Abstract
Australian bean common mosaic virus (BCMV) isolates were sequenced, and the sequences were compared to global BCMV and bean common mosaic necrosis virus (BCMNV) sequences and analysed for conserved potyviral motifs to generate in planta RNA-interference (RNAi) resistance. Thirty-nine out of 40 previously reported potyvirus motifs were conserved among all 77 BCMV/BCMNV sequences. Two RNAi target regions were selected for dsRNA construct design, covering 920 bp of the nuclease inclusion b (NIb) protein and 461 bp of the coat protein (CP). In silico prediction of the effectiveness of these constructs for broad-spectrum defence against the 77 BCMV and BCMNV sequences was done via analysis of putative 21-nucleotide (nt) and 22-nt small-interfering RNAs (siRNAs) generated from the target regions. The effectiveness of both constructs for siRNA generation and BCMV RNAi-mediated resistance was validated in Nicotiana benthamiana transient assays.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bean common mosaic virus (BCMV) and bean common mosaic necrosis virus (BCMNV) are closely related potyviruses that globally infect common bean (Phaseolus vulgaris) and a range of legumes [65]. As with potyviruses, BCMV and BCMNV are single-stranded positive-sense RNA viruses that form rod-shaped virions [65]. The genome is approximately 10 kb long and possesses a 3’-terminal poly(A) tail [6, 15]. It has an open reading frame (ORF) that is translated into a polyprotein that self-cleaves into 10 functional proteins: P1, helper component-protease (HC-Pro), P3, 6K1, cylindrical Inclusion (CI), 6K2, nuclease inclusion a (NIa)-viral genome-linked protein (VPg), NIa-Pro, nuclease inclusion b (NIb) and coat protein (CP) [50, 65]. An additional highly conserved potyvirus protein, Pretty Interesting Polyprotein ORF (PIPO), is produced through a frameshift within the P3 region and is involved in viral cell-to-cell movement [12]. The best characterised of these include HC-Pro, NIb and CP. HC-Pro is involved in polyprotein maturation, aphid-mediated virus transmission, and disruption of RNA silencing pathways [22, 65]. NIb is the viral RNA-dependent RNA polymerase responsible for genome replication [50, 65]. The CP is essential for virus assembly and is required for viral amplification, aphid-mediated transmission, and systemic and cell-to-cell movement [23, 51, 57, 61].
BCMV and BCMNV can be transmitted through seed or mechanical inoculation, or spread by aphids in a non-persistent manner, with the aphids retaining the virus on their stylets for a limited time [54, 58, 65]. Infection can result in yield losses as high as 100% [13, 31, 53, 59, 64]. BCMV and BCMNV have similar symptoms in common bean, including mosaic, leaf curling, dwarfing and chlorosis [16]. The most prominent difference between BCMV and BCMNV symptomology is observed in bean plants that contain the dominant I resistance gene [65]. In these plants, BCMV does not normally trigger black root disease, a hypersensitive systemic necrotic reaction that results in the death of the plant, except for two strains grown at temperatures above 30 °C (temperature-dependent) [25, 58]. However, all BCMNV strains trigger black root disease in these plants independently of temperature [58].
Disease management for BCMV currently relies on breeding for genetic resistance in bean varieties using the dominant I resistance gene. However, these cultivars become susceptible to lethal black root disease upon infection with BCMNV [25]. BCMNV is endemic throughout central and east Africa [55, 60], and this limits the benefits of bean varieties whose resistance to BCMV is provided by the I gene [65]. As a result, breeding efforts are focused on creating cultivars that include the dominant I gene and a pyramid of recessive genes linked to BCMV and BCMNV, which include bc-u, bc-1, bc12, bc-2, bc22 and bc-3 [25, 45, 46]. However, these recessive genes are virus-strain-specific, thus limiting the breeding of broad-scale BCMV- and BCMNV-resistant bean cultivars based on one of the recessive genes alone.
As an alternative, RNA-interference (RNAi)-mediated crop protection is emerging as a powerful tool to combat plant pests and diseases, including those caused by viruses [14, 19, 27, 33]. RNAi is conserved across the plant and animal kingdoms and is crucial for growth, development, and host defence against viruses and transposons [4, 5, 8, 11, 36]. In plants, the RNAi pathway is triggered by double-stranded RNA (dsRNA), which is processed into small-interfering RNA (siRNA) by DICER-like enzymes. The siRNA is then incorporated into a RNA-induced silencing complex, which degrades any complementary RNA [5]. When targeted against viral RNA, these siRNAs can therefore prevent virus replication and transmission. RNAi is most often utilized through genetic engineering, but to date, there are no known reports of RNAi-based transgenic resistance to BCMV or BCMNV. This is likely due to the recalcitrance of Phaseolus vulgaris to current transformation techniques, as well as regulatory issues surrounding GMO acceptance in many countries [28]. In 2011, the first GMO bean, known as Embrapa 5.1, targeted the AC1 viral gene of bean golden mosaic virus (BGMV) [7] and was approved for cultivation and consumption in Brazil [1].
There are currently no reports of BCMNV in Australia [65]; however, BCMV has caused a global epidemic and has been detected across Australia [42, 52, 53]. Despite this distribution, only one Australian BCMV isolate has been fully sequenced to date (GenBank accession no. EU761198, accessed January 2018). In 1992, two isolates of BCMV were collected from French bean displaying mosaic symptoms at the Australian Tropical Crops Genetic Resources Centre, Biloela, Queensland. This study represents the first report of the full genomic sequences of these Australian BCMV isolates, along with the design and validation of RNAi-mediated resistance to BCMV.
Materials and methods
BCMV sources and maintenance
Two Australian BCMV isolates (labelled 424 and 425) (family Potyviridae, genus Potyvirus, species Bean common mosaic virus) were obtained by the Department of Agriculture and Fisheries, Queensland, Australia, and collected from symptomatic P. vulgaris at the Australian Topical Crops Genetic Resources Centre, Biloela, Queensland, in 1992. Revival, maintenance, and propagation were conducted by mechanical sap inoculation (0.01 M phosphate buffer [pH 7.0] with carborundum [21]) onto Nicotiana benthamiana grown under glasshouse conditions (average temperature, 25° C with natural light). N. benthamiana infected with isolates 424 and 425 did not show symptoms, but the infection was confirmed using a PathoScreen BCMV-specific antigen-coated plate (ACP) enzyme-linked immunosorbent assay (ELISA) kit (Agdia). The Australian BCMV isolates 424 and 425 are herein designated BCMV 1 and BCMV 2, respectively.
BCMV 1 and BCMV 2 sequencing
Total RNA from BCMV-1- and BCMV-2-infected N. benthamiana was extracted using TRIzol Reagent (Life Technologies), and 1 µg was used as template for cDNA synthesis using either Invitrogen SuperScript III First-Strand Synthesis SuperMix (Life Technologies) or a SensiFast cDNA synthesis kit (BioLine) in accordance with the manufacturers’ instructions. Full-length viral sequences were determined using the primer-walking method [56]. PCR primers are listed in Supplementary Table 1. PCR amplification was conducted with either One Taq DNA Polymerase (New England Biolabs) or MyTaq DNA Polymerase (Bioline) as per the manufacturer’s instructions. The One Taq thermal cycling conditions were 94 °C for 3 min, followed by 30 cycles of 94 °C for 30 s, 45-68 °C for 30 s, and 68 °C for 1 min, with a final extension of 68 °C for 5 min. The My Taq thermal cycling conditions were 95 °C for 30 s, followed by 30 cycles of 95 °C for 15 s, 45-68 °C for 15 s, and 72 °C for 10 s with no final extension. PCR details for BCMV 1 and BCMV 2 are shown in Supplementary Fig. 1 and 2. PCR amplicons were visualised on a 1% TAE agarose gel and extracted using a QIAquick Gel Extraction Kit (QIAGEN). Purified bands were either sequenced directly or ligated into pGEM-T (Promega) and used to transform One Shot TOP10 Electrocomp Escherichia coli cells (Invitrogen) with blue/white selection. Plasmids were extracted using a QIAprep Spin Miniprep Kit (QIAGEN) and sequenced using M13 and SP6 primers. Sequencing was conducted in triplicate by capillary separation Sanger sequencing using the Australian Genome Research Facility (Brisbane, Australia).
A 5’ RACE System for Rapid Amplification of cDNA Ends V2.0 (Invitrogen) was used as per the manufacturer’s instructions with primer BCMV 68 as a gene-specific primer (Supplementary Table 1). A 3’ RACE System for Rapid Amplification of cDNA Ends (Invitrogen) was used as per the manufacturer’s instructions with primer BCMV 33 as a gene-specific primer (Supplementary Table 1). The 5’UTR and 3’UTR were sequenced a minimum of six times. The full-length genomic sequences of BCMV 1 and BCMV 2 were assembled from overlapping fragments using the Map to Reference tool in Geneious Pro R7 (Biomatters; default parameters) with the Australian BCMV full genomic sequence EU761198 as the reference, and they were then edited manually. Full genomic sequences were submitted to the GenBank database under accession numbers MH220847 and MH220846.
Bioinformatic analysis
Multiple alignments of the nucleotide (nt) and translated amino acid (aa) sequences of BCMV 1 and BCMV 2 with 62 available BCMV and 13 BCMNV genome sequences (GenBank, accessed January 2018) were made using the MUSCLE alignment tool in Geneious Pro R7 (default parameters; iterations set to 8). Phylogenetic analysis based on nt MUSCLE alignment was conducted using the Geneious Pro R7 tree builder using the Tamura-Nei genetic distance model and 100 bootstrap replicates). Three potato virus Y (PVY) sequences (KJ603225, HQ912911 and FJ214726) were included as the out-group.
The mean pairwise percent identity values for the alignments were calculated using a built-in feature of the Geneious program. All pairs of bases in the same column were given a score of one when identical and divided by the total number of pairs to give the average pairwise percent identity.
The conservation of potyvirus motifs identified in the literature (Table 2) was assessed within the aligned BCMV and BCMNV aa sequences. Based on the motif analysis and the degree of sequence conservation across all 77 sequences, two target regions, showing ≥84.0% average pairwise nucleotide sequence identity and the highest conserved motif abundance, were chosen for dsRNA construct design: a 920-bp portion of the NIb region (BCMV 1 nt position 7,639 – 8,558) and a 461-bp portion of the CP region (BCMV 1 nt position 9,312 – 9,772). Custom in-house scripts were used to identify all putative siRNA sequences of a set length (21 nt and 22 nt) that could be generated from the two dsRNA targets. The number of these siRNAs that exactly matched the reference genome sequence of every viral isolate at least once was then determined and used as an additional indicator to infer the effectiveness of the RNAi.
RNAi construct assembly and cloning
Double-stranded RNA constructs were designed to include the sense and antisense regions of the 461-bp CP dsRNA target or the 920-bp NIb dsRNA target (positions as above) separated by a 446-bp intron fragment corresponding to nt position 1757 to 2196 of the tobacco peroxidise gene (GenBank accession no. D11396.1). The resulting dsRNA constructs, BCMVNIb-dsRNA and BCMVCP-dsRNA, which were constructed using a pUC57 backbone with additional NcoI and NheI restriction sites flanking the dsRNA hairpin DNA, were obtained from GenScript, U.S.A. The pUC57/BCMVNIb-dsRNA and pUC57/BCMVCP-dsRNA constructs were used to transform One Shot TOP10 Electrocomp Escherichia coli cells (Thermo Fisher Scientific), and plasmid was extracted using a QIAprep Spin Miniprep Kit (QIAGEN) as per the manufacturers’ instructions.
The DNA fragments encoding the dsRNA hairpins were excised from the pUC57 backbone with NcoI and NheI (New England Biolabs) and resolved by gel electrophoresis on a 1% TAE agarose gel. The correct-sized band was gel purified and subcloned into an intermediate vector, L4440, and the construct was used to transform One Shot TOP10 Electrocomp E. coli cells (Thermo Fisher Scientific). Positive colonies were selected by blue/white section on AMP100 agar plates with ITPG and X-Gal and miniprepped as above. The BCMVNIb-dsRNA and BCMVCP-dsRNA fragments were then excised from L4440 with XbaI and KpnI (New England Biolabs), gel-purified, and subcloned into similarly digested and purified pART7 [17]. The pART7 plasmids were introduced into E. coli, selected and miniprepped as above. They were then digested with NotI (New England Biolabs), and the gel-purified insert containing the hairpin, 35S promoter and OCS terminator was cloned into the NotI site of the pART27 binary expression vector [17]. The pART27 plasmids were transformed, selected with SPEC50, miniprepped and checked via enzyme digestion. The resulting expression vectors (pART27/BCMVNIb-dsRNA and pART27/BCMVCP-dsRNA) were used to transform into electrocompetent Agrobacterium tumefaciens GV3101 as described previously [34]. A schematic map of the cloning vectors is shown in Supplementary Fig. 3. GV3101 pUQC214 for expression of GFP-dsRNA, was kindly provided by Bernard Carroll at The University of Queensland, Australia [10].
Agroinfiltration for siRNA generation and RNAi-mediated resistance
To detect siRNA generation from the dsRNA constructs in planta, N. benthamiana plants were infiltrated in duplicate with GV3101 blank (no vector), GV3101 pART27/BCMVNIb-dsRNA or GV3101 pART27/BCMVCP-dsRNA as described by Mann et al. [38]. Two leaf discs of 8 mm were collected from the agroinfiltrated leaves at three and seven days post-infiltration (only at three days for GV3101 blank). Total RNA was extracted using TRIzol Reagent (Life Technologies), and 60 µg was separated by 15% (wt/vol) denaturing urea polyacrylamide gel electrophoresis (PAGE), and transferred to a Hybond-N membrane (Roche) using a trans-blot SD semi-dry transfer unit (Bio-Rad) for northern blot analysis. Blots were probed at 37 °C overnight with digoxigenin (DIG)-labelled oligonucleotide probes (BCMV CP middle (F) and BCMV NIb middle (F), Supplementary Table 1). Blots were processed as per the manufacturer’s recommendations using a proprietary buffer set (Roche), and the DIG BCMVNIb-dsRNA or BCMVCP-dsRNA was detected by exposure to Super RX X-ray film (Fuji) using a chemiluminescent detection kit (Roche) as per the manufacturer’s instructions.
To assess conferral of RNAi-mediated protection against BCMV in planta, one leaf per plant was infiltrated with GV3101 pART27/BCMVNIb-dsRNA or GV3101 pART27/BCMVCP-dsRNA as described by Mann et al. [38], or with GV3101 pUQC214 (GFP-dsRNA)[10] as an off-target dsRNA control targeted to GFP. A fourth group, BCMV only, was used as the non-infiltrated infection control. Agroinfiltrated leaves and similarly positioned leaves of the plants of the BCMV-only group were challenged with 50 µL of BCMV sap inoculum three days post-infiltration. A 7-day postinfection, BCMV-specific ACP-ELISA was used as above to detect the presence of BCMV. Plants were considered infected when the absorbance reading at 405 nm was three times that of the negative control plants. All plants for agrobacterium experiments were grown and maintained in growth cabinets at 26 °C, 65% humidity and 16 h/8 day/night.
Results
Sequencing and phylogenetic analysis
The complete genome sequences of the two Australian isolates, BCMV 1 (GenBank accession no. MH220847) and BCMV 2 (GenBank accession no. MH220846), are 10,039 and 10,037 nt in length, respectively, excluding the 3’ poly(A) tail. Pairwise alignments of the BCMV 1 and BCMV 2 sequences showed 99.8% pairwise identity at the nucleotide level. As with other potyviruses, both isolates contain an open reading frame (ORF) encoding the 10 expected proteins (P1, HC-Pro, P3, 6K1, CI, 6K2, NIa-VPg, NIa-Pro, NIb and CP) flanked by a 5’ untranslated region (UTR) and a 3’UTR with another small ORF, PIPO, within the P3 cistron [43, 50].
A phylogenetic analysis of 62 BCMV and 13 BCMNV complete genome sequences from GenBank (accessed January 2018) revealed two primary groups separating as BCMV and BCMNV (Fig. 1). Less diversity was observed among the 13 BCMNV sequences than among the 64 BCMV sequences, with the majority of BCMNV sequences sampled from U.S.A. (eight out of nine reported sample locations) and one from East Timor. In the BCMV group, two major clades were delineated (cluster 1 and cluster 2). Both clusters could be broken down further into cluster1a, cluster1b, cluster2a and cluster 2b, as shown in Fig. 1. BCMV 1 and BCMV 2 grouped in Cluster 1a with the only other Australian isolate (EU761198) and 13 others collected from around the globe: U.S.A., Australia, India, Germany, South Korea and China. Also within this cluster, the NL1 and NL7 (Netherlands) and US1 and US10 (U.S.A.) strains can be observed. Cluster1b could be further broken into clusters 1b.1 and 1b.2. Cluster 1b.2 was mainly populated by isolates from one study conducted in China, while 1b.1 included isolates collected from China and South Korea as well as the peanut stripe strain (PSt). Cluster 2a was populated with isolates from China plus one blackeye cowpea mosaic strain (BICM). Cluster2b contained samples from Colombia and the U.S.A. The U.S.A. sequences were all RU1 strain (Russian) and the Colombian strain was the NL4 strain.
Phylogenetic tree of bean common mosaic virus (BCMV) and bean common mosaic necrosis virus (BCMNV) isolates. Sixty-two full-length genomic sequences are shown for BCMV, and 13 for BCMNV. The two Australian isolates sequenced in this study, BCMV 1 and BCMV 2, are shown in blue. Scale bar: 0.08 nucleotide substitutions per site. Analysis based on complete genome sequences available through GenBank (January 2018) and outgroup potato virus Y (PVY) as shown in red. Countries where samples were known to have been collected are listed
The mean pairwise percent identity of the BCMV 1 and BCMV 2 nt and aa sequences to all other complete GenBank BCMV and BCMNV sequences is shown in Supplementary Table 2. Looking at the pairwise percent identity between the full genome and mature peptide regions of all 64 BCMV sequences, the mean nt sequence identity was 87.0% across the full genome, and it ranged from 79.2% to 92.0% in the individual protein coding regions. The amino acid sequence conservation in each of the peptide regions ranged from 75.5% to 97.6% (Table 1). The 13 BCMNV sequences showed higher overall sequence conservation, with 88.9% mean nt sequence identity across the full genome, ranging from 92.2% to 98.5% in the mature peptide regions. Amino acid sequence conservation ranged from 91.4% to 99.3% in the peptide regions (Table 1). When all 77 sequences were compared, the mean nt sequence identity dropped to 80.8% for the full genome and 68.4% to 85.2% for the mature peptide regions. The amino acid sequence conservation was 61.6% to 91.3% across all sequences (Table 1). In nt sequence comparisons of BCMV only, BCMNV only, and both BCMV and BCMNV, the P1 mature peptide region showed the highest variability, while the CP showed the highest conservation (Table 1). All other peptide regions showed similar pairwise nt sequence identity values. These results suggest that P1 would not be a suitable RNAi target, CP should make a strong RNAi target, and the other protein regions require further analysis.
Potyvirus motif analysis
To design effective RNAi constructs against BCMV, conserved potyvirus motifs, suggesting functional conservation, were searched for within BCMV 1 and BCMV 2 and across the 62 BCMV and 13 BCMNV GenBank sequences (Table 2). BCMV and BCMNV sequences showed high conservation of potyviral motifs, and most were found within the HC-Pro, CI, NIb and CP regions. Thirty-nine out of 40 conserved potyvirus motifs were found in BCMV 1 and BCMV 2, which were exactly conserved in both sequence and position (Table 2).
As expected based on the pairwise comparisons, the P1 protein showed the highest variability (Table 2). Briefly, two P1 motifs, IXFG and FLXG, were found in 51 and 60 BCMV sequences and 12 and 10 BCMNV sequences, respectively. The GXSG motif required for protease activity was completely conserved in all sequences. Another P1 motif involved in protease activity, FIVRG/FMIIRG, showed a clear difference between BCMV and BCMNV sequences. The BCMV sequences showed high conservation for the FMIIRG motif, while BCMNV maintained the more common FIVRG motif [9, 47]. The HX8DX32SGX22RG motif was found in all BCMV 1 and BCMV 2 sequences, with changes to the ‘X’ aa spacer length [9, 47]. It was also observed that all BCMNV sequences contained a D-to-S substitution. These results highlight that the region encoding the mature P1 peptide would most likely be the least efficient region to target for RNAi.
Within HC-Pro, seven motifs were completely conserved in all 62 BCMV and 13 BCMNV sequences; CX8CX18CX2C (putative zinc finger binding motif) [32], KLSC (aphid transmission – known as KITC in other potyviruses) [65], FRNKX12CDNQLD (symptomatology) [3], CCCVT (long distance movement) [43], PTK (aphid transmission) [65], GYCY (cysteine proteinases) [9], and CX72H (protease activity) [32] (Table 2). The HAKRFF motif, which is possibly involved in cell-to-cell movement [37], was found in all sequences, including BCMV 1 and BCMV 2, as HSKRFF. The motifs NIFLAML and AELPRILVDH [37] were found in the HC-Pro region of all 13 BCMNV sequences and 61 BCMV sequences (NVFLAML in KP903372 and ADLPRILVDH in GQ219793). The LXKA motif, which is known for silencing suppressor activity [63], was found in 11 BCMNV sequences, while all 62 BCMV sequences contained a change to LXLA. The less-conserved motif IGN, which is involved in cell-to-cell and long-distance movement [30], was not found in any BCMV 1 or BCMV 2 sequences or in any BCMV or BCMNV sequences from GenBank.
In the P3 protein, the EPYX7SPX2LXAX2NXGX2EX5N motif [30] was found in a derivative form, DPYX7SPX2LXHXRXRX2EX5W [9], in BCMV 1 and BCMV 2 (Table 2). The derivative form was also found in 61 BCMV sequences (DPYX7SPX2SXHXRXRX2EX5W in KC478389), and in all 13 BCMNV sequences, it was DPYX7SPX2LXHXRXKX2EX5W. Within the CI protein, four motifs involved in helicase activity were completely conserved across the BCMV and BCMNV sequences: DEXH, KVSATPP, LVYV and VATNIIENGVTL [18] (Table 2). Another motif involved in helicase activity, GERIQRLGRVGR, was found in all but one of the BCMNV sequences (GERIQRPGRVGR in AY864314). The VLLLEPTRPL motif, also involved in helicase activity, was observed in all 13 BCMNV sequences. BCMV 1 and BCMV 2 and all BCMV sequences except one retained the motif as VLLCERTRPL (VLLCGRTRPL in KC832502). Still within the CI protein, the GAVGASGKST motif, which is involved in NTP binding [29], was observed in all BCMNV sequences and in 60 BCMV sequences (GAVDSGKST in KC83501 and GAGGCGKKS in KC832502). In the NIa-Pro region, HX34DX67GXCG [29] was completely conserved across all sequences (Table 2).
Within the NIb protein, five motifs were conserved in all 62 BCMV and 13 BCMNV sequences from GenBank: SLKAEL (RNA polymerase activity) [43], CVDDFN [66], CHADGS (RNA-dependant polymerase activity) [43], GDD (RNA-dependant polymerase activity) [43], and [A/S]M[I/V]E[S/A]WG as AMIEAWG [66] (Table 2). The GNNSGQPSTVVDNTLMV motif, which is associated with RNA polymerase activity [43], was found in all but one sequence (KC832501 – GNNSGQPSTVVDNTLVV). The FTAAP[L/I][D/E] motif [66]was found in all BCMV sequences, including BCMV 1 and BCMV 2, as FTAAPMD. All 13 BCMNV sequences contained the standard motif FTAAPID.
Within the CP, a high degree of motif conservation across the BCMV and BCMNV sequences was observed except for one BCMV sequence, KT175568. BCMV KT175568 contained only two out of the seven highly conserved potyvirus motifs (Table 2). One of these two motifs, the W[V/T]MMDG[D/E/N] motif [66] was observed in all sequences, and the second motif, QMKAAA [29], was found in all 62 BCMV and 10 BCMNV sequences (QMKAAR in AY138897, NC_004047 and U19287). When excluding this outlier, three motifs were found in all GenBank sequences: MVWCI[E/D]NGTSP as MVWCIDNGTSP [29], AFDF [29] and E[N/D]TERH as ENTERH [66]. The [P/R/A]YMPRYG motif [66] was observed as PYMPRYG in all 61 BMCV sequences (KT175568 outlier as PYMLRYG); however, all 13 BCMNV sequences contained the motif FYMPRYG.
The DAG motif, which is associated with aphid transmission [65], was found in 59 BCMV sequences and 11 BCMNV sequences (Table 2). The three BCMV sequences without the DAG motif (AY112735, KT175568 and KF919299) were substituted to DAS, suggesting these three isolates had lost their aphid transmissibility [35]. The two BCMNV sequences (HG792063 and HQ229993) were converted to NAG, also suggesting a loss of aphid transmissibility [24].
RNAi construct design
The 39 conserved potyvirus motifs were used as guide to design dsRNA constructs to target conserved regions of the viral genome encoding critical functions that are less likely than other regions to mutate over time (Fig. 2a). These regions are hypothesised to offer the best chance for broad-spectrum dsRNA-mediated protection from BCMV and related potyviruses due to their facultative functional importance, and thus sequence conservation. Using a minimum pairwise sequence identity value of 84%, NIb and CP were found to be the best RNAi target regions (Table 1). A 920-bp region from NIb and a 461-bp region from CP were selected for further analysis. Within the 920-bp dsRNA NIb target region, all NIb motifs listed in Table 2 were included (Fig. 2b). Within the 461-bp dsRNA CP target region, all CP motifs included in Table 2 aside from the DAG motif were included (Fig. 2c). All 64 BCMV and 13 BCMNV sequences were aligned to the NIb and CP target regions, with 85.1% and 91.0 % average pairwise nt sequence identity, respectively (Supplementary Fig. 4 and 5).
Schematic diagram indicating the motif positions in a) the full length of the Australian BCMV isolates, b) the zoomed-in view of the NIb mature peptide region, and c) the CP mature peptide region. Green bars indicate mature peptides, purple bars indicate the 39 conserved motifs (motifs containing ‘X’ aa spacers have multiple purple bars placed only on the conserved aa), and orange bars indicate the dsRNA target areas used to design two highly conserved dsRNA constructs for RNAi-mediated protection against BCMV
The complete set of 21-nt and 22-nt siRNAs that could potentially be generated from each NIb and CP dsRNA targeted region was predicted. The conservation of these 21-nt and 22-nt siRNAs across all BCMV and BCMNV sequences was then used to determine the degree to which global BCMV and BCMNV isolates could be targeted. Double-stranded-RNA-derived 21- and 22-nt siRNAs that exactly matched each viral sequence were identified (Fig. 3). Within BCMV 1, 900 and 899 putative 21-nt and 22-nt siRNAs, respectively, were generated from the 920-bp NIb dsRNA target, and 816 and 811 putative 21-nt and 22-nt siRNAs, respectively, were generated within BCMV 2. In both BCMV 1 and BCMV 2, 438 and 437 putative 21-nt and 22-nt siRNAs, respectively, were generated from the 461-bp CP dsRNA target. In sequences closely related to BCMV 1 and BCMV 2 (KU896809-KP903372, Fig. 1), the number of conserved putative 21-nt and 22-nt siRNAs for the NIb dsRNA target ranged from 103 to 816 and 96 to 811, respectively, across all cluster 1a isolates. Meanwhile, the number of conserved 21-nt and 22-nt sequences in the CP dsRNA target ranged from 97 to 399 and 89 to 396, respectively (Fig. 3, BCMV cluster 1a).
Number of potential siRNA hits generated from the designed dsRNA targeted regions, NIb (920 bp) and CP (461 bp) against the individual sequences corresponding to the 62 BCMV and 13 BCMNV sequences from GenBank (accessed January 2018). Panel a represents the number of 21-nt matches. Panel b represents the number of 22-nt matches. The lower panel shows the phylogenetic cluster. Tabulated data are available in Supplementary Table 3
Within the analysis of the conserved 21-nt and 22-nt sequences in phylogenetic cluster 2 (KF919300-KC832502, Fig. 1), a difference between the two dsRNA targets can be observed. The number of potential 21-nt and 22-nt hits for the NIb dsRNA target ranged from 18 to 173 and 15 to 163, respectively, while the CP dsRNA target ranged from 126 to 385 and 118 to 382, respectively (Fig. 3, BCMV cluster 2). Similar results were also observed for the potential siRNA generated in the BCMV sequences in cluster 1b (KM051428-AY968604, Fig. 1), where the NIb dsRNA target ranged from 27 to 81 and 24 to 74 conserved putative 21-nt and 22-nt siRNAs, respectively, while the CP dsRNA target ranged from 75 to 201 and 68 to 193, respectively (Fig. 3, BCMV cluster 1b). Within the BCMNV sequences, which show less sequence conservation than BCMV, a clear difference between the two dsRNA targets can again be observed. The NIb dsRNA target has very poor coverage, only hitting one sequence a minimum of 21 nt and 22 nt (three and two, respectively), while the CP dsRNA target could potentially hit all BCMNV sequences ranging from 18 to 37 and 15 to 34, respectively (Fig. 3, BCMNV cluster). As such, the NIb dsRNA target will likely not work against the current BCMNV sequences, while the CP dsRNA target has a small range of potential siRNAs generated against all BCMNV sequences. This analysis of potential siRNA generation from the two constructs suggests that although the NIb dsRNA target is larger, the CP dsRNA target may provide RNAi-mediated resistance to a wide range of BCMV strains, with the potential to broadly silence BCMNV as well.
RNAi-mediated protection against BCMV
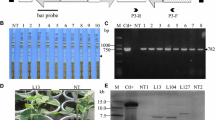
The designed dsRNA-targeted regions were used to make the dsRNA constructs BCMVNIb-dsRNA and BCMVCP-dsRNA for in planta experiments. BCMVNIb-dsRNA and BCMVCP-dsRNA were transiently expressed in Nicotiana benthamiana via agroinfiltration and tested for siRNA production and RNAi-mediated protection against BCMV within the plant system. The 920-bp NIb and 461-bp CP target regions were synthesized as dsRNA hairpins containing the 446-bp tobacco protease intron (GenBank accession D11396.1) as the hairpin loop. To test for siRNA generation, agrobacteria expressing BCMVNIb-dsRNA were infiltrated into N. benthamiana plants, and total RNA was collected at three and seven days post-infiltration and subjected to northern blot analysis. Analysis of the total RNA probed with the BCMVNIb-middle DIG-labelled oligonucleotide probe confirmed typical siRNA generation of 21 and 22 nt size at both time points (Fig. 4a). Analysis of BCMVCP-dsRNA probed with BCMVCP-middle DIG-labelled oligonucleotide probe also revealed typical 21 nt and 22 nt siRNA generation (Fig. 4b). Agrobacteria with a blank pART27 construct (no dsRNA) lacking siRNA homologous to the BCMV-specific oligonucleotide probes, were used as a negative control (Fig. 4).
Small interfering RNA (siRNA) generation of BCMVNIb-dsRNA (920 bp) and BCMVCP-dsRNA (461 bp) in agroinfiltrated Nicotiana benthamiana. N. benthamiana plants were agroinfiltrated with a) GV3101/pART27BCMVNIb-dsRNA (n = 2) and b) GV3101/pART27BCMVCP-dsRNA (n = 2). Total RNA from the infiltrated tissue was collected at 3 and 7 days post-agroinfiltration. Northern blot analysis of total RNA resolved on a PAGE gel and probed with the BCMV NIb middle or BCMV CP middle DIG-labelled oligonucleotide probe shows that 21- and 22-nt siRNA can be observed at both time points in BCMVNIb-dsRNA and BCMVCP-dsRNA samples. Plants agroinfiltrated with GV3101 pART27/blank were used as negative controls. The lower panel shows equal loading of RNA
N. benthamiana plants were agroinfiltrated with the BCMV-specific dsRNA and challenged with BCMV 2 to examine the efficiency of RNAi-mediated resistance. Over three trials, plants were agroinfiltrated with either GFP-dsRNA (nonspecific dsRNA) or BCMVNIb-dsRNA and BCMVCP-dsRNA, or left untouched (BCMV only group). Three days after agroinfiltration, all plants were challenged with BCMV 2 on the infiltrated leaf, or, in the BCMV-only group, a similar leaf. An antigen-coated plate (ACP) enzyme-linked immunosorbent assay (ELISA) was used to detect the presence of BCMV in the infiltrated tissue seven days after viral inoculation. Plants that were agroinfiltrated with BCMVNIb-dsRNA and BCMVCP-dsRNA showed protection against BCMV 2, as overall, only 15% and 8% of plants were infected, respectively (Table 3). Both the BCMV-only and GFP-dsRNA plants showed high infection rates of 92% and 100%, respectively. The lack of RNAi-mediated protection in the nonspecific dsRNA control GFP-dsRNA demonstrates that homology to the targeted virus is required to generate RNAi-mediated protection.
Discussion
In this study, we present complete genome sequences for two new Australian BCMV isolates. Previously, only one Australian BCMV isolate had been fully sequenced (GenBank accession no. EU761198, accessed January 2018) even though BCMV has been detected across Australia [42, 52, 53]. The nt and aa sequences of these two Australian BCMV sequences were compared to those of 62 BCMV and 13 full genomic BCMNV sequences obtained from GenBank (accessed January 2018). Analysis of 40 highly conserved potyvirus motifs within BCMV and BCMNV sequences was used to design two dsRNA targets (BCMVNIb-dsRNA and BCMVCP-dsRNA). Using a custom in-house script, the potential 21-nt and 22-nt siRNA hits produced by the designed dsRNA targets against individual BCMV and BCMNV sequences in the GenBank database were calculated. The scoring of potential 21-nt and 22-nt siRNA hits revealed that the BCMVNIb-dsRNA target might not be effective for BCMNV RNAi-mediated protection (Fig. 3). To target BCMNV effectively, new targets should be considered. Northern blot analysis of siRNA generation for both dsRNA targets showed typical siRNA generation (Fig. 4). Mechanical inoculation studies demonstrated that BCMVNIb-dsRNA and BCMVCP-dsRNA were able to protect plants from BCMV infection (Table 3).
Development of a successful dsRNA target for BCMV paves a new direction for plant protection away from current protective methods that rely on genetic BCMV resistance [25]. With the development of Embrapa 5.1, a genetically engineered bean resistant to BGMV, technology exists to create GMOs resistant to BCMV. However, the development of Embrapa 5.1, was not cost- or time-efficient, as only one plant exhibited resistance to the virus from 18 putative transgenic lines consisting of 2,706 plants [7]. Recently, a novel method for delivering dsRNA has emerged as an attractive alternative to transgenic RNAi plants [41]. Mitter et al. [41] were able to topically apply dsRNA loaded onto designer, non-toxic, degradable layered double hydroxide clay nanoparticles, called BioClay, to protect tobacco plants from cucumber mosaic virus for at least 20 days after viral challenge in sprayed and newly emerged unsprayed leaves. This sustainable and easy-to-adopt technology could be translated with BCMV-specific dsRNA to afford crop protection.
References
International Service for the Acquisition of Agri-Biotech Applications (ISAAA) (2018) Bean (Phaseolus vulgaris) GM Events
Aleman-Verdaguer M-E, Goudou-Urbino C, Dubern J, Beachy RN, Fauquet C (1997) Analysis of the sequence diversity of the P1, HC, P3, NIb and CP genomic regions of several yam mosaic potyvirus isolates: implications for the intraspecies molecular diversity of potyviruses. J Gen Virol 78:1253–1264
Anuradha C, Balasubramanian V, Selvarajan R (2015) Sequence motif comparison and homology modeling of helper component proteinase (HC–Pro) of banana bract mosaic virus.
Bartel D (2004) MicroRNAs: genomics, biogensis, mechanism and function. Cell 116:281–297
Baulcombe D (2004) RNA silencing in plants. Nature 431:356–363
Bhadramurthy V, Bhat A (2009) Biological and molecular characterization of Bean common mosaic virus associated with vanilla in India. Indian J Virol 20:70–77
Bonfim K, Faria JC, Nogueira EO, Mendes ÉA, Aragão FJ (2007) RNAi-mediated resistance to Bean golden mosaic virus in genetically engineered common bean (Phaseolus vulgaris). Mol Plant-microbe Interact 20:717–726
Borges F, Martienssen RA (2015) The expanding world of small RNAs in plants. Nat Rev Mol Cell Biol 16:727
Bravo E, Calvert LA, Morales FJ (2008) The complete nucleotide sequence of the genomic RNA of bean common mosaic virus strain nl4. Rev Acad Colomb Cienc Exactas 32:37–46
Brosnan C, Mitter N, Christie M, Smith N, Waterhouse P, Carroll B (2007) Nuclear gene silencing directs reception of long-distance mRNA silencing in Arabidopsis. Proc Natl Acad Sci 104:14741–14746
Burand JP, Hunter WB (2013) RNAi: Future in insect management. J Invertebr Pathol 112:S68–S74
Cheng G, Dong M, Xu Q, Peng L, Yang Z, Wei T, Xu J (2017) Dissecting the molecular mechanism of the subcellular localization and cell-to-cell movement of the sugarcane mosaic virus P3N-PIPO. Sci Rep 7:9868
Damayanti T, Susilo D, Nurlaelah S, Sartiami D, Okuno T, Mise K (2008) First report of Bean common mosaic virus in yam bean [Pachyrhizus erosus (L.) Urban] in Indonesia. J Gen Plant Pathol 74:438–442
Duan C-G, Wang C-H, Guo H-S (2012) Application of RNA silencing to plant disease resistance. Silence 3:5
El-Sawy MA, Mohamed HAE, Elsharkawy MM (2013) Serological and molecular characterisations of the Egyptian isolate of Bean common mosaic virus. Arch Phytopathol Plant Protect 47:1–13
Flores-Estévez N, Acosta-Gallegos J, Silva-Rosales L (2003) Bean common mosaic virus and Bean common mosaic necrosis virus in Mexico. Plant disease 87:21–25
Gleave AP (1992) A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 20:1203–1207
Gong D, Wang J-H, Lin Z-S, Zhang S-Y, Zhang Y-L, Yu N-T, Xiong Z, Liu Z-X (2011) Genomic sequencing and analysis of Chilli ringspot virus, a novel potyvirus. Virus Genes 43:439–444
Gordon KH, Waterhouse PM (2007) RNAi for insect-proof plants. Nat Biotechnol 25:1231–1232
Guyatt K, Proll D, Menssen A, Davidson A (1996) The complete nucleotide sequence of bean yellow mosaic potyvirus RNA. Arch Virol 141:1231–1246
Hamid A, Ahmad M, Padder B, Shah M, Saleem S, Sofi T, Mir A (2013) Pathogenic and coat protein characterization confirming the occurrence of Bean common mosaic virus on common bean (Phaseolus vulgaris) in Kashmir, India. Phytoparasitica 42:1–6
Ivanov K, Eskelin K, Lõhmus A, Mäkinen K (2014) Molecular and cellular mechanisms underlying potyvirus infection. J Gen Virol 95:1415–1429
Ivanov KI, Mäkinen K (2012) Coat proteins, host factors and plant viral replication. Curr Opin Virol 2:712–718
Kamenova I, Lohuis H, Peters D (2002) Loss of aphid transmissibility of plum pox virus isolates. Biotechnol Biotechnol Equip 16:48–54
Kelly J, Afanador L, Haley S (1995) Pyramiding genes for resistance to Bean common mosaic virus. Euphytica 82:207–212
Knierim D, Menzel W, Winter S (2017) Analysis of the complete genome sequence of euphorbia ringspot virus, an atypical member of the genus Potyvirus. Arch Virol 162:291–293
Koch A, Biedenkopf D, Furch A, Weber L, Rossbach O, Abdellatef E, Linicus L, Johannsmeier J, Jelonek L, Goesmann A (2016) An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog 12:e1005901
Kwapata K, Nguyen T, Sticklen M (2012) Genetic transformation of common bean (Phaseolus vulgaris L.) with the gus color marker, the bar herbicide resistance, and the barley (Hordeum vulgare) HVA1 drought tolerance genes. Int J Agron 2012:1–8
Li F, Xu D, Abad J, Li R (2012) Phylogenetic relationships of closely related potyviruses infecting sweet potato determined by genomic characterization of Sweet potato virus G and Sweet potato virus 2. Virus Genes 45:118–125
Li Y, Jia A, Qiao Y, Xiang J, Zhang Y, Wang W (2017) Virome analysis of lily plants reveals a new potyvirus. Arch Virol 163:1–4
Li YQ, Liu ZP, Yang YS, Zhao B, Fan ZF, Wan P (2014) First report of Bean common mosaic virus infecting Azuki bean (Vigna angularis Ohwi & Ohashi) in China. Plant Disease 98:1017
Liang WX, Song LM, Tian GZ, Li HF, Fan ZF (2006) The genomic sequence of Wisteria vein mosaic virus and its similarities with other potyviruses. Arch Virol 151:2311–2319
Lilley C, Davies L, Urwin P (2012) RNA interference in plant parasitic nematodes: a summary of the current status. Parasitology 139:630–640
Lin J-J (1995) Electrotransformation of Agrobacterium. 171–178
Lopez-Moya J, Wang R, Pirone T (1999) Context of the coat protein DAG motif affects potyvirus transmissibility by aphids. J Gen Virol 80:3281–3288
Maillard P, Ciaudo C, Marchais A, Li Y, Jay F, Ding S, Voinnet O (2013) Antiviral RNA interference in mammalian cells. Science 342:235–238
Mangrauthia SK, Jain R, Praveen S (2008) Sequence motifs comparisons establish a functional portrait of a multifunctional protein HC-Pro from papaya ringspot potyvirus. J Plant Biochem Biotechnol 17:201–204
Mann KS, Johnson KN, Dietzgen RG (2015) Cytorhabdovirus phosphoprotein shows RNA silencing suppressor activity in plants, but not in insect cells. Virology 476:413–418
Maoka T, Hataya T (2005) The complete nucleotide sequence and biotype variability of Papaya leaf distortion mosaic virus. Phytopathology 95:128–135
Mishra R, Verma RK, Gaur RK (2015) Analysis of genome comparison of two Indian isolates of Cowpea aphid-borne mosaic virus from India. Virus Genes 51:306–309
Mitter N, Worrall EA, Robinson KE, Li P, Jain RG, Taochy C, Fletcher SJ, Carroll BJ, Lu G, Xu ZP (2017) Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat Plants 3:16207
Moghal S, Francki R (1976) Towards a system for the identification and classification of potyviruses: I. Serology and amino acid composition of six distinct viruses. Virology 73:350–362
Moradi Z, Mehrvar M, Nazifi E, Zakiaghl M (2017) Iranian johnsongrass mosaic virus: the complete genome sequence, molecular and biological characterization, and comparison of coat protein gene sequences. Virus Genes 53:77–88
Moura MF, Marubayashi JM, Mituti T, Gioria R, Kobori RF, Pavan MA, Krause-Sakate R (2012) Comparative analysis of coding region for the coat protein of PepYMV and PVY isolates collected in sweetpepper. Summa Phytopathol 38:93–96
Mukeshimana G, Paneda A, Rodríguez-Suárez C, Ferreira JJ, Giraldez R, Kelly JD (2005) Markers linked to the bc-3 gene conditioning resistance to bean common mosaic potyviruses in common bean. Euphytica 144:291–299
Pasev G, Kostova D, Sofkova S (2014) Identification of genes for resistance to Bean common mosaic virus and Bean common mosaic necrosis virus in snap bean (Phaseolus vulgaris L.) breeding lines ssing conventional and molecular methods. J Phytopathol 162:19–25
Perotto MC, Pozzi EA, Celli MG, Luciani CE, Mitidieri MS, Conci VC (2018) Identification and characterization of a new potyvirus infecting cucurbits. Arch Virol 163:719–724
Puli’uvea C, Khan S, Chang W-L, Valmonte G, Pearson MN, Higgins CM (2017) First complete genome sequence of vanilla mosaic strain of Dasheen mosaic virus isolated from the Cook Islands. Arch Virol 162:591–595
Rajamäki M, Merits A, Rabenstein F, Andrejeva J, Paulin L, Kekarainen T, Kreuze J, Forster R, Valkonen J (1998) Biological, serological, and molecular differences among isolates of potato A potyvirus. Phytopathology 88:311–321
Revers F, Garcia J (2015) Molecular biology of potyviruses. Adv Virus Res 92:101–199
Rojas MR, Zerbini FM, Allison RF, Gilbertson RL, Lucas WJ (1997) Capsid protein and helper component-proteinase function as potyvirus cell-to-cell movement proteins. Virology 237:283–295
Saqib M, Jones RAC, Cayford B, Jones MGK (2005) First report of Bean common mosaic virus in Western Australia. Plant Pathol 54:563
Saqib M, Nouri S, Cayford B, Jones RAC, Jones MGK (2010) Genome sequences and phylogenetic placement of two isolates of Bean common mosaic virus from Macroptilium atropurpureum in north-west Australia. Aust Plant Pathol Soc 39:184–191
Sastry KS (2013) Mechanism of seed transmission. Seed-borne plant virus diseases. Springer, Heidelberg, pp 85–100
Sengooba TN, Spence NJ, Walkey DGA, Allen DJ, Femi Lana A (1997) The occurrence of Bean common mosaic necrosis virus in wild and forage legumes in Uganda. Plant Pathol 46:95–103
Shapter FM, Waters DL Genome walking T cereal genomics, pp 133–146
Shukla DD, Frcnkel M, Ward CW (1991) Structure and function of the potyvirus genome with special reference to the coat protein coding region. Can J Plant Pathol 13:178–191
Silbernagel MJ, Mink GI, Zhao RL, Zheng GY (2001) Phenotypic recombination between bean common mosaic and bean common mosaic necrosis potyviruses in vivo. Arch Virol 146:1007–1020
Singh SP, Schwartz HF (2010) Breeding common bean for resistance to diseases: a review. Crop Sci 50:2199–2223
Spence N, Walkey D (1995) Variation for pathogenicity among isolates of Bean common mosaic virus in Africa and a reinterpretation of the genetic relationship between cultivars of Phaseolus vulgaris and pathotypes of BCMV. Plant Pathol 44:527–546
Urchqui-Inchima S, Haenni A-L, Bernardi F (2001) Potyvirus proteins: a wealth of functions. Virus Res 74:157–175
Valli A, López-Moya JJ, García JA (2007) Recombination and gene duplication in the evolutionary diversification of P1 proteins in the family Potyviridae. J Gen Virol 88:1016–1028
Velasco L, Salem N, Willemsen A, Lapidot M, Mansour AN, Rubio L, Galipienso L (2016) Genetic variation and evolutionary forces shaping Cucumber vein yellowing virus populations: risk of emergence of virulent isolates in Europe. Plant Pathol 65:847–856
Verma P, Gupta U (2010) Immunological detection of Bean common mosaic virus in French bean (Phaseolus vulgaris L.) leaves. Indian j Microbiol 50:263–265
Worrall EA, Wamonje FO, Mukeshimana G, Harvey JJ, Carr JP, Mitter N (2015) Bean common mosaic virus and Bean common mosaic necrosis virus: relationships, biology, and prospects for control. Adv Virus Res 93:1–46
Zheng L, Wayper PJ, Gibbs AJ, Fourment M, Rodoni BC, Gibbs MJ (2008) Accumulating variation at conserved sites in potyvirus genomes is driven by species discovery and affects degenerate primer design. PLoS One 3:e1586
Acknowledgments
The authors would like to thank Queensland Alliance of Agriculture and Food Innovation (QAAFI), Australia and the University of Queensland, Australia, for their support.
Funding
This study was funded by the Accelerated Partnership Grant, Queensland Government (2014000652), awarded to N.M. with Nufarm Australia Limited as the industry partner. E.A.W. PhD programme with N.M. is supported by a scholarship from the University of Queensland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Data availability
The datasets generated and/or analysed during the current study are available in the NCBI GenBank repository, https://www.ncbi.nlm.nih.gov. Only complete genomes of BCMV and BCMNV were used.
Additional information
Handling Editor: F. Murilo Zerbini.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Worrall, E.A., Hayward, A.C., Fletcher, S.J. et al. Molecular characterization and analysis of conserved potyviral motifs in bean common mosaic virus (BCMV) for RNAi-mediated protection. Arch Virol 164, 181–194 (2019). https://doi.org/10.1007/s00705-018-4065-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-4065-6