with PLE (200 µg/ml) than in the control, increasing significantly from 213.57 ± 28.98 pg/ml to 343.341 ± 12.35 pg/ml (Fig. 6) (*, p < 0.05).
Haemolytic activity
The haemolytic activity of PLE at different concentrations was measured in human blood. Distilled water was used as a positive control, and 0.5% saline was used as a negative control. PLE extract did not exhibit haemolytic activity at any of the doses tested (40 mg/ml, 20 mg/ml, 10 mg/ml, 5 mg/ml and 2.5 mg/ml) in comparison to distilled-water-treated cells (Fig. 7A) (***, p < 0.001).
Haemolytic and anti-haemolytic activity of PLE. (A) Haemolytic activity of different concentrations of PLE (2.5-40 mg/ml) was measured in human blood samples (n = 3). The graph represents the mean values of optical density ± SE. Saline and double-distilled water were used as negative and positive controls, respectively. The optical density of PLE-treated hPBMCs was significantly lower than that of distilled-water-treated hPBMCs. ***, p < 0.001 vs. positive control. (B) Anti-haemolytic activity of PLE. The anti-haemolytic activity of different concentrations of PLE (2.5-40 mg/ml) was measured in human blood samples (n = 3). The graph represents the mean values ± SE. H2O2 and ascorbic acid were used as negative and positive controls, respectively. Different concentrations of PLE-treated hPBMCs showed significantly higher anti-hemolytic activity in comparison to H2O2-treated cells. ***, p < 0.001 vs. negative control
Anti-haemolytic activity
Reactive oxygen species are a major cause of oxidative damage and hemolysis of RBCs. We determined the effect of PLE on hydrogen peroxide (H2O2)-induced oxidation of RBCs by anti-hemolytic assay. PLE treatment resulted in significantly higher anti-haemolytic activity in comparison to the negative control (H2O2) at 40 mg/ml, 20 mg/ml, 10 mg/ml, 5 mg/ml and 2.5 mg/ml. PLE showed significantly higher anti-haemolytic activity than ascorbic acid (positive control) when tested at 40 mg/ml, 20 mg/ml and 10 mg/ml (***, p < 0.001 vs. negative control) (Fig. 7B).
Platelet augmentation activity of PLE
The platelet augmentation effect of PLE was investigated in a two-phase experiment. In the first phase, the doses of cyclophosphamide and PLE were standardized to ensure survival of the rats. Cyclophosphamide was given subcutaneously (s/c) at 50 mg/kg body weight (100 µl of 0.4 M cyclophosphamide) for two consecutive days starting from day 1 after retro-orbital bleeding, and PLE extract was administered orally at 200 mg/kg body weight of rats. The average platelet counts of the control, cyclophosphamide-treated, PLE-only, prophylactic, and therapeutic groups were 595.33 ± 15.9 × 103/mm3, 623.833 ± 55.6 × 103/mm3, 572 ± 25.61 × 103/mm3, 559.333 ± 31.3 × 103/mm3, and 576.333 ± 24.09 × 103/mm3, respectively, on the first day of the experiment before any treatment. Platelet counts within the cyclophosphamide and therapeutic group started to fall after day 3 (239.5 ± 40.63 × 103/mm3 in the cyclophosphamide group and 252.33 ± 20.36 × 103/mm3 in the therapeutic group) and considerable thrombocytopenia developed after 7 days (200.5 ± 31.26 × 103/mm3 and ± 172.66 ± 14.1 × 103/mm3 in the therapeutic group) (***, p < 0.001). PLE was given from day one until day 6 in the prophylactic group after cyclophosphamide treatment on day 1. Interestingly PLE treatment did not result in a reduction in platelet count in this group (415 ± 13.2 × 103/µl) on day 4 and (466.83 ± 41.07 × 103/mm3) on day 7, and it was found to be significantly increased in comparison to the cyclophosphamide-only-treated group on day 7 (###, p < 0.001). In the therapeutic group, PLE was given from day 7 after cyclophosphamide treatment, and platelet counts were observed to increase on day 11 (389 ± 47.3 × 103/mm3) and reach a normal level at day 14 (578.33 ± 57 × 103/mm3) (##, p < 0.01 in comparison to the cyclophosphamide-only-treated group on day 7) (Fig. 8A). The platelet count was also found to be significantly increased in the PLE group only in comparison to the control on day 7 (690 ± 101.7 × 103/mm3) (*, p < 0.05).
Effect of PLE on hematological parameters in cyclophosphamide-treated rats (A) Effect of PLE on platelet count in a cyclophosphamide-treated rat. Cyclophosphamide treatment resulted in thrombocytopenia in rats on day 7, so the platelet count decreased significantly in the cyclophosphamide-only, and therapeutically treated rats in comparison to control rats on day 1 (***, < 0.001). PLE treatment in the prophylactic group prevented the platelet count from falling sharply and in fact increased the platelet count significantly in the prophylactically treated group on day 7 (###, p < 0.001) in comparison to the cyclophosphamide-only-treated group on day 7. Similarly, PLE treatment increased the platelet count in blood plasma of thrombocytopenic rats in the therapeutic group on day 14 in comparison to the cyclophosphamide-only-treated group of rats of day 7 (##, p < 0.001). Similarly, PLE treatment also increased the platelet count in the PLE-only-treated group on day 7 in comparison to control rats on day 1 (*, p < 0.5). (B) Effect of PLE on WBC count in cyclophosphamide-treated rats, determined using a haematology analyzer. Cyclophosphamide treatment significantly decreased the WBC count in the cyclophosphamide-only and therapeutically treated rats on day 7 in comparison to control rats on day l (**, p < 0.01). PLE treatment increased the WBC count in both the prophylactic and therapeutic groups. (C) Effect of PLE on RBC count in cyclophosphamide-treated rats. Cyclophosphamide treatment significantly decreased the RBC count in cyclophosphamide-only and therapeutically treated rats on day 7 (***, p < 0.001) in comparison to control rats on day 1. PLE treatment was found to increase the RBC count in prophylactic rats significantly (##, p < 0.01) in comparison to cyclophosphamide-only-treated rats on day 7. It also increased the RBC count in therapeutically treated rats on day 14 in comparison to cyclophosphamide-only-treated rats on day 7
PLE treatment increases the WBC count in rats with cyclophosphamide-induced thrombocytopenia
The white blood cell (WBC) count was also found to increase in PLE-treated thrombocytopenic rats. The average WBC count in control, cyclophosphamide-only-treated, PLE-only treated, prophylactic PLE and therapeutic PLE rats was 7.10 ± 0.98 × 103/mm3, 7.23 ± 0.75 × 103/mm3, 6.35 ± 0.84 × 103/mm3, 7.55 ± 0.82 × 103/mm3, 6.88 ± 0.64 × 103/mm3, respectively, on day 1 of the experiment. The WBC count significantly decreased to 5.13 ± 1.09 × 103/mm3 on day 4 (*, p < 0.05 in comparison to the control on day 1) and 2.67 ± 0.42 × 103/mm3 on day 7 (**, p < 0.01 in comparison to the control on day 1) in cyclophosphamide-only-treated rats. Similarly it also decreased in therapeutically PLE-treated rats to 2.07 ± 0.31 × 103/mm3 on day 4 (***, p < 0.001 in comparison to the control on day 1) and 2.73 ± 0.44 × 103/mm3 on day 7 (**, p < 0.01 in comparison to the control on day 1), while it increased to 5.05 ± 0.43 × 103/mm3 on day 14 after PLE treatment. PLE treatment was also found to increase the WBC count in prophylactically-treated rats, as the WBC count first decreased to 3.69 ± 0.84 × 103/mm3 (***, p < 0.001 in comparison to the control on day 1) on day 4 due to cyclophosphamide treatment, but it gradually increased to 4.08 ± 0.45 × 103/mm3 on day 7, 5.28 ± 0.86 × 103/mm3 on day 11, and 6.83 ± 1.32 × 103/mm3 on day 14 (Fig. 8B).
PLE treatment increases the RBC count in rats with cyclophosphamide-induced thrombocytopenia
The RBC count was also found to increase in PLE-treated thrombocytopenic rats (Fig. 8C). Cyclophosphamide treatment significantly decreased the RBC count in cyclophosphamide-only-treated rats from 7.23 ± 0.22 × 106/mm3 on day 1 to 5.65 ± 0.22 × 106/mm3 on day 7 (***, p < 0.001 in comparison to the control rats on day 1). It also decreased the RBC count in therapeutically treated rats from 7.86 ± 0.40 × 106/mm3 on day 1 to 4.84 ± 0.24 × 106/mm3 on day 7 (***, p < 0.001 in comparison to control rats on day 1). PLE treatment increased the RBC count in the prophylactic group of rats to 7.03 ± 0.21 × 106/mm3 on day 7 (##, p < 0.01 in comparison to the cyclophosphamide-only group on day 7) and to 6.64 ± 0.21 × 106/mm3 on day 14 in the therapeutic group.
Histopathological analysis of rat liver, kidney and spleen
The histology of the liver, spleen and kidney showed normal morphology in the control, PLE-only, prophylactic and therapeutic groups of rats. Hepatocytes in control rats, which received saline orally for fourteen days, showed normal cell morphology with well-preserved cytoplasm and nucleus. In contrast, cyclophosphamide-treated rats showed altered staining of the nucleii. The liver morphology of rats treated with PLE only, the prophylactically treated group, and the therapeutic group was similar to that of the control group (Fig. 9A). Renal tubules in control rats showed normal cell structure lined by columnar epithelium, while cyclophosphamide-treated cells showed renal tubules with edematous changes in the epithelial cell lining. The PLE-only, prophylactically treated and therapeutically treated rats shows normal renal tubules (Fig. 9B). The histopathology of the spleen showed that neither cyclophosphamide nor PLE treatment in rats with cyclophosphamide-induced thrombocytopenia caused significant changes in splenocyte morphology (Fig. 9C).
A. Effect of PLE on the histology of hepatocytes in the liver of thrombocytopenic rats, examined by hematoxylin and eosin staining. Photomicrographs of (a) hepatocytes of a control rat showing normal cell morphology with well-preserved cytoplasm and nucleus, (b) hepatocytes of a cyclophosphamide-treated thrombocytopenic rat showing altered staining characteristic of nuclei, (c) hepatocytes of a rat showing normal cell morphology that received PLE extract only, (d) hepatocytes of cyclophosphamide-treated thrombocytopenic rats treated prophylactically with PLE, also showing normal morphology of hepatocytes, and (e) hepatocytes of cyclophosphamide-treated rats treated therapeutically with PLE, showing normal cell morphology. B. Effect of PLE on histology of renal tubules of the kidney in thrombocytopenic rats. Photomicrographs of (a) renal tubules of control rats showing normal cell structure lined by columnar epithelium, (b) renal tubules of cyclophosphamide-treated thrombocytopenic rats, showing edematous changes in the epithelial cell lining of the renal tubules, (c) renal tubule of PLE-only-treated rats, showing normal morphology, (d) normal morphology of renal tubules of kidney of thrombocytopenic rats prophylactically treated with PLE, and (e) renal tubules of thrombocytopenic rats treated therapeutically with PLE. C. Effect of PLE on the histology of splenocytes in the spleen of a cyclophosphamide-treated rat. Photomicrographs of splenocytes of (a) control rats, (b) cyclophosphamide-only-treated rats, (c) PLE-only-treated rats, (d) thrombocytopenic rats treated prophylactically with PLE, and (e) thrombocytopenic rats treated therapeutically with PLE, showing normal morphology of splenocytes
Estimation of IL-6 and TPO in rat plasma of thrombocytopenic rats by ELISA
The cytokine IL-6 has been reported to stimulate thrombopoiesis through thrombopoietin (TPO). We evaluated the expression of IL-6 and TPO in plasma of rats with cyclophosphamide-induced thrombocytopenia. It was found that the IL-6 level increased significantly in the PLE-treated group in comparison to the control group from 291.462 ± 10.11 pg/ml to 523.17 ± 25.7 pg/ml on day 14 (***, p < 0.001). Similarly, it was found to increase significantly in the therapeutic group to 465.81 ± 70.07 on day 14 (*, p < 0.05) (Fig. 10A). The TPO level was found to increase significantly in the PLE-only-treated group on day14 (218.52 ± 11.7 pg/ml) in comparison to the control group (46.371 ± 11.4 pg/ml) (*, p < 0.01) (Fig. 10B)
A. Effect of PLE on IL-6 secretion in a cyclophosphamide-treated rat. PLE treatment significantly increased IL-6 secretion in the PLE-only treated group of rats on day 14 in comparison to control rats on day 1 (***, p < 0.001). Similarly, PLE treatment was found to significantly increase IL-6 secretion in the therapeutic group also in comparison to control rats on day 14 (*, p < 0.05). B. Effect of PLE on TPO secretion in cyclophosphamide-treated rats. PLE treatment significantly increased TPO secretion in PLE-only-treated rats on day 14 in comparison to control rats on day 1 (***, p < 0.001)
Discussion
Plant-derived antivirals could be a potential source for drug development. Working on this aspect, we have found that papaya leaf extract has potential anti-dengue properties. A few reports have suggested that PLE has anti-thrombocytopenic activity in mice and humans [28, 31]. Recently, Zunjar et al. reported that carpaine, found in alkaloidal fraction of PLE, is responsible for anti-thrombocytopenic activity of PLE [37], but the anti-dengue activity of PLE has not been investigated. The present study was designed to investigate the immunomodulatory aspects of PLE and its role in anti-dengue activity and platelet augmentation.
PLE was prepared from shade-dried young leaves of papaya plants, as shade drying of leaves limits the biochemical changes in the leaves by reducing moisture content. LC-MS analysis of PLE showed the presence of various phytochemicals, including amino acids, carbohydrates, vitamins phenolic compounds, alkaloids, and flavonoids. PLE has been used in the cosmetic industry for its anti-ageing effect due to the presence of a large variety and number of amino acids and vitamins such as vitamins C and E for keeping skin and hair healthy. PLE is a rich source of flavonoids such as myricetin, quercetin, hesperitin, and 7-hydroxy flavanone, which are responsible for its anti-oxidant activity. PLE also contains phenolic compounds such as caffeic acid and catechin, which play an important role in its various immune functions. Carpaine, an alkaloid that is the most abundant component of PLE, has been reported by Zunjar et al. [37] to be responsible for the platelet augmentation activity of papaya leaves.
The antiviral properties of PLE were investigated in vitro in THP-1 cells. When DENV-infected THP-1 cells were treated with PLE, DENV envelope protein expression decreased significantly. As the envelope protein is primarily responsible for receptor binding, haemagglutination of erythrocytes, and induction of neutralizing antibodies in dengue disease [12], a significant reduction in envelope protein expression indicates that PLE has anti-dengue activity. Besides playing a pivotal role in dengue replication, NS1 has been reported to be released into the plasma, where it is highly immunogenic. Virions released from infected cells might also directly damage endothelial cells, and uptake of the non-structural protein NS1 by hepatocytes might promote viral infection of the liver [1]. Sun et al. has reported that NS1 elicits platelet-bound antibodies, leading to accelerated clearance by phagocytes, complement-mediated lysis, and the activation of platelets, which in turn leads to the development of thrombocytopenia in mice [32]. Patients with dengue fever with an elevated level of NS1 antigen have a higher risk of developing thrombocytopenia and, consequently, severe dengue [22]. PLE was found to significantly decrease DENV NS1 protein expression, confirming the anti-dengue activity of PLE. It was also found to decrease the intracellular viral load in DENV-infected THP-1 cells, again confirming its anti-dengue activity.
Interferons play an important role in virus inhibition during the early stages of infection. Type I interferons inhibit dengue virus infection by preventing the accumulation of negative-strand RNA [9]. PLE was found to increase the expression of IFN-α in dengue-infected and PLE-treated THP-1 cells, which strengthens our hypothesis.
The erythrocyte membrane is a model system that is used for many in vitro investigations of drug and membrane interactions [4]. PLE did not show haemolytic activity, even a dose of up to 40 mg/ml. In fact, it showed significant anti-haemolytic activity in hydrogen-peroxide-treated RBCs. It exhibited even higher anti-haemolytic activity than the positive control ascorbic acid at concentrations of 40, 20 and 10 mg/ml. These results show that PLE has membrane-stabilizing effects on RBCs. Any compound or drug that stabilizes the plasma membrane may effectively enhance the survival of platelets and thereby reduce the morbidity and mortality of patients with DENV infections.
The currently available mouse model (AG129) has the limitations of low viral load and a short period of viraemia and therefore cannot be used to mimic the platelet count decrease observed in severe dengue. Instead, we induced thrombocytopenia chemically in rats, using cyclophosphamide, which is a well-established model to study thrombocytopenia.
PLE was found to exhibit a significant increase in platelet count at both the prophylactic level and the therapeutic level. Interestingly, PLE, if given prophylactically, prevents the platelet count from decreasing to dangerous levels, even in thrombocytopenic rats, and thereby prevents bleeding manifestations. It also increases the platelet count therapeutically and restores it to a normal level by day 14 after six doses. It was also found to significantly increase the platelet count in control animals in a dose-dependent manner. It should be noted that an increase in platelet count above the normal level is also dangerous, so it is not advised for PLE to be taken in large doses by an individual with a normal platelet count.
Severe dengue virus infection is also associated with liver failure, especially in children [20]. The histopathology of thrombocytopenic rats showed liver damage, which was not found in the PLE-only, prophylactic or therapeutic group, indicating that PLE also prevents liver damage. However, the dose of cyclophosphamide used in this study was low, so this liver damage was not as severe as is observed in clinical patients. The same was the case with histopathological sections of kidney. The cyclophosphamide group showed edematous changes in the epithelial cell lining of renal tubules, but PLE prevented these changes in the other three groups, showing its role in prevention of kidney damage.
TPO is a cytokine that specifically regulates megakaryocytopoiesis and platelet production by activating the TPO receptor, c-mpl [8]. PLE was found to increase TPO and IL-6 cytokine levels in plasma of thrombocytopenic rats. This suggests that Carica papaya leaf juice has platelet increasing effect and can enhance haemopoiesis and thrombopoiesis in animals.
In summary, our data show that PLE is an excellent antiviral drug, as it decreases the intracellular DENV load and prevents thrombocytopenia. Our results thus give scientific support to the possibility of developing PLE as an antiviral compound against DENV, which could be considered for the development of an effective therapeutic drug against DENV infection.
Abbreviations
- DENV:
-
Dengue virus
- PLE:
-
Papaya leaf extract
- DHF:
-
Dengue hemorrhagic fever
- DSS:
-
Dengue shock syndrome
- WHO:
-
World Health Organization
- FBS:
-
Fetal bovine serum
- UPLC:
-
Ultra performance liquid chromatography
- LC-MS:
-
Liquid chromatography mass spectrometry
- hPBMCs:
-
Human peripheral blood mononuclear cells
- IFN-α:
-
Interferon alpha
- MTT:
-
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyltetrazoliumbromide
- HE:
-
Hematoxylin and eosin
- TPO:
-
Thrombopoietin
References
Alcon-LePoder S, Drouet MT, Roux P, Frenkiel MP, Arborio M, Durand-Schneider AM, Maurice M, Le Blanc I, Gruenberg J, Flamand M (2005) The secreted form of dengue virus nonstructural protein NS1 is endocytosed by hepatocytes and accumulates in late endosomes: implications for viral infectivity. J Virol 79:11403–11411
Aguiar M, Stollenwerk N (2018) Dengvaxia: age as surrogate for serostatus. Lancet Infect Dis 18:245
Aruoma OI, Colognato R, Fontana I, Gartlon J, Miglior L, Koike K, Coecke S, Lamy E, Mersch-Sundermann V, Laurenza I, Benzi L, Yoshino F, Kobayashi K, Lee MC (2006) Molecular effects of fermented papaya preparation on oxidative damage, MAP kinase activation and modulation of the benzo[a]pyrene mediated genotoxicity. Biofactors 26:147–159
Awe EO, Makinde JM, Adeloye OA, Banjoko SO (2009) Membrane stabilizing activity of Russelia equisetiformis, Schlecht and Chan. J Nat Prod 2:3–9
Azeredo de EL, Monteiro RQ, de-Oliveira Pinto LM (2015) Thrombocytopenia in dengue: interrelationship between virus and the imbalance between coagulation and fibrinolysis and inflammatory mediators. Mediat Inflamm 2015:313842
Baskaran C, Ratha BV, Velu S, Kumaran K (2012) The efficacy of Carica papaya leaf extract on some bacterial and a fungal strain by well diffusion method. Asian Pac J Trop Dis 2:S658–S662
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI (2013) The global distribution and burden of dengue. Nature 496:504–507
de Sauvage FJ, Hass PE, Spencer SD, Malloy BE, Gurney AL, Spencer SA, Darbonne WC, Henzel WJ, Wong SC, Kuang WJ et al (1994) Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature 369:533–538
Diamond MS, Roberts TG, Edgil D, Lu B, Ernst J, Harris E (2000) Modulation of dengue virus infection in human cells by alpha, beta, and gamma interferons. J Virol 74:4957–4966
Emeruwa AC (1982) Antibacterial substance from Carica papaya fruit extract. J Nat Prod 45:123–127
Guy B, Barrere B, Malinowski C, Saville M, Teyssou R, Lang J (2011) From research to phase III: preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine 29:7229–7241
Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martínez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW (2010) Dengue: a continuing global threat. Nat Rev Microbiol 8(12 Suppl):S7–S16
Halstead SB (1988) Pathogenesis of dengue: challenges to molecular biology. Science 239:476–481
Halstead SB (2017) Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine 35:6355–6358
Hasheen FM (2007) Antibacterial activity of Carica papaya extract. Oxford University Press, New York, pp 15–25
Henchal EA, Putnak JR (1990) The dengue viruses. Clin Microbiol Rev 3:376–396
Honda S, Saito M, Dimaano EM, Morales PA, Alonzo MT, Suarez LA, Koike N, Inoue S, Kumatori A, Matias RR, Natividad FF, Oishi K (2009) Increased phagocytosis of platelets from patients with secondary dengue virus infection by human macrophages. Am J Trop Med Hyg 80:841–845
Jassim SAA, Naji MA (2003) Novel antiviral agents: a medicinal plants perspective. J Appl Microbiol 95:412–427
Kho LK, Wulur H, Himawan T (1972) Blood and bone marrow changes in dengue haemorrhagic fever. Paediatr Indones 12:31–39
Khongphatthanayothin A, Mahayosnond A, Poovorawan Y (2013) Possible cause of liver failure in patient with dengue shock syndrome. Emerg Infect Dis 19:1161–1163
Latifah S, AbdKadir A, Yaakob H, Zulkifli RM (2013) Potential anti-dengue medicinal plants: a review. J Nat Med 67:677–689
Libraty D, Young PR, Pickering D, EndyTP Kalayanarooj S, Green S (2002) High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 186:1165–1168
Mehdipour S, Yasa N, Dehghan G, Khorasani R, Mohammadirad A, Rahimi R, Abdollahi M (2006) Antioxidant potentials of Iranian Carica papaya juice in vitro and in vivo are comparable to alpha-tocopherol. Phytother Res 20:591–594
Mishra KP, Sharma N, Diwaker D, Ganju L, Singh SB (2013) Plant derived antivirals: a potential source of drug development. J Virol Antivir Res 2:2
Noriko O, Nam HD, Emi K, Akira K, Sathoshi I, Chikao M (2010) Aqueous extract of Carica papaya leaves exhibit anti tumour activity and immunomodulatory effects. J Etnopharmacol 27:760–767
Ojha A, Nandi D, Batra H, Singhal R, Annarapu GK, Bhattacharyya S, Seth T, Dar L, Medigeshi GR, Vrati S, Vikram NK, Guchhait P (2017) Platelet activation determines the severity of thrombocytopenia in dengue infection. Sci Rep 7:41697
Parle M, Guruditta G (2011) Basketful benefits of papaya. Int Res J Pharm 2:6–12
Sathasivam K, Ramanathan S, Mansor SM, Haris MR, Wernsdorfer WH (2009) Thrombocyte counts in mice after the administration of papaya leaf suspension. Wien Klin Wochenschr 21(Suppl 3):19–22
Sharma N, Mishra KP, Ganju L (2016) Salidroside exhibits anti-dengue virus activity by upregulating host innate immune factors. Arch Virol 161:3331–3344
Silva JAT, Rashid Z, Nhut DT, Sivakumar D, Gera A, Souza MT, Tennant P (2007) Papaya (Carica papaya L.) biology and biotechnology. Tree For Sci Biotechnol 1:47–73
Subenthiran S, Choon CT, Cheong CK, Thayan R, Teck BM, Muniandy KP (2013) Carica papaya leaves juice significantly accelerates the rate of increase in platelet count among patients with dengue fever and dengue haemorrhagic fever. Evid Based Complement Altern Med 2013:616737
Sun DS, King CC, Huang HS, Shih YL, Lee CC, Tsai WJ, Yu CC, Chang HH (2007) Antiplatelet autoantibodies elicited by dengue virus non-structural protein 1 cause thrombocytopenia and mortality in mice. J Thromb Haemost 5:2291–2299
WHO (1997) Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. World Health Organization, Geneva
WHO (2009) Dengue: guidelines for diagnosis, treatment, prevention and control. World Health Organization, Geneva
Yunita F, Hanani E, Kristianto J (2012) The effect of Carica papaya L. leaves extract capsules on platelets count and hematocrit level in dengue fever patient. Int J Med Aromat Plants 2:573–578
Zandi K, Teoh BT, Sam SS, Wong PF, Mustafa MR, Abubakar S (2012) Novel antiviral activity of baicalein against dengue virus. BMC Complement Altern Med 12:214
Zunjar V, Dash RP, Jivrajani M, Trivedi B, Nivsarkar M (2016) Antithrombocytopenic activity of carpaine and alkaloidal extract of Carica papaya Linn. leaves in busulfan induced thrombocytopenic Wistar rats. J Ethnopharmacol 181:20–25
Acknowledgements
The authors thank the Defence Research & Development Organization (DRDO), Government of India, for financial support. NS thanks the Council of Scientific and Industrial Research for providing a fellowship in the form of a Junior and Senior Research Fellowship. She also thanks Dr. Sachin Kolte (Associate Professor, MD Pathology, Safdarjung Hospital, Delhi, India) in helping in analyzing histopathological slides of different organs of rats.
Funding
This study was funded by the Defence Research & Development Organization (DRDO) [DIP-264]. NS thanks the Council of Scientific and Industrial Research CSIR for providing a fellowship in the form of a Junior and Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. All animal protocols were approved by the DIPAS Institutional Animal Ethics Committee (IAEC/DIPAS/2015-25, 18/10/2015).
Additional information
Handling Editor: Victor Hugo Aquino.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, N., Mishra, K.P., Chanda, S. et al. Evaluation of anti-dengue activity of Carica papaya aqueous leaf extract and its role in platelet augmentation. Arch Virol 164, 1095–1110 (2019). https://doi.org/10.1007/s00705-019-04179-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-019-04179-z