Abstract
The aim of this study was to evaluate the long-term effectiveness of first-line antiretroviral therapy in HIV/AIDS patients in Southeast China. A total of 450 eligible patients were selected to initiate first-line antiretroviral therapy from February 2005 through August 2009. During the study period from 2009 through 2013, each subject received clinical and laboratory monitoring for effectiveness, safety and toxicity once every 3 months in the first year, and once every 6 months in the following years. The response to first-line antiretroviral therapy was evaluated through body weight gain and immunological and virological outcomes. During the mean follow-up period of 70.86 ± 28.9 months, the overall mortality was 14.2 %. The mean body weight and CD4+ counts increased significantly following antiretroviral therapy as compared to baselines across the follow-up period, and the rate of immunological effectiveness was over 85 % in all subjects at 2 to 5 years of treatment. The rate of inhibition of HIV virus was 87.67 %, 89.32 %, 91.73 %, 92.8 % and 91.63 % across the study period. In addition, significant differences were detected after treatment as compared to baselines, and Pearson correlation analysis revealed a positive correlation between immunological effectiveness and viral inhibition. Forty-eight percent of the subjects changed antiretroviral drugs once, and 16.22 % twice, and 31 patients switched from first-line to second-line antiretroviral therapy. Long-term antiretroviral therapy remains effective for treatment of HIV/AIDS, resulting in higher mean body weight, effective viral inhibition and a higher CD4 count. Immunological effectiveness of antiretroviral therapy positively correlates with HIV viral inhibition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acquired immunodeficiency syndrome (AIDS), caused by human immunodeficiency virus (HIV), continues to be a major global public-health and social issue. In 2013, there were 35 million people living with HIV/AIDS, and it was estimated that 1.5 million died of AIDS-related diseases worldwide [1]. In China, AIDS remains a big challenge to public health and has been defined as one of the priorities for communicable-disease control by the central government [2]. It was estimated that a total of 436,800 people were living with HIV or AIDS in China by the end of 2013, and 136,300 had died from the disease [3].
Currently, there is no cure for HIV infection or AIDS [4]. However, effective antiretroviral therapy is found to sustain suppression of HIV replication and reconstitution of the immune response [5, 6], and this leads to a dramatic decrease in AIDS-related morbidity and mortality [7–9]. Since effective treatment with antiretroviral drugs can control the virus, people living with HIV/AIDS can enjoy healthy and productive lives [10]. Based on the standard World Health Organization (WHO) clinical and laboratory criteria, people with stage III/IV disease and/or a CD4 count of < 200 cells/ml require antiretroviral therapy [11].
China’s national free antiretroviral treatment program began in 2003 [12]. Initial implementation and scale-up of the free antiretroviral treatment concentrated on former blood donors in the central provinces [13]. Since 2005, however, with the changes in the HIV/AIDS epidemic, the treatment coverage has extended across the country, and more and more HIV/AIDS patients who acquired the virus via sex and drug use are included in the program [14]. At the end of 2009, a total of 80,000 patients in China were estimated to be receiving antiretroviral therapy [15, 16]. However, following the extensive implementation of antiretroviral treatment, there is great concern about the emergence of drug-resistant HIV strains [17]. HIV drug resistance is considered one of the strongest predictors of treatment failure [18–20], since it can render existing therapies ineffective, thereby increasing the risk of viral rebound and opportunistic infections [21–23]. Therefore, a national HIV drug resistance surveillance and monitoring network was established in China in 2004 in order to monitor the development of resistance of HIV strains to antiretroviral agents [24]. In China, the prevalence of drug-resistant HIV strains has been reported to increase with the continuation of antiretroviral therapy [25], and in Henan province of central China, the prevalence of drug-resistant HIV strains was found to increase sharply from 17.6 % to 45.4 % and 62.3 % in patients undergoing antiretroviral therapy beyond three and six months, respectively [26].
In Fujian province, southeast coast of mainland China, HIV is predominantly transmitted through sexual contact [27]. Since 2005, free antiretroviral therapy has been implemented for HIV/AIDS patients across the province [28]. During the 10-year period from 2005 to 2014, a total of 3158 patients had been treated, and there are still 2708 patients undergoing treatment. In the current study, a 5-year longitudinal evaluation was carried out with aim of assessing the long-term effectiveness of first-line antiretroviral therapy through monitoring of clinical, immunological and virological parameters in HIV/AIDS patients in Fujian Province, Southeast China.
Subjects and methods
Subjects
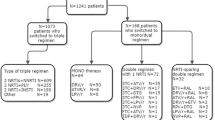
During the period from February 2005 through August 2009, 450 treatment-naïve patients who met the inclusion criteria of the Chinese national antiretroviral treatment guidelines were selected for antiretroviral therapy. These included a CD4+ T lymphocyte (CD4) count of < 200 cells/μl, a total lymphocyte count of < 1200 cells/μl, or HIV clinical stage III/IV according to the World Health Organization [11, 16] Each subject was evaluated prior to initiation of antiretroviral therapy. For those receiving antiretroviral therapy, subsequent clinical and laboratory monitoring for effectiveness, safety and toxicity was performed once every 3 months in the first year, and once every 6 months in the following years. Among the 450 patients, 345 (76.7 %) were followed up for 5 years or longer, while 105 (23.3 %) dropped out of treatment during the follow-up period due to death (64, 14.2 %), withdrawal (14, 3.1 %), transfer to other provinces (18, 4.0 %), and loss of follow-up (9, 2.0 %).
Treatment protocol
According to the WHO recommendations on antiretroviral therapy for HIV infections [11], the first-line antiretroviral therapy consisted of two nucleoside reverse transcriptase inhibitors (NRTIs) and one non-nucleoside reverse transcriptase inhibitor (NNRTI), including zidovudine (AZT) or stavudine (d4T) + lamivudine (3TC) + nevirapine (NVP) or efavirenz (EFV). This regimen, however, was not employed until 2005, when 3TC was market-available in China. As a result, 15 patients received antiretroviral therapy on initial treatment, including seven patients (1.56 %) treated with a didanosine (ddI) regimen (DDI + 3TC + EFV for one case, DDI + D4T + NVP for 5 cases, and DDI + AZT + NVP for one case) and eight patients (1.78 %) treated with indinavir (IDV) regimen (3TC + IDV + D4T for 4 cases, 3TC + IDV + AZT for one case, Atripla + IDV for one case, and EFV + IDV for 2 cases). The second-line antiretroviral therapy consisted of two NRTIs + a ritonavir-boosted protease inhibitor (PI), TDF/AZT + 3TC + lopinavir/ritonavir (LPV/r).
Laboratory tests
At baseline and each follow-up visit, all subjects were weighed, interviewed about their adherence to therapy and treatment-related side effects and asked to provide blood specimens. These specimens were used to measure the CD4 count and viral load and for routine blood and liver function testing. The main information collected included gender, age, and the probable route of infection.
The HIV diagnosis was confirmed using a Western blot assay (MP Diagnostics™ HIV BLOT 2.2, MP Biomedicals, Asia Pacific Pte. Ltd., Singapore). Patients were considered anti-HIV-1 antibody positive if the following two criteria were met: (1) presence of at least two env bands (gp41 and gp160/gp120) or simultaneous emergence of at least one env band and p24 band and (2) meeting the diagnostic criteria proposed by the manufacturer of the Western blot kit [14].
The CD4 count was measured using a BD TriTEST™ CD3-FITC/CD4-PE/CD45-PerCP Kit (BD Biosciences; Franklin Lakes, NJ, USA) on a BD FACSCalibur system (BD Biosciences; Franklin Lakes, NJ, USA). The plasma HIV-1 RNA concentration was determined using a Versant® HIV-1 RNA 3.0 Assay (bDNA; Bayer HealthCare; Tarrytown, NY, USA) on a Bayer Versant® 340 bDNA Analyzer (Bayer HealthCare; Tarrytown, NY, USA) following the manufacturer’s instructions.
Assessment of efficacy of antiretroviral therapy
The efficacy of the antiretroviral therapy provided to patients was assessed using the WHO criteria for clinical, immunological and virological failure. As the most sensitive indicator for demonstrating the efficacy of antiretroviral therapy against HIV [29], body weight gain was used in this study to evaluate the response to antiretroviral therapy. The criteria for immunological failure included a CD4 count drop to < 100 cells/mm3, a 50 % reduction in baseline CD4 from the treatment peak value, or a decrease to the baseline CD4 level or below, and the criterion for virological failure was a plasma viral load of more than 1000 copies/ml when the patient had received antiretroviral therapy for at least 6 months [30].
Genotypic testing of HIV-1 drug resistance
Samples with an HIV viral load of 1000 copies/ml or greater were selected for HIV-1 drug resistance testing by using an in-house RT-PCR protocol as described previously [31]. To detect HIV drug resistance mutations, the HIV-1 pol gene (protease amino acids 1–99 and reverse transcriptase amino acids 1–252) was amplified, and its sequence was compared to those in the Stanford HIV Drug Resistance Database (http://hivdb.stanford.edu/). We included mutation results that conferred low-, intermediate- and high-level resistance.
Ethical considerations
This study was approved the Ethics Review Committee of Fujian Provincial Center for Disease Control and Prevention (permission no. FJCDCERC-2004004). Written informed consent was obtained from all participants with a detailed description of the purpose and potential benefits of this study, and the confidentiality of the respondents’ information and blood test results was guaranteed.
Statistical analysis
All measurement data were expressed as mean ± standard deviation (SD), and all statistical analysis was performed using the statistical software SPSS version 15.0 (SPSS Inc.; Chicago, IL, USA). Differences in mean values among groups were tested for statistical significance by one-way analysis of variance (ANOVA), while comparisons of mean values between groups was done by Student’s t-test. The association between immunological effectiveness and viral inhibition was evaluated using Pearson correlation analysis. A P-value less than 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics of patients receiving antiretroviral therapy
The 450 HIV/AIDS patients undergoing antiretroviral therapy included 300 men (66.7 %) and 150 women (33.3 %), and the mean age was 45.24 ± 10.92 years (range, 21 to 82 years). Of these patients, 65.6 % were from 30 to 50 years old. Among the 450 subjects, 69.1 % were married or cohabiting, and 76 % were infected with HIV through sexual contact (2.4 % homosexual transmission and 73.6 % heterosexual transmission). There were 302 patients (67.1 %) with a CD4 count of < 150 cells/mm3 on admission (Table 1).
Follow-up and survival of patients receiving antiretroviral therapy
All subjects had a mean follow-up period of 70.86 ± 28.9 months. There were 377 subjects who were followed up for 5 year or longer, with a 2 % loss to follow-up, and 345 subjects are still under therapy, with 76.7 % adherence to therapy. The overall mortality was 14.2 % (64/450) during the entire follow-up period, with the most deaths observed within the first year post-treatment (51.6 %), indicating a reduction in deaths due to HIV/AIDS during the treatment period (Table 2).
Change of body weight caused by antiretroviral therapy
During the follow-up period, the mean body weight of the patients increased significantly each year following antiretroviral therapy as compared to baseline (all P-values < 0.001), with the greatest increase (5.26 %) seen in the second year of therapy. Over 60 % of the patients showed an increase in body weight during the follow-up period. One-way ANOVA revealed a significant difference in the mean body weight during the 5-year follow-up period (F = 15.66, P < 0.001); however, no significant differences were seen in the body weight between years (Table 3).
Immunological outcome of antiretroviral therapy
Following antiretroviral therapy, CD4 counts increased significantly, compared to those at baseline (all P-values < 0.001), as revealed by paired t-test. In the first year of antiretroviral therapy, the mean CD4 count increased by 147 cells/mm3, and it increased by over 200 cells/mm3 in the following 4 years, with the greatest increase (293 cells/mm3) detected following the 5-year treatment (Fig. 1A). During the 5-year treatment period, the number of patients with CD4 count elevation of > 150 cells/mm3 gradually increased with the extension of treatment, from 39.24 % in the first year to 75.07 % in the fifth year of treatment. There was a significant difference in CD4 count for the different years of treatment (F = 294.2, P < 0.001). The mean CD4 count increased from 116.41 cells/mm3 at baseline to 413.54 cells/mm3 following 5 years of antiretroviral therapy (Fig. 1B). Except for years four and five of treatment, there were significant differences in the mean CD4 count between the years of treatment and between the year of treatment and baseline. According to the WHO criteria for identification of immunological failure, the highest rate (19.24 %) of immunological failure was observed in the first year of antiretroviral therapy, and the rate was uniformly < 15 % in the following four years, indicating over 85 % immunological effectiveness (Table 4).
Virological outcome of antiretroviral therapy
Our findings showed that antiretroviral therapy resulted in a marked inhibition of HIV replication, and the percentage of HIV viral inhibition (< 1000 copies/ml) was 87.67 % following one-year treatment. The rate of viral suppression was found to increase with the continuation of antiretroviral therapy and reached 91.63 % after 5 years of treatment. Pearson correlation analysis revealed that the immunological effectiveness was associated with viral inhibition 2, 3, 4 and 5 years post-therapy (all P values < 0.01), while no correlation was detected in the first year of treatment (R = 0.135, P = 0.104) (Table 5).
Change of treatment regimen and drug resistance
During the follow-up period, 216 patients (48 %) changed their antiretroviral treatment regimen once, predominantly because of adverse effects (71.76 %), and to a much lesser extent because of to treatment failure (only 4.63 %). With the extension of first-line antiretroviral therapy, 73 subjects (16.22 %) changed their antiretroviral agents twice, mainly because of side effects (61.64 %), and the rate of treatment failure increased to 13.7 % among these patients. During the follow-up period, 31 patients (8.98 %) switched to second-line antiretroviral therapy, including 25 patients (5.56 %) due to resistance to first-line antiretroviral therapy. HIV-1 drug resistance testing revealed resistance to multiple NRTIs and NNRTIs at various levels, but the HIV-1 strains remained susceptible to PIs. Treatment with PI-containing regimens continued to result in viral inhibition.
Discussion
Although antiretroviral therapy is not a cure for HIV/AIDS, most HIV-infected individuals without treatment will eventually develop progressive immunosuppression, leading to AIDS-defining illnesses and premature death [1]. The major purpose of antiretroviral therapy is to prevent HIV-associated morbidity and mortality [32]. Effective antiretroviral therapy may reduce the HIV viral load, improve immune function, delay the onset of AIDS, enhance the quality of life, lower the risk of both AIDS-defining and non-AIDS-defining complications, and reduce the impact of HIV transmission in the community [33, 34]. Following long-term, extensive, repeated application of antiretroviral therapy, however, there is an increasing prevalence of HIV drug resistance [35–37]. It is therefore of great importance to monitor the effectiveness of first-line antiretroviral therapy in regions where the treatment regimen is widely implemented, because firstly, there is concern that continued use of antiretroviral therapy may give rise to populations of drug-resistant HIV strains, and secondly, the treatment regimens may be intermediately changed if drug resistance is detected.
Worldwide, a high level of HIV drug resistance has been reported among patients undergoing first-line antiretroviral therapy, notably in African countries [37–41]. In China, an increasing prevalence of drug resistance has been detected among HIV/AIDS patients with first-line antiretroviral therapy. In Hubei province, the prevalence of NRTI- and NNRTI-resistance mutations was 24.3 %, 57.1 % and 63.3 % at 3-6, 9-12, and 20-24 months post-treatment, respectively [42]. Data from the Chinese National HIV Drug Resistance Surveillance and Monitoring Network between 2003 and 2012 showed that the proportion of patients with drug resistance to NNRTIs, NRTIs and PIs was 52.9 %, 76.5 % and 4.4 %, respectively [43]. Among the 718 HIV-1-infected individuals with first-line antiretroviral therapy living in the provinces of Henan, Heilongjiang, Jilin, Liaoning, Neimenggu, Shanxi and Yunnan, the overall prevalence of drug-resistance mutations was 37.8 % [44]. A recent meta-analysis to describe the trends in emergent HIV drug resistance to first-line antiretroviral therapy among Chinese HIV-infected patients revealed that the pooled prevalence of HIV drug resistance from longitudinal cohort studies was 10.79 % and 80.58 % after 12 and 72 months of antiretroviral therapy, and the pooled prevalence of HIV drug resistance increased from 11.1 % after 0-12 months to 22.92 % after 61-72 months of antiretroviral therapy [25]. Until now, however, there has been little information on the effectiveness of and resistance to first-line antiretroviral therapy among HIV/AIDS patients in Fujian Province, Southeast China. The current study was therefore, in a longitudinal cohort, designed to evaluate the 5-year effectiveness of first-line antiretroviral therapy among HIV/AIDS patients.
In the current study, the longitudinal cohorts had a mean follow-up period of 70.86 ± 28.9 months, 83.8 % were followed up for 5 years or longer, and 76.7 % adhered to antiretroviral therapy. Across the follow-up period, the overall mortality was 14.2 %, with deaths predominantly observed during the first year of treatment (51.6 %). It has been demonstrated that early initiation of antiretroviral therapy lowers mortality in HIV-infected patients [45–47]. Previous studies have demonstrated that a high baseline CD4 cell count is associated with a low risk of AIDS-associated illness and death among HIV patients initiating antiretroviral therapy [48–51]. Therefore, the central government of China has gradually increased the absolute CD4 count cutoff from 200 cells/mm3 in 2005 to 350 cells/mm3 in 2012 and 500 cells/mm3 in 2015 for patients starting antiretroviral therapy [52, 53].
In this study, the efficacy of antiretroviral therapy was evaluated using body weight, CD4 count and HIV viral load. A 5-year follow-up showed that antiretroviral therapy markedly suppressed HIV replication, improved immune function, and increased body weight. In addition, a significant correlation was observed between immunological effectiveness and viral inhibition following antiretroviral therapy, except the first year, suggesting that the elevation in CD4 count was consistent with the decline in HIV viral load in most patients undergoing antiretroviral therapy. However, inconsistency was observed in some patients between immunological and virological outcomes following antiretroviral therapy, which may be attributed to age, baseline viral load, baseline CD4 count, treatment regimen, or adherence to therapy. Such a postulation is consistent with previous reports, demonstrating that the patient’s characteristics (such as age, viral load and CD4 count), treatment regimens and adherence affected the immunological and virological response to antiretroviral therapy [54–60].
Our findings showed that the change of first-line antiretroviral treatment regimens was mainly due to drug-related side effects, and the switch to second-line antiretroviral therapy was predominantly due to failure of first-line antiretroviral treatment due to drug resistance. HIV drug resistance testing showed resistance to NRTIs and NNRTIs at various levels, similar to other reports from China [61–63]. This may be associated with the wide implementation of free therapy with two NRTIs and one NNRTI. With the initiation of second-line antiretroviral therapy, more and more HIV/AIDS patients with failure in first-line therapy switch to a second-line antiretroviral therapy that includes PIs. Therefore, there is a high risk of emergence and spread of PI-resistant HIV strains, to which much attention should be paid. Follow-up of drug-resistant patients showed that low adherence to antiretroviral therapy was the major cause of HIV drug resistance. An assessment of the adherence to the therapy is therefore recommended prior to switching to second-line antiretroviral therapy, and the adherence to second-line antiretroviral therapy should be improved. Otherwise, drug resistance, or even treatment failure is unavoidable even if second-line antiretroviral therapy is employed.
The results suggest that the efficacy of currently recommended free first-line antiretroviral drugs remains satisfactory for the treatment of HIV/AIDS in Fujian Province, Southeast China; however, we cannot ignore the emergence and spread of drug-resistant HIV strains of drug-resistant strains, which may be detrimental for the sustainability of the therapy.
Conclusions
The results of this study demonstrate that the long-term efficacy of first-line antiretroviral therapy against HIV/AIDS appears satisfactory in Fujian Province, Southeast China, and the therapy is effective for increasing body weight, reducing the HIV viral load, and increasing the CD4 count. However, treatment failure and drug resistance were still detected. We therefore should not reduce our vigilance regarding the emergence of drug-resistant HIV strains. Fortunately, drug resistance can be easily detected [64–66]. Further periodical studies monitoring both the efficacy of first-line antiretroviral therapy and the development and epidemiology of drug-resistant HIV strains in Fujian province are still required. If resistance to first-line antiretroviral therapy is detected in HIV/AIDS patients, second-line antiretroviral therapy should be implemented immediately to delay the progression of HIV-associated illness and prevent the spread of HIV drug resistance.
References
Maartens G, Celum C, Lewin SR (2014) HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet 384:258–271
Shao Y, Jia Z (2012) Challenges and opportunities for HIV/AIDS control in China. Lancet 379:804
Murray CJ, Ortblad KF, Guinovart C et al (2014) Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384:1005–1070
Simon V, Ho DD (2006) Abdool Karim Q. HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. Lancet 368:489–504
Thaker HK, Snow MH (2003) HIV viral suppression in the era of antiretroviral therapy. Postgrad Med J 79:36–42
Kaufmann GR, Zaunders J, Cooper DA (1999) Immune reconstitution in HIV-1 infected subjects treated with potent antiretroviral therapy. Sex Transm Infect 75:218–224
Palella FJ Jr, Delaney KM, Moorman AC et al (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338:853–860
Hogg RS, Heath KV, Yip B et al (1998) Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA 279:450–454
Hogg RS, Yip B, Kully C et al (1999) Improved survival among HIV-infected patients after initiation of triple-drug antiretroviral regimens. CMAJ 160:659–665
Günthard HF, Aberg JA, Eron JJ et al (2014) Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA 312:410–425
World Health Organization (2006) Antiretroviral therapy of HIV Infection in adults and adolescents: towards universal access, recommendations for a public health approach. WHO, Geneva. http://www.who.int/hiv/pub/guidelines/en/. Accessed 7 July 2007
Zhang F, Haberer JE, Wang Y et al (2007) The Chinese free antiretroviral treatment program: challenges and responses. AIDS 21:S143–S148
Zhang FJ, Pan J, Yu L et al (2005) Current progress of China’s free ART program. Cell Res 15:877–882
Luo L, Li TS (2011) Overview of antiretroviral treatment in China: advancement and challenges. Chin Med J (Engl) 124:440–444
Zhang F, Dou Z, Yu L et al (2008) The effect of highly active antiretroviral therapy on mortality among HIV-infected former plasma donors in China. Clin Infect Dis 47:825–833
Zhang F, Dou Z, Ma Y et al (2009) Five-year outcomes of the China National Free Antiretroviral Treatment Program. Ann Intern Med 151:241–251
Solomon DA, Sax PE (2015) Current state and limitations of daily oral therapy for treatment. Curr Opin HIV AIDS 10:219–225
Wainberg MA, Friedland G (1998) Public health implications of antiretroviral therapy and HIV drug resistance. JAMA 279:1977–1983
Kozal MJ (2009) Drug-resistant human immunodefiency virus. Clin Microbiol Infect 15:69–73
DeGruttola V, Dix L, D’Aquila R et al (2000) The relation between baseline HIV drug resistance and response to antiretroviral therapy: re-analysis of retrospective and prospective studies using a standardized data analysis plan. Antivir Ther 5:41–48
Clavel F, Hance AJ (2004) HIV drug resistance. N Engl J Med 350:1023–1035
Lucas GM, Gallant JE, Moore RD (2004) Relationship between drug resistance and HIV-1 disease progression or death in patients undergoing resistance testing. AIDS 18:1539–1548
Hogg RS, Bangsberg DR, Lima VD et al (2006) Emergence of drug resistance is associated with an increased risk of death among patients first starting HAART. PLoS Med 3:e356
Xing H, Ruan Y, Li J et al (2013) HIV drug resistance and its impact on antiretroviral therapy in Chinese HIV-infected patients. PLoS One 8:e54917
Liu H, Ma Y, Su Y et al (2014) Emerging trends of HIV drug resistance in Chinese HIV-infected patients receiving first-line highly active antiretroviral therapy: a systematic review and meta-analysis. Clin Infect Dis 59:1495–1502
Li JY, Li HP, Li L et al (2005) Prevalence and evolution of drug resistance HIV-1 variants in Henan, China. Cell Res 15:843–849
Xia P, Okumura J, Yan P et al (2013) Steps towards preventive HIV treatment in Fujian, China: problems identified via an assessment of initial antiretroviral therapy provision. PLoS One 8:e76483
Zhuang MZ, Xia PC, Wu SL et al (2011) Analysis of AIDS antiretroviral therapy in Fujian Province from 2005 to 2009. Strait J Prev Med 17:5–7
Madec Y, Szumilin E, Genevier C et al (2009) Weight gain at 3 months of antiretroviral therapy is strongly associated with survival: evidence from two developing countries. AIDS 23:853–861
Rutherford GW, Anglemyer A, Easterbrook PJ et al (2014) Predicting treatment failure in adults and children on antiretroviral therapy: a systematic review of the performance characteristics of the 2010 WHO immunologic and clinical criteria for virologic failure. AIDS 28:S161–S169
Shao YM (2010) The strategy and methodology for HIV drug resistance surveillance and detection. People’s Medical Publishing House, Beijing
Piacenti FJ (2006) An update and review of antiretroviral therapy. Pharmacotherapy 26:1111–1133
The International AIDS Society Scientific Working Group on (2012) HIV Cure. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol 12:607–614
Thompson MA, Aberg JA, Hoy JF et al (2012) Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA 308:387–402
Hosseinipour MC, Gupta RK, Van Zyl G et al (2013) Emergence of HIV drug resistance during first- and second-line antiretroviral therapy in resource-limited settings. J Infect Dis 207:S49–S56
Sigaloff KC, Calis JC, Geelen SP et al (2011) HIV-1-resistance-associated mutations after failure of first-line antiretroviral treatment among children in resource-poor regions: a systematic review. Lancet Infect Dis 11:769–779
Manasa J, Lessells RJ, Skingsley A et al (2013) High-levels of acquired drug resistance in adult patients failing first-line antiretroviral therapy in a rural HIV treatment programme in KwaZulu-Natal, South Africa. PLoS One 8:e72152
Dagnra AY, Vidal N, Mensah A et al (2011) High prevalence of HIV-1 drug resistance among patients on first-line antiretroviral treatment in Lomé, Togo. J Int AIDS Soc 14:30
Hamers RL, Sigaloff KC, Kityo C et al (2013) Emerging HIV-1 drug resistance after roll-out of antiretroviral therapy in sub-Saharan Africa. Curr Opin HIV AIDS 8:19–26
Hamers RL, Wallis CL, Kityo C et al (2011) HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. Lancet Infect Dis 11:750–759
Stadeli KM, Richman DD (2013) Rates of emergence of HIV drug resistance in resource-limited settings: a systematic review. Antivir Ther 18:115–123
Luo M, Liu H, Zhuang K et al (2009) Prevalence of drug-resistant HIV-1 in rural areas of Hubei province in the People’s Republic of China. J Acquir Immune Defic Syndr 50:1–8
Leng XB, Liang SJ, Ma YL et al (2014) HIV virological failure and drug resistance among injecting drug users receiving first-line ART in China. BMJ Open 4:e005886
Zhang M, Han XX, Cui WG et al (2008) The impacts of current antiretroviral therapy regimens on Chinese AIDS patients and their implications for HIV-1 drug resistance mutation. Jpn J Infect Dis 61:361–365
Anglemyer A, Rutherford GW, Easterbrook PJ et al (2014) Early initiation of antiretroviral therapy in HIV-infected adults and adolescents: a systematic review. AIDS 28:S105–S118
Violari A, Cotton MF, Gibb DM et al (2008) Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 359:2233–2244
Thorner A, Rosenberg E (2003) Early versus delayed antiretroviral therapy in patients with HIV infection: a review of the current guidelines from an immunological perspective. Drugs 63:1325–1337
Opportunistic Infections Project Team of the Collaboration of (2012) Observational HIV Epidemiological Research in Europe (COHERE) in EuroCoord. CD4 cell count and the risk of AIDS or death in HIV-Infected adults on combination antiretroviral therapy with a suppressed viral load: a longitudinal cohort study from COHERE. PLoS Med 9:e1001194
Mills EJ, Bakanda C, Birungi J et al (2011) Mortality by baseline CD4 cell count among HIV patients initiating antiretroviral therapy: evidence from a large cohort in Uganda. AIDS. 25:851–855
Olubajo B, Mitchell-Fearon K, Ogunmoroti O (2014) A comparative systematic review of the optimal CD4 cell count threshold for hiv treatment initiation. Interdiscip Perspect Infect Dis 2014:625670
Moore DM, Hogg RS, Yip B et al (2006) CD4 percentage is an independent predictor of survival in patients starting antiretroviral therapy with absolute CD4 cell counts between 200 and 350 cells/microL. HIV Med 7:383–388
Ministry of Health (2005) National handbook for free treatment of HIV/AIDS with antiretroviral drugs, 2nd edn. People’s Medical Publishing House Co., LTD., Beijing
Ministry of Health (2012) National handbook for free treatment of HIV/AIDS with antiretroviral drugs, 3rd edn. People’s Medical Publishing House Co., LTD., Beijing
Hadland SE, Milloy MJ, Kerr T et al (2012) Young age predicts poor antiretroviral adherence and viral load suppression among injection drug users. AIDS Patient Care STDS 26:274–280
O’Neil CR, Palmer AK, Coulter S et al (2012) Factors associated with antiretroviral medication adherence among HIV-positive adults accessing highly active antiretroviral therapy (HAART) in British Columbia, Canada. J Int Assoc Physicians AIDS Care (Chic) 11:134–141
Nolan S, Milloy MJ, Zhang R et al (2011) Adherence and plasma HIV RNA response to antiretroviral therapy among HIV-seropositive injection drug users in a Canadian setting. AIDS Care 23:980–987
Cescon AM, Cooper C, Chan K et al (2011) Factors associated with virological suppression among HIV-positive individuals on highly active antiretroviral therapy in a multi-site Canadian cohort. HIV Med 12:352–360
Althoff KN, Justice AC, Gange SJ et al (2010) Virologic and immunologic response to HAART, by age and regimen class. AIDS 24:2469–2479
May MT, Hogg RS, Justice AC et al (2012) Heterogeneity in outcomes of treated HIV-positive patients in Europe and North America: relation with patient and cohort characteristics. Int J Epidemiol 41:1807–1820
Lima VD, Harrigan R, Murray M et al (2008) Differential impact of adherence on long-term treatment response among naive HIV-infected individuals. AIDS 22:2371–2380
Su Y, Zhang F, Liu H et al (2014) The prevalence of HIV-1 drug resistance among antiretroviral treatment naïve individuals in mainland China: a meta-analysis. PLoS One 9:e110652
Wu J, Norris J, Liu HX et al (2014) The prevalence of HIV drug resistance among treatment-failure individuals and treatment-naïve individuals in China: a meta-analysis. Biomed Environ Sci 27:858–871
Hua J, Lin H, Ding Y et al (2015) HIV drug resistance in newly diagnosed adults in a rural prefecture of eastern China. Epidemiol Infect 143:663–672
Vandamme AM, Houyez F, Bànhegyi D et al (2001) Laboratory guidelines for the practical use of HIV drug resistance tests in patient follow-up. Antivir Ther 6:21–39
Vandamme AM, Camacho RJ, Ceccherini-Silberstein F et al (2011) European recommendations for the clinical use of HIV drug resistance testing: 2011 update. AIDS Rev 13:77–108
Haubrich R, Demeter L (2001) International perspectives on antiretroviral resistance. Clinical utility of resistance testing: retrospective and prospective data supporting use and current recommendations. J Acquir Immune Defic Syndr 26:S51–S59
Acknowledgments
This study was supported by a grant from the Scientific and Research Project of Fujian Provincial Department of Health for Young Investigators (grant no. 2013-1-14) and the Personal Supporting Projects of Health Department in Fujian Province of China (2015-ZQN-ZD-11). We are thankful to all participants for their kind cooperation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
S. Wu and Y. Qiu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wu, S., Qiu, Y., Yan, P. et al. Effectiveness of first-line antiretroviral therapy in HIV/AIDS patients: A 5-year longitudinal evaluation in Fujian Province, Southeast China. Arch Virol 160, 2693–2701 (2015). https://doi.org/10.1007/s00705-015-2583-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-015-2583-z