Abstract
Autophagy is an intrinsic cellular process that can degrade cytoplasmic components. It has been reported that several pathogens hijack this process to facilitate their replication. Coxsackievirus B3 (CVB3), a member of the family Picornaviridae, induces autophagy upon infection. However, the details of CVB3-induced autophagy remain a subject of debate. This study applied a combination of multiple assays for the measurement of autophagy and demonstrated that CVB3 induces a complete autophagic flux. Experiments with infected HEK293A cells revealed that autophagosomes were induced upon CVB3 infection. Most of these autophagosomes were mCherry positive in mCherry-GFP-LC3 cells. Conversely, mCherry-positive autophagosomes were rescued to green positive when treated with the acidification inhibitors chloroquine (CQ) and bafilomycin A1 (BAF), suggesting that autophagosomes fused with late endosomes or lysosomes. The co-localization of LC3-positive puncta with lysosome-associated membrane protein 1 (LAMP1) or LysoTracker confirmed that the autophagosomes fused primarily with lysosomes. Interestingly, the disruption of autophagosome formation by 3-methyladenine (3-MA) or ATG5 siRNA treatment during viral infection significantly decreased CVB3 replication. However, inhibitors of lysosomal acidification, fusion, or degradation did not affect viral replication. Therefore, autolysosomes may not be critical for viral replication in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coxsackievirus B3 (CVB3), which belongs to the family Picornaviridae, is a non-enveloped single-stranded RNA virus with a genome of approximately 7,400 nt in length [12]. It is considered a common infectious pathogen that induces viral myocarditis, which often progresses into chronic myocarditis and even dilated cardiomyopathy [3, 17, 27]. During CVB3 infection, viral particles enter cells via the coxsackievirus and adenovirus receptor. The uncoated genomic RNA functions as a template for genome replication and protein synthesis. The single polyprotein translated from the genomic RNA is further processed by viral protease into viral capsid and nonstructural proteins for virion packaging [8, 16, 18]. Effective preventive vaccines against CVB3 have not yet been developed. Thus, the mechanisms of host–virus interplay during infection must be clarified to facilitate the development of efficient therapeutic strategies to control CVB3 infection effectively.
Autophagy, a highly conserved protein degradation system in eukaryotic cells, is a homeostatic process that controls the quantity and quality of cytoplasmic biomass [14, 22]. This process involves the formation of a double lipid bilayer membrane structure known as an autophagosome, which engulfs long-lived cytoplasmic macromolecules and damaged organelles and then coordinates the fusion of the autophagosome with a lysosome or a vacuole for degradation. Viral infection processes are closely related to the autophagy of host cells, and autophagy can serve in innate immunity and the adaptive immune response against intracellular pathogens, including human immunodeficiency virus 1 (HIV-1), hepatitis B virus (HBV) and herpes simplex virus 1 (HSV-1) [10, 13, 15].
Despite the role of autophagic signaling in host defense, several pathogens can subvert autophagy and maximize its advantages [6, 9]. For example, the replication cycle of positive-strand RNA viruses can be promoted by some aspects of the autophagic pathway. Positive-strand RNA viruses are causative agents of many diseases, including myocarditis, encephalitis, and hand, foot, and mouth disease. CVB3 can induce autophagy both in vitro and in vivo [1, 4, 26, 30]. However, there are conflicting findings that need to be resolved. A previous study showed that CVB3 infection triggers autophagosome formation in 293A cells, which are important for viral replication [30]. It is less certain whether the autophagosome is fused with a lysosome in the subsequent process of autophagy, although the inhibition of lysosomal fusion results in enhanced viral replication. In another study, using a mouse model of CVB3 infection, it was found that CVB3 induces the formation of small autophagy-like vesicles and permits amphisome formation in pancreatic acinar cells. However, the fusion of autophagosomes with lysosomes is greatly reduced by viral infection. As a consequence, large autophagy-related structures called megaphagosomes are formed [1, 4]. A recent study also showed that CVB3 protease 2A can cleave the p62 protein, which is an autophagy receptor targeting ubiquitinated proteins for degradation [23]. Thus, the interpretation of lysosome protein degradation in these studies should be reconsidered by measuring the p62 protein level.
Confocal microscopy with mCherry-GFP fluorescent-protein-tagged LC3 is an efficient tool that is used to investigate the conversion of autophagosomes into autolysosomes [7]. In this study, fluorescent proteins combined with autophagy chemical inhibitors or inducers were used in HEK293A cells, and CVB3-induced LC3-targeting vacuoles were characterized under a confocal microscope. We demonstrate that CVB3 infection induces a complete autophagy flux, and autophagosomes alone are sufficient for efficient viral replication. This study provides further insights into CVB3-induced autophagy.
Materials and methods
Viruses and plasmids
CVB3 (Nancy strain) was maintained by passage in HeLa cells. CVB3-eGFP was produced by transfecting HeLa cells with a plasmid containing the CVB3 genome and the eGFP gene [2, 28]. Virus titers were determined using a 50 % tissue culture infectious dose (TCID50) assay on HeLa cell monolayers and calculated by the Reed–Muench method [19]. Plasmids encoding mCherry-LC3 (#40827) were purchased from Addgene. The plasmid mCherry–GFP-LC3 was kindly provided by Prof. William Jackson (Medical College of Wisconsin, USA).
Cell culture and transfection
HeLa cells and HEK293A cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; HyClone) supplemented with 10 % fetal bovine serum, 4 mM L-glutamine, 100 U of penicillin, and 100 μg of streptomycin per ml. These cells were cultured at 37 °C in 5 % CO2. Transfection was performed with the indicated plasmids using Lipofectamine 2000 Reagent (Invitrogen) in accordance with the manufacturer’s guidelines. HEK293A cells were transiently transfected mCherry-GFP-LC3 or mcherry-LC3 in a 10-cm dish to generate a stable cell line. After two rounds of sorting by BD FACSAriaTM III flow cytometry, more than 90 % of the cells continuously expressed GFP or mCherry.
Antibodies and chemical compounds
For Western blot analysis, a primary anti-LC3B antibody from Sigma-Aldrich (L7543) was used at a dilution of 1:2 000. A rabbit polyclonal anti-GAPDH antibody from Hangzhou Goodhere Biotechnology, anti-Atg5 (Atg5-Atg12 complex), and anti-p62/SQSTM1 antibodies from MBL International (PM050 and PM045) were used at a dilution of 1:1000. The secondary antibodies Alexa Fluor 680-goat anti-rabbit IgG and Alexa Fluor 680-goat anti-mouse IgG from Jasckson ImmunoResearch were used at a dilution of 1:10000. For immunofluorescence, a mouse monoclonal anti-LAMP1 antibody from Santa Cruz (sc-20011) was used at a dilution of 1:200. A rabbit polyclonal anti-EEA1 antibody from Cell Signaling Technology (#2411S) was used at a dilution of 1:500. The secondary antibodies DyLight 633- or 488-labeled anti rabbit IgG and DyLight 488-labeled anti-mouse IgG from KPL were used at a dilution of 1:200. The rabbit polyclonal anti-CVB3 VP1 (1:2000) antibody was a gift from Dr. Fei Deng (Wuhan Institute of Virology, Chinese Academy of Science).
Rapamycin (100 nM, R706203) was purchased from Sangon Biotech. CQ (50 nm, C6628), 3-MA (10 mM, M9281), pepstatin A (2 μg/mL, P5318), and leupeptin trifluoroacetate salt (10 μM, L2023) were procured from Sigma-Aldrich. BAF (100 nM, sc-201550), vinblastine sulfate (10 μM, ICM1035), E-64d (10 μM, E123225), and LysoTracker Green DND-26 (L-7526) were obtained from Santa Cruz, Gene Operation, Aladdin, and Life Technologies, respectively. Cell cytotoxicity was measured by CCK8 assay to ensure that there was no obvious cytotoxicity due to treatment with chemical compounds.
Western blot analysis
The cells were harvested and washed twice with phosphate-buffered saline (PBS). Afterward, the cell lysate was separated by 12 % SDS-PAGE and then transferred to PVDF membranes (Millipore). The membranes were blocked with 5 % nonfat milk in 1× PBS-0.1 % Tween 20 for 2 h and incubated with the indicated primary antibodies for 2 h at room temperature or overnight at 4 °C. The membranes were washed three times with 1× PBS-0.1 % Tween 20 and incubated with secondary antibodies at room temperature for 2 h. Immunoreactive bands were visualized using an enhanced Odyssey Imaging System (Gene Company Limited).
Indirect immunofluorescence and confocal microscopy
mCherry-GFP-LC3 or mCherry-LC3 stable cell lines were infected with CVB3 at an MOI of 1.5 in a culture chamber for confocal imaging (Livefocus) to detect autophagosomes. The cells were fixed at 8 h postinfection with or without chemical inhibitors and permeabilized with paraformaldehyde buffer which containing 10 ml 10× PBS, 33.4 ml 11 % formaldehyde, 0.6 ml 30 % Triton X-100, and 56 ml ddH2O in total 100 ml volume for 30 min at 37 °C. For immunofluorescent staining, 1× PBS containing 1 % BSA solution was used to block the fixed cells for 30 min at 37 °C. The cells were then incubated with the indicated primary antibodies for 2 h at room temperature and with secondary antibodies (KPL) for 1 h at room temperature. The nuclei were stained with DAPI (Beyotime) for 10 min at room temperature, and the cells were viewed using a laser scanning confocal microscope (Nikon A1).
Atg5 siRNA assay
HEK293A cells in a six-well plate were transfected with a mixture of 50 nM siRNA targeting the human Atg5 gene (siRNA1 5′-CAA AGA AGU UUG UCC UUC UGC UAU U-3′; siRNA2 5′-AAU AGC AGA AGG ACA AAC UUC UUU G-3′) and siRNA N. C. FAM (Invitrogen), a scrambled siRNA, using Lipofectamine. Two days post-transfection, the cells were collected for Atg5 blotting or infected with CVB3-eGFP.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software. Data are presented as the mean and standard deviation. The data were statistically analyzed using a two-tailed independent Student’s t-test, and the level of significance was set at P < 0.05.
Results
Autophagosomes are induced by CVB3 infection
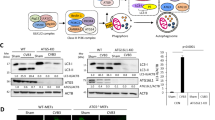
The Atg8/LC3 protein is an ubiquitin-like protein that can be conjugated to phosphatidyl ethanolamine, which is a reliable marker associated with autophagosome formation. The conversion of nonlipidated LC3-I to lipidated LC3-II is usually considered an indicator of autophagic activity [7]. We initially determined whether CVB3 infection triggers the formation of autophagosomes, and the conversion of endogenous LC3-I to LC3-II was assessed by immunoblotting. Even at a low MOI of 0.75, the LC3-II level notably increased at 8 h postinfection (Fig. 1A and B). This indicated that autophagosome formation exhibited a cumulative increase as infection progressed.
Formation of autophagosomes during CVB3 infection. (A) HEK293A cells were infected with CVB3 at various MOIs as indicated. Viral replication and the induction of autophagy were evaluated via western blotting of VP1 and conversion of LC3I to LC3II, respectively, at 8 h postinfection. (B) HEK293A cells were infected with CVB3 (MOI = 1.5) as indicated. Viral replication and the induction of autophagy were evaluated via western blotting of VP1, and conversion of LC3I to LC3II, respectively, at different time points as indicated
CVB3 infection induces the formation of acidic autophagic vesicles
The formation and accumulation of autophagosomes is an intermediate step of autophagic flux that appears to correlate with the induction of autophagy. The formation of the autophagosome is followed by fusion with lysosomes and the degradation of the contents, resulting in a complete flux. Alternatively, before fusing with a lysosome, an autophagosome can fuse with an endosome to produce an amphisome [4]. The level of the autophagic substrate SQSTM1/p62, an adaptor protein that interacts with LC3, is generally considered an indicator of autophagy pathway degradation [30]. Consistent with previous findings [23], our results indicated that CVB3 can promote the degradation of p62 at 8 h postinfection (data not shown). However, viral protease 2Apro is responsible for CVB3-induced p62 cleavage, as described in a previous study [23]. Thus, the degradation of p62 in the present study may not correspond to the CVB3-induced autophagy flux.
We then used the special tandem reporter plasmid mCherry-GFP-LC3. The GFP of this tandem autophagosome reporter is sensitive to a low-pH (acidic) environment, whereas mCherry remains intact in the acidic environment. Therefore, the fusion of autophagosomes with late endosomes or lysosomes results in the loss of GFP fluorescence and the appearance of the red fluorescence of mCherry [25]. In the positive control, the majority of the LC3-positive autophagic vacuoles in DMEM-starved cells were mCherry positive. However, when we treated the DMEM-starved cells with CQ to block acidification of the lysosome, many GFP-positive autophagosomes were detected and appeared yellow in merged images (Supplementary Data 1). We then infected mCherry-GFP-LC3 stable cell lines with CVB3. It appeared that there were few GFP-positive autophagic vacuoles, but a large number of mCherry-positive autophagic vacuoles remained detectable compared to mock-infected cells (Fig. 2). This observation indicated that CVB3 infection may induce lysosomal fusion, which occurs in the late stage of autophagy flux.
Formation of acidic vacuoles during CVB3 infection. HEK293A cells were infected with CVB3 at an MOI of 1.5 with or without inhibitor treatment. At 8 h postinfection, the cells were fixed and the nuclei were stained with DAPI before imaging. The chemical inhibitors CQ, BAF and vinblastine were used at a concentration of 50 nM, 100 nM and 10 μM, respectively. The bar represents 10 μm
To confirm the above observation, we treated CVB3-infected cells with the chemical inhibitors CQ, BAF and vinblastine. The results showed that the majority of autophagic vacuoles become positive for both mCherry and GFP when vacuole acidification was inhibited by CQ or BAF and when microtubule assembly was prevented via vinblastine treatment. This confirms that autophagosomes can be processed further to acidic autophagic vacuoles upon CVB3 infection (Fig. 2).
CVB3-induced autophagic vacuoles can fuse with lysosomes
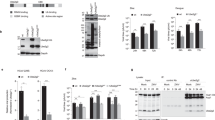
CVB3-induced acidic autophagic vacuoles can be generated through the fusion of autophagosomes with endosomes to form amphisomes, or with lysosomes to form autolysosomes [4]. We stained cells with LAMP1 and LysoTracker Green to observe the subcellular colocalization of CVB3-induced autophagic vacuoles in detail. The mCherry-LC3 stable cell line was also generated to monitor the autophagic vacuoles, given that GFP is sensitive in the acidic vacuoles. A yellow punctuate distribution was observed with LAMP1 or LysoTracker Green, thereby confirming that the CVB3-induced autophagic vacuoles colocalize with lysosomes (Fig. 3). However, the mCherry-positive vacuoles did not colocalize well (Fig. 3) when we tracked endosome colocalization with early endosome antigen 1 (EEA1) or the late endosome/amphisome marker mannose-6-phosphate receptor (MPR) [11]. Similar results were obtained when we investigated colocalization of LAMP1 or EEA1 with mCherry-LC3 upon starvation. These data demonstrate that autophagosomes primarily fuse with lysosomes during CVB3 infection, representing the late stage of autophagy. However, we cannot exclude that a small proportion of the autophagosomes fuse with endosomes to form amphisomes.
Colocalization of CVB3-induced autophagic vacuoles with lysosomes. The stable cell line HEK293A expressing mCherry-LC3 was infected with CVB3 at an MOI of 1.5. At 8 h postinfection, the cells were fixed and treated with Lysotracker or stained for the lysosome marker LAMP1, the endosome marker EEA1, or the endosome/amphisome marker MPR. DAPI was used for nuclear staining before imaging. The bar represents 10 μm
CVB3 hijacks autophagosomes but not autolysosomes for efficient viral replication
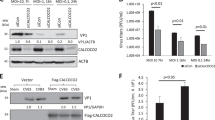
To investigate the relationship between autophagy and viral replication, we treated cells with various inhibitors targeting different stages of autophagy. These inhibitors included 3-MA, an inhibitor of autophagosome formation, and CQ/BAF, which are inhibitors of lysosome acidification. The results revealed that the VP1/GAPDH ratio is notably decreased in 3-MA-treated cells compared with that in CVB3-infected cells (Fig. 4A). However, the VP1/GAPDH ratio did not change considerably with CQ/BAF treatment. Similarly, virus titration showed that the viral titer decreased approximately tenfold upon 3-MA treatment (Fig. 4B). These results were also further confirmed by flow cytometry analysis using CVB3-eGFP virus. The disruption of autophagosome formation via Atg5 siRNAs confirmed that autophagosome formation induced by CVB3 infection is critical for viral replication (Fig. 4C). Particularly, we observed that treatment with lysosome protease inhibitors (pepstatin A + E-64d + leupeptin) had no influence on CVB3 replication (Fig. 4D). Therefore, the initial formation and accumulation of autophagosomes is crucial for CVB3 replication, but later formation of autolysosomes is not, although CVB3 infection induces autophagy flux.
Autophagosomes are critical for efficient viral replication. (A) HEK 293A cells were treated with 10 mM 3-MA, 50 nM CQ, or 100 nM BAF before infection with CVB3 at an MOI of 1.5. The induction of autophagy and viral replication were evaluated by blotting of LC3IIB and VP1 at 8 hours postinfection. (B) Measurement of viral titers in the culture medium of infected cells after the treatments shown in panel A. (C) The Atg5-siRNA silencing efficiency was measured via western blotting using anti-Atg5 antibody 48 hours post-transfection (upper panel). HEK293A cells with Atg5-siRNA silencing were infected with CVB3-eGFP at an MOI of 1, and at 12 and 24 h postinfection, virus replication was measured by flow cytometry. (D) 293A cells were treated as indicated with chemical compounds and then infected with CVB3-eGFP at an MOI of 3. Viral replication was measured by flow cytometry at 12 h postinfection. The results are presented as the mean ± SD of three separate experiments. **, p < 0.01; ***, p < 0.001
Discussion
Autophagy can play an important role in protecting host cells during virus infection, and several viruses have developed strategies to evade or even exploit this homeostatic pathway. Many positive-strand RNA viruses exploit the autophagic process for viral RNA replication [29, 30]. Studies on poliovirus, a member of the family Picornaviridae, have shown that both autophagosome formation and maturation of the autophagic vacuole are used to promote two separate and distinct steps in the virus life cycle [20, 24]. In this study, we used multiple assays in HEK293A cells to show that picornavirus CVB3 induces mCherry-positive autophagic vacuoles. Treatment with the lysosomal acidification inhibitors chloroquine (CQ) and bafilomycin A1 (BAF) rescued the GFP-positive vacuoles. This finding indicated that the induced vacuoles were processed from autophagosomes in a low-pH (acidic) environment. These vacuoles were co-localized with lysosome-associated membrane protein 1 (LAMP1) and LysoTracker. Inhibitors of lysosomal protease, fusion, and acidification did not affect viral replication. However, viral replication was significantly decreased by treatment with 3-methyladenine (3-MA) or Atg5 siRNA, indicating that autophagosomes are critical for replication.
Although some studies have shown that CVB3 can induce autophagy both in vitro and in vivo [1, 4, 26, 30], it was not certain whether CVB3 infection induces a complete autophagic flux. Autophagy is a complicated process, and therefore, no individual assay is guaranteed to be the most appropriate in every situation. To interpret the results, the use of multiple assays, such as LC3 turnover detection via Western blot and Tandem mCherry-GFP-LC3 via fluorescence microscopy in combination with autophagic inhibitors, is strongly recommended for monitoring autophagy [7]. We initially demonstrated by mCherry-GFP confocal microscopy, which is a fluorescence assay particularly designed to monitor autophagic flux [5], that most of the vesicles induced by CVB3 infection in stable mCherry-GFP-LC3 cells were mCherry positive. These vesicles then became positive for both mCherry and GFP when treated with lysosomal inhibitors, indicating that these vesicles may fuse with lysosomes in a low-pH environment. These observations were confirmed by immunostaining, showing that mCherry-positive puncta colocalized well with LysoTracker and LAMP1, but not with the early-endosome marker EEA1 or the late-endosome/amphisome marker MPR. Therefore, the autophagosomes induced by CVB3 infection predominantly fuse with lysosomes in cell culture. Another report showed that CVB3 induces amphisome formation in pancreatic acinar cells in a mouse model of CVB3 infection [4]. The different cell types or methods used may account for this discrepancy. However, we still cannot exclude the formation of amphisomes because a small number of the LC3-positive vesicles colocalized with EEA1 and MDR via immunostaining.
It has been proposed that remodeled autophagic intracellular membranes serve as a structural platform for replication and assembly of picornaviruses [21]. The interruption of autophagosome formation either by 3-MA- or by Atg5-specific siRNA decreases viral replication. This finding is in accordance with that of a previous study [30]. In contrast to earlier results showing that the inhibition of autophagosomes and lysosomes by LAMP2 siRNA enhances CVB3 replication [30], our results with lysosomal fusion, protease, and acidification inhibitors revealed that the degradation of autolysosomes and lysosomal fusion are not essential for virus production. The maturation of infectious poliovirus particles requires the acidification of autophagic vesicles. In these vesicles, the capsid protein VP0 is cleaved into VP4 and VP2, and as a result, the infectious 150S virion is formed [20]. Treatment with lysosomal inhibitors in vitro did not significantly reduce the CVB3 titer; however, further studies should determine whether CVB3-induced autolysosomes participate in the viral life cycle in vivo. Virions that are resistant to acidic degradation in autolysomes might be better suited to escape digestion in the gastrointestinal tract during natural infection.
In conclusion, we show that complete autophagic flux is induced in CVB3-infected HEK293A cells. Autophagosomes, but not autolysosomes, are required for efficient viral replication. These vesicles may provide relatively stable sites for cytoplasmic virus replication. Further studies on autophagy would improve our knowledge of the pathogenesis of CVB3-induced viral myocarditis and provide insights into the development of novel antiviral strategies against CVB3 infection.
References
Alirezaei M, Flynn CT, Wood MR, Whitton JL (2012) Pancreatic acinar cell-specific autophagy disruption reduces coxsackievirus replication and pathogenesis in vivo. Cell Host Microbe 11:298–305
Cornell CT, Kiosses WB, Harkins S, Whitton JL (2006) Inhibition of protein trafficking by coxsackievirus b3: multiple viral proteins target a single organelle. J Virol 80:6637–6647
Feldman AM, McNamara D (2000) Myocarditis. N Engl J Med 343:1388–1398
Kemball CC, Alirezaei M, Flynn CT, Wood MR, Harkins S, Kiosses WB, Whitton JL (2010) Coxsackievirus infection induces autophagy-like vesicles and megaphagosomes in pancreatic acinar cells in vivo. J Virol 84:12110–12124
Kimura S, Noda T, Yoshimori T (2007) Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3:452–460
Kirkegaard K, Taylor MP, Jackson WT (2004) Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat Rev Microbiol 2:301–314
Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Zschocke J, Zuckerbraun B (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8:445–544
Krausslich HG, Nicklin MJ, Lee CK, Wimmer E (1988) Polyprotein processing in picornavirus replication. Biochimie 70:119–130
Kudchodkar SB, Levine B (2009) Viruses and autophagy. Rev Med Virol 19:359–378
Kyei GB, Dinkins C, Davis AS, Roberts E, Singh SB, Dong C, Wu L, Kominami E, Ueno T, Yamamoto A, Federico M, Panganiban A, Vergne I, Deretic V (2009) Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J Cell Biol 186:255–268
Lee YR, Hu HY, Kuo SH, Lei HY, Lin YS, Yeh TM, Liu CC, Liu HS (2013) Dengue virus infection induces autophagy: an in vivo study. J Biomed Sci 20:65
Lindberg AM, Stalhandske PO, Pettersson U (1987) Genome of coxsackievirus B3. Virology 156:50–63
Liu B, Fang M, Hu Y, Huang B, Li N, Chang C, Huang R, Xu X, Yang Z, Chen Z, Liu W (2014) Hepatitis B virus X protein inhibits autophagic degradation by impairing lysosomal maturation. Autophagy 10:416–430
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147:728–741
Orvedahl A, Alexander D, Talloczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib DA, Levine B (2007) HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 1:23–35
Palmenberg AC (1990) Proteolytic processing of picornaviral polyprotein. Annu Rev Microbiol 44:603–623
Pauschinger M, Phan MD, Doerner A, Kuehl U, Schwimmbeck PL, Poller W, Kandolf R, Schultheiss HP (1999) Enteroviral RNA replication in the myocardium of patients with left ventricular dysfunction and clinically suspected myocarditis. Circulation 99:889–895
Racaniello VR (2007) Picornaviridae: the virus and their replication. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Straus SE, Martin MA, Roizman B (eds) Fields virology, 5th edn. Lippincott, Williams & Wilkins, Philadelphia
Reed L, Muench H (1938) A simple method of estimating fifty per cent endpoint. Am J Hyg 27:493–497
Richards AL, Jackson WT (2012) Intracellular vesicle acidification promotes maturation of infectious poliovirus particles. PLoS Pathog 8:e1003046
Richards AL, Jackson WT (2013) How positive-strand RNA viruses benefit from autophagosome maturation. J Virol 87:9966–9972
Rubinsztein DC, Shpilka T, Elazar Z (2012) Mechanisms of autophagosome biogenesis. Curr Biol 22:R29–R34
Shi J, Wong J, Piesik P, Fung G, Zhang J, Jagdeo J, Li X, Jan E, Luo H (2013) Cleavage of sequestosome 1/p62 by an enteroviral protease results in disrupted selective autophagy and impaired NFKB signaling. Autophagy 9:1591–1603
Singh R, Cuervo AM (2012) Lipophagy: connecting autophagy and lipid metabolism. Int J Cell Biol 2012:282041
Sun MX, Huang L, Wang R, Yu YL, Li C, Li PP, Hu XC, Hao HP, Ishag HA, Mao X (2012) Porcine reproductive and respiratory syndrome virus induces autophagy to promote virus replication. Autophagy 8:1434–1447
Tabor-Godwin JM, Tsueng G, Sayen MR, Gottlieb RA, Feuer R (2012) The role of autophagy during coxsackievirus infection of neural progenitor and stem cells. Autophagy 8:938–953
Tam PE (2006) Coxsackievirus myocarditis: interplay between virus and host in the pathogenesis of heart disease. Viral Immunol 19:133–146
Tong L, Lin L, Zhao W, Wang B, Wu S, Liu H, Zhong X, Cui Y, Gu H, Zhang F, Zhong Z (2011) Destabilization of coxsackievirus b3 genome integrated with enhanced green fluorescent protein gene. Intervirology 54:268–275
Wileman T (2007) Aggresomes and pericentriolar sites of virus assembly: cellular defense or viral design? Annu Rev Microbiol 61:149–167
Wong J, Zhang J, Si X, Gao G, Mao I, McManus BM, Luo H (2008) Autophagosome supports coxsackievirus B3 replication in host cells. J Virol 82:9143–9153
Acknowledgments
The authors would like to thank Prof. William Jackson at Medical College of Wisconsin for providing the mCherry-GFP-LC3 plasmid. The authors also thank Prof. Zhaohua Zhong at Harbin Medical University for providing the CVB3-eGFP plasmid. The anti-CVB3-VP1 polyclonal antibody was kindly provided by Dr. Fei Deng at the Wuhan Institute of Virology, Chinese Academy of Science.
This work was supported by grants from Major State Basic Research Development Program of China (2013CB531502, 2013CB530501), the National Natural Science Foundation of China (31270977, 81101257, 31470848), the Scientific Research Foundation for Returned Overseas Chinese Scholars from State Education Ministry to C.D., and Jiangsu Provincial Innovative Research Team. The authors declare that there are no conflicting financial interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
X. Shi and Z. Chen contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.

705_2016_2896_MOESM1_ESM.jpg
Supplementary data 1 HEK293A mCherry-GFP-LC3 stable cells starved with or without 50 nM CQ treatment. At 8 h post-starvation, the cells were observed under confocal microscopy. The bar represents 10 μm. (JPEG 2103 kb)
Rights and permissions
About this article
Cite this article
Shi, X., Chen, Z., Tang, S. et al. Coxsackievirus B3 infection induces autophagic flux, and autophagosomes are critical for efficient viral replication. Arch Virol 161, 2197–2205 (2016). https://doi.org/10.1007/s00705-016-2896-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-016-2896-6