Abstract
A new duck Tembusu virus (TMUV), also known as BYD virus, has been identified as the causative agent for the emerging duck egg-drop syndrome in mainland China. The rapid spread and wide distribution of the new TMUV in mainland China result in heavy loss to the poultry industry and pose great threats to public health. Rapid and sensitive detection methods are critical for prevention and control of TMUV infections. In this study, a reverse-transcription loop-mediated isothermal amplification assay (RT-LAMP) and an SYBR Green-I–based real-time RT-PCR assay specific for the duck TMUV were developed and validated with laboratory and field samples, respectively. The detection limits were 1 × 10−4 and 1 × 10−3 PFU per reaction for the RT-LAMP assay and real-time RT-PCR assay, respectively. The specificities were analyzed with other related members of the genus Flavivirus, and no cross-reaction was observed. Furthermore, both assays were evaluated with field samples, and they exhibited high sensitivity and specificity. In addition, the real-time RT-PCR assay worked well in viral load analysis, which revealed that the spleen may be the primary target for the replication of new duck TMUV in ducks. The TMUV-specific RT-LAMP and real-time RT-PCR assays will provide useful tools for the diagnosis and epidemiological surveillance of TMUV infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Duck farming represents an important agro-business in China and some Asia countries. However, since April 2010, a severe infectious disease, termed duck egg-drop syndrome (DEDS), has emerged in egg-laying and breeder ducks and spread around the major duck-breading regions in mainland China in a few months. Currently, DEDS has been reported in Zhejiang, Fujian, Jiangsu, Jiangxi, Hebei, Shandong, Guangxi, Guangdong, and Anhui provinces, and more than 10 million ducks were affected, resulting in heavy loss to poultry industry [14, 18]. DEDS is characterized by a sudden onset with heavy egg production decline. Affected ducks develop clinical symptoms including high fever, diarrhea, loss of appetite, and retarded growth. The disease exhibits high pathogenicity, with an almost 100 % infection rate, and mortality varies from 5 % to 30 % in ducks [14, 18]. The epidemic of DEDS has rapidly become a great threat to the poultry industry in mainland China.
A new virulent variant of Tembusu virus (TMUV), named BYD virus (BYDV) after the name of the district in which it was first isolated, has recently been identified as the causative agent of DEDS [14]. BYDV shows high amino acid sequence homology and a close antigenic relationship to the known TMUV isolate, which is a member of the Ntaya virus group within the genus Flavivirus, family Flaviviridae [2, 14, 18]. The origin, natural host and transmission cycle of the new duck TMUV remain elusive, and special attention should be paid to its threat to public health.
Currently, no vaccine or control measure is available for duck TMUV infection. Early, rapid and sensitive detection methods are critical for prevention and control of duck TMUV infections. Currently, the conventional RT-PCR method is used to detect new TMUV strains [14]. A real-time RT-PCR assay based on the Taqman® probe technique was developed recently and exhibited a sensitivity of 50 copies per reaction [17]. However, these methods require specific, expensive equipment and skilled technicians, which limits their applications in resource-limited laboratories, especially in rural animal health stations.
The loop-mediated isothermal amplification (LAMP) method, first described in 2000 [10], is a rapid, simple and field-adaptable technique. This technique employs the principle of a strand displacement reaction while the amplification reaction runs under isothermal conditions without sophisticated and expensive thermal cyclers. The LAMP primers are designed to recognize four to six distinct regions of the target gene, which gives them the potential for high specificity. Furthermore, LAMP exhibits extremely high amplification efficiency of at least 0.4 μg/μl DNA yield in less than one hour. Previously, LAMP methods have been developed for detection of a number of viral pathogens, including West Nile virus (WNV), dengue virus (DENV), Japanese encephalitis virus (JEV), enterovirus type 71 (EV71), H5N1 avian influenza virus (AIV), duck plague virus, and Muscovy duck parvovirus [6–8, 12]. All of these results demonstrated the superiority of LAMP as a routine field-detection method for viral infectious diseases.
In this study, a RT-LAMP assay together with a SYBR Green-I–based real-time RT-PCR assay was developed for detection of the new duck TMUV. Both assays are rapid, sensitive and specific, providing powerful tools for early and rapid diagnosis of duck TMUV infections.
Materials and methods
Field samples
A total of 27 field samples including spleen, liver and kidney were collected from 15 ducks with DEDS from two poultry farms in Hebei and Jiangxi provinces. Negative control sample, including brain and liver samples, were collected from four healthy ducks from the same farm. The ducks were taken to the laboratory and sacrificed, and tissue samples were collected. A 10 % suspension was made from homogenized sample with Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, CA, USA). After centrifuging at 10,000 g for 10 min, the upper suspension was collected for further assay.
The field studies were approved by each farm, and the field samples were appropriately coded to be anonymous. All animal experimental procedures were carried out in strict accordance with the guidelines of the Animal Experiment Committee of the State Key Laboratory of Pathogen and Biosecurity.
Cells and viruses
The following six flavivirus strains were used for specificity evaluation in this study: TMUV strain byd-1, DENV type 2 (DENV2) strain 43, WNV strain Chin-01, JEV strain SA14, tick-borne encephalitis virus (TBEV) strain Senzhang, and yellow fever virus (YFV) strain 17D. All experiments with live WNV were performed in biosafety level 3 laboratories.
Baby hamster kidney cells (BHK-21; ATCC CCL-10) were maintained in DMEM (Life Technologies) supplemented with 10 % fetal bovine serum (FBS) (Life Technologies). All flaviviruses were passaged in BHK-21 cells for 3-5 days to produce the final stocks. Briefly, a monolayer of BHK-21 cells was infected by the above flaviviruses for 1 hour and then incubated in DMEM supplemented with 2 % FBS in a 5 % CO2 incubator at 37 °C until 75 % to 100 % cytopathic effect was observed. The viral culture supernatants were collected and stored at -80 °C until use.
RNA extraction
Total RNA was extracted from 200 μl sample suspension or viral culture supernatant using a Purelink RNA Mini Kit (Life Technologies) according to the instructions of the manufacturer. The extracted RNA was eluted in a final volume of 40 μl in RNase-free water and stored at −80 °C until use. For evaluation of the detection limit of the RT-LAMP and real-time RT-PCR, serial RNA samples were extracted from tenfold serial dilutions of viral culture from 104 to 10−4 PFU/ml.
Primer design for RT-LAMP and real-time RT-PCR
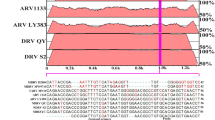
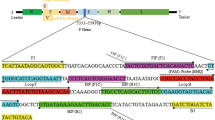
The nucleotide sequence of the complete genome of TMUV strain byd-1 was retrieved from the Genbank dataBase (GenBank accession number JF312912) and then input into the online software LAMP Primer Explorer (http://primerexplorer.jp/e/). A total of 25 primer sets for TMUV-specific RT-LAMP, comprising two outer primers (F3 and B3) and two inner primers (FIP and BIP) were designed accordingly. Subsequently, potential mismatched bases were taken into consideration by using the BLAST program (http://blast/ncbi.nlm.nih.gov/blast.cgi), and six primer sets that exhibited minor mismatches were chosen. After tests with different dilutions of TMUV viral RNA, the best primer set was selected, and a loop primer (LF) was then designed using LAMP Primer Explorer. The primers for TMUV-specific real-time RT-PCR assay were designed using Primer Express 3.0 (life technologies). Four primer sets were screened and then validated using the BLAST program. After evaluation with different dilutions of TMUV viral RNA, the best primer pair was selected. All of the primers used in this study (Table 1) were synthesized by Invitrogen Shanghai Ltd.
RT-PCR
The conventional RT-PCR reaction was performed using a PrimeScript® One-Step RT-PCR Kit (Takara, Dalian, China) with specific primers TV-3(f) and TV-3(r) for TMUV as described previously by Su et al. [14]. The primers amplified a 400-bp segment of the envelope gene of the TMUV genome. The 50 μl reaction mixture contained 2 μl of total RNA, 25 μl of 2× One step buffer, 2 μl of PrimeScript 1 Step Enzyme Mix, 2 μl each of forward and reverse primer (10 μM), and 17 μl of RNase-free water. The reaction conditions were set at 50 °C for 30 min and 94 °C for 2 min, followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 70 °C for 30 s, with a final extension step of 72 °C for 7 min. Finally, RT-PCR products were analyzed by agarose gel electrophoresis.
RT-LAMP assay
The RT-LAMP reaction was carried out in a total of 25 μl reaction mixture containing 2.5 μl of total RNA, 1.6 μM each of FIP and BIP primers, 0.2 μM each of F3 and B3 primers, 0.8 μM LF primer, 1.4 mM deoxynucleotide triphosphates, 0.8 M betaine, 0.1 % (w/v) Tween 20, 10 mM of (NH4)2SO4, 8 mM MgSO4, 10 mM KCl, 20 mM Tris-HCl (pH 8.8), 16 units of Bst DNA polymerase (NEB, MA, USA), and 0.125 units of AMV reverse transcriptase (Promega, WI, USA) in accordance with the published protocol [11].
RT-LAMP amplification was monitored in real time in a LoopAmp real-time turbidimeter LA-320 (Eiken Chemical, Tokyo, Japan) at 63 °C for 30 min (the optical density data of each reaction were recorded every 6 s), and this was followed by heating at 80 °C for 5 min to terminate the reaction. The threshold of turbidity for a positive sample was defined as 0.1 according to the instructions for the instrument. The time of positivity (Tp) was determined as the time when the turbidity value increased above the threshold.
For visual detection of the endpoint, 1 μl of calcein (1.25 nM) was added to the reaction mixture, which was then incubated at 63 °C. When the reaction was complete, a color change was observed under normal light and UV light.
Real-time RT-PCR
Real-time RT-PCR was performed using a One-Step SYBR® PrimeScript® Plus RT-PCR Kit (Takara, Dalian, China) in the LightCycler 2.0 system (Roche, Penzberg, Germany). The reaction mixture of 20 μl contained 2 μl of total RNA, 10 μl of 2× One Step SYBR RT-PCR Buffer 4, 1.2 μl of Ex Taq® HS mix, 0.4 μl of PrimeScript® PLUS RTase Mix, 0.8 μl each of forward and reverse primer (10 μM), and 4.8 μl of RNase-free water. The reaction was performed for 5 min at 42 °C, followed by 20 s at 95 °C, with a subsequent 40 cycles of amplification (95 °C for 5 s, 60 °C for 20 s; fluorescence was recorded at 60 °C), and this was followed by a melting analysis cycle (95 °C for 0 s; 65 °C for 15 s; 95 °C for 15 s by slowly increasing the temperature 0.1 °C/second).
Sensitivity and specificity of the assays
The sensitivity of the RT-LAMP and real-time RT-PCR assays was analyzed using RNA extracted from a tenfold dilution series of viral culture ranging from 104 to 10−4 PFU/ml. The standard curve of the real-time RT-PCR was established by plotting the log of each standard of known concentration in the dilution series against the Ct values for that concentration. The specificities of the assays were evaluated by cross-reactivity tests with five other related members of the genus Flavivirus, including DENV2 strain 43, WNV strain Chin-01, JEV strain SA14, TBEV train Senzhang, and YFV strain 17D.
Evaluation with field samples
The RT-LAMP and real-time RT-PCR assays were evaluated with 35 field samples. The Tp value of 30 min was set as the cutoff for a positive result in the RT-LAMP assay, while Ct ≤ 37 was detected to be positive for the real-time RT-PCR assay. Restriction analysis of RT-LAMP products was carried out to confirm the specific amplification. All samples were also tested by conventional RT-PCR assay, and the PCR products were sequenced.
Results
Sensitivity and specificity of RT-LAMP and real-time RT-PCR
A series of TMUV RNA dilutions ranging from 102 to 10−6 PFU per reaction mixture were used to analyze the sensitivity of the RT-LAMP and real-time RT-PCR. The results showed that TMUV-infected samples from ducks can be readily detected by the RT-LAMP assay (Fig. 1), and the detection limit of the TMUV-specific RT-LAMP is 1 × 10−4 PFU per reaction (Tp = 27.1 min). Based on the result of the sensitivity analysis, the duration of the RT-LAMP reaction was optimized to be 30 min. The amplification products of RT-LAMP can be observed under natural light or UV light. The color of the positive reaction changed from brown to green under natural light (Fig. 1B and D), and bright green fluorescence was observed under UV light (Fig. 1C and E). In the case of negative samples, the color of reaction mixture was retained under natural light as well as UV light. In the specificity test, there was no visible amplification or color change in the RT-LAMP reactions with viral RNA from DENV, JEV, WNV, TBEV or YFV, which demonstrated the high specificity of the RT-LAMP for detection of duck TMUV. (Fig. 1D and E).
Sensitivity and specificity of the RT-LAMP assay. (A) Amplification curves of RT-LAMP with a series of RNA ranging from 101 to 10−6 PFU per reaction. The detection limit is 1 × 10−4 PFU per reaction (orange line). (B) and (C) direct visual detection of RT-LAMP with RNA extracted from serial dilution of viral culture. Calcein (1 μl, 1.25 nM) was added to each reaction mixture for visual detection of the endpoint. The colors of the positive reactions (from 101 to 10−4 PFU per reaction) changed from brown to green under natural light (B), and to bright green fluorescence under UV light (C). (D) and (E) direct visual detection of RT-LAMP with RNA extracted from viral culture of TMUV, WNV, DEN2, JEV, TBEV and YFV. The specific color change was observed for RT-LAMP with TMUV RNA. No color change was seen in RT-LAMP with other viral RNA under natural light or UV light (color figure online)
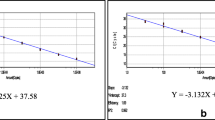
The results of the real-time RT-PCR showed good correlation with those of the RT-LAMP assay, and the detection limit was 1 × 10−3 PFU per reaction (Ct = 34.9) (Fig. 2A). The standard curve analysis showed that the assay had a wide linear range (from 10−3 PFU to 102 PFU per reaction, R2 = 0.998) (Fig. 2B). The specificity of real-time the RT-PCR assay was evaluated with other five flaviviruses, including DENV-2, WNV, JEV, TBEV and YFV. There was no specific amplification observed, demonstrating the high specificity of the SYBR Green-I–based real-time RT-PCR assay for detection of duck TMUV (Fig. 2C).
Sensitivity and specificity of the real-time RT-PCR assay. (A) Amplification curves of real-time RT-PCR with a series of viral RNA ranging from 102 to 10−4 PFU/ml. The x-axis represents cycles, and the y-axis represents the fluorescence data. The detection limit is 1 × 10−3 PFU per reaction. (B) Standard curve of the real-time RT-PCR assay. RNA standards ranging from 102 to 10−3 PFU/ml were plotted against the threshold cycle. The equation of the regression curve (y) and the coefficient of determination (R2) were calculated. (C) Real-time RT-PCR results for TMUV, WNV, DEN2, JEV, TBEV and YFV. An amplification curve was observed in the case of the positive sample (orange line). No amplification was seen with other viral RNAs (color figure online)
Evaluation with field samples
Field samples were tested using the RT-LAMP and real-time RT-PCR assays. The results of the RT-LAMP (Fig. 3) and real-time RT-PCR assays in comparison with the conventional RT-PCR are shown in Table 2. Samples collected from healthy ducks all tested negative using all three assays. Of the 27 samples collected from ducks suspected to be infected with TMUV, 25 that were positive for TMUV by conventional RT-PCR assay agreed with the results of the RT-LAMP and real-time RT-PCR assays. However, one of two samples that were negative by conventional RT-PCR assay was positive by both RT-LAMP and real-time RT-PCR assays, and this was further confirmed by gel electrophoresis of the RT-LAMP products as well as melting curve analysis in the real-time RT-PCR. These results showed that both RT-LAMP and real-time RT-PCR were more sensitive than conventional RT-PCR.
Visual detection of field samples by RT-LAMP. RNA extracted from field samples was detected using the RT-LAMP assay. Calcein (1 μl, 1.25 nM) was added to each reaction mixture for visual detection of the endpoint. RT-LAMP products were observed visually under natural light (A) and under UV light (B). A plus sign indicates the positive control (viral RNA), and a minus sign indicates the negative control (water). The reactions exhibiting a green color under natural light and bright green fluorescence under UV light were considered positive
Comparison of viral loads in different tissues
The viral loads of TMUV in 16 different tissue samples were determined using the real-time RT-PCR assay (Fig. 4). The peak viral load of 3.18 log10 PFU/ml was observed in the spleen samples, with a median of 2.34 log10 PFU/ml (IQR: 1.79 to 2.69 log10 PFU/ml). The median viral load in the liver samples were 0.05 log10 PFU/ml (IQR: −0.74 to 0.56 log10 PFU/ml), while a median of 0.26 log10 PFU/ml was found in the brain samples (IQR: −0.64 to 1.25 log10 PFU/ml). The results showed that the spleen tissues exhibited higher viral loads compared to livers and brains in the TMUV-infected ducks.
Discussion
In mainland China and other Asia countries, most poultry farms are still run in the traditional way, and breeders have limited knowledge of infectious diseases. The poultry industry faces serious threats from infectious diseases of fowl. As an emerging infectious disease of ducks, DEDS, caused by the new duck TMUV, spreads rapidly and results in high mortality in ducks. No vaccine or control measure is currently available due to the limited information about TMUV. Moreover, very significantly, TMUV is reported to be the first flavivirus that can cause severe disease in ducks [5, 14]. Previous studies showed that only a few flaviviruses, such as WNV and Usutu virus, were reported to causes mild disease in water fowl or ducks [3, 5]. Interestingly, Sitiawan virus, which has also been identified as a virulent variant of TMUV, can cause encephalitis in chickens [9]. Although most human-pathogenic flaviviruses are transmitted by mosquitoes or ticks, the transmission cycle and pathogenicity of the new duck TMUV for humans is still unknown. However, considering its rapid spread and wide distribution in mainland China, the potential threat to public health should not be ignored. Simple and rapid detection methods are therefore important not only for the poultry industry but also for public health.
In this study, a RT-LAMP and a SYBR Green-I–based real-time RT-PCR assay were developed for rapid detection of the new duck TMUV. Unlike the published Taqman assay, the SYBR-based real-time RT-PCR assay does not need a specific probe, so its cost per reaction is much lower. The RT-LAMP assay is one of the best choices for field detection. It has the highest sensitivity of all of the developed methods for detection of TMUV, and no cross-reactivity with other flaviviruses was observed. Additionally, the amplification reaction in the RT-LAMP assay is done under isothermal conditions, and a simple constant-temperature water bath can fulfill the requirement. The results of the duck TMUV-specific RT-LAMP assay are also easy to judge by observing the color change under natural light and UV light. As shown in Fig. 1B and C, when the LAMP is performed with serial dilutions of viral RNA, the reaction results in a distinct color change that distinguishes positive from negative samples even when the viral RNA concentration is near the detection limit. The RT-LAMP assay is therefore a perfect fit for township veterinary stations lacking skilled technicians and diagnostic apparatus. In short, both of the assays developed in the current study exhibited high sensitivity and specificity and can be used for rapid and early detection of duck TMUV, separately or in combination. In the case of combined application for detection of TMUV, the RT-LAMP assay is appropriate for visual field screening, while the SYBR-based real-time RT-PCR assay is appropriate for laboratory tests, with the melting curve analysis for discriminating the specific amplification product from nonspecific ones.
When our report was submitted, two other RT-LAMP assays with sensitivities of 4 and 20 copies per reaction were also reported [15, 16]. There is no detailed information about the relationship between viral RNA copy number and infectious units of TMUV. Considering that the number of RNA copies per infectious unit (PFU) was found to be around 1000 in previous studies of DENV and YFV [1, 13], the sensitivity of the two RT-LAMP assays can be roughly estimated to be 4 × 10−3 and 2 × 10−2 PFU per reaction, respectively. In contrast, the assays developed in the current study exhibit higher sensitivity, with 1 × 10−4 and 1 × 10−3 PFU per reaction for the RT-LAMP assay and the real-time RT-PCR assay, respectively. In addition, the RT-LAMP assay is faster, and the result can be obtained within 30 min, compared to 45 or 50 min in the case of the published assays. More importantly, the RT-LAMP assay developed in the current study was shown to have practical application in visual detection of field samples. The results show a high potential of the RT-LAMP assay for pen-side testing. Considering that the number of samples tested in this study was not sufficient, further studies using more field samples from different endemic areas are needed. Meanwhile, the improved method of RNA extraction from field samples should also be taken into consideration.
Sequence alignment and analysis indicated that there was no mismatch between the primers and nucleotide sequences of other related viral duck pathogens. The cross-reactivity of the RT-LAMP assay and real-time RT-PCR assay was evaluated with five other flaviviruses. The DENV, JEV and TBEV strains used in the tests are endemic flavivirus of public health importance in mainland China, and WNV can cause disease both in poultry and humans [4]. Both assays showed good specificity, which were further confirmed using field samples collected from ducks.
There is little information about the tissue distribution of the new duck TMUV. By using the SYBR Green-I real-time RT-PCR assay, the viral loads in different tissues were measured in the study. Higher viral loads were observed in spleen samples than in liver and brain samples, which agrees with the findings of tissue distribution of TMUV in experimentally infected ducks [18]. Considering that splenomegaly and severe necrosis of the spleen are important clinical features of infected ducks [14, 18], duck spleen seems to be one of the preferred targets for replication of the new duck TMUV. This result also suggested a good choice for sampling for TMUV detection.
In conclusion, a TMUV-specific RT-LAMP assay and a SYBR Green-I–based real-time assay were developed and evaluated with laboratory and field samples with high sensitivity and high specificity. These assays have the potential to provide useful tools for detection of duck TMUV, especially in a resource-limited setting, and they will play important roles in the early diagnosis and epidemiological surveillance of new duck TMUV infection.
References
Barban V, Girerd Y, Aguirre M, Gulia S, Petiard F, Riou P, Barrere B, Lang J (2007) High stability of yellow fever 17D–204 vaccine: a 12-year restrospective analysis of large-scale production. Vaccine 25:2941–2950
Cao Z, Zhang C, Liu Y, Ye W, Han J, Ma G, Zhang D, Xu F, Gao X, Tang Y, Shi S, Wan C, He B, Yang M, Lu X, Huang Y, Diao Y, Ma X (2011) Tembusu virus in ducks, china. Emerg Infect Dis 17:1873–1875
Chvala S, Bakonyi T, Hackl R, Hess M, Nowotny N, Weissenbock H (2006) Limited pathogenicity of usutu virus for the domestic goose (Anser anser f. domestica) following experimental inoculation. J Vet Med B Infect Dis Vet Public Health 53:171–175
Gao X, Nasci R, Liang G (2010) The neglected arboviral infections in mainland China. PLoS Negl Trop Dis 4:e624
Hubalek Z (2004) An annotated checklist of pathogenic microorganisms associated with migratory birds. J Wildl Dis 40:639–659
Ji J, Du LQ, Xie QM, Cao YC, Zuo KJ, Xue CY, Ma JY, Chen F, Bee YZ (2009) Rapid diagnosis of duck plagues virus infection by loop-mediated isothermal amplification. Res Vet Sci 87:53–58
Ji J, Xie QM, Chen CY, Bai SW, Zou LS, Zuo KJ, Cao YC, Xue CY, Ma JY, Bi YZ (2010) Molecular detection of Muscovy duck parvovirus by loop-mediated isothermal amplification assay. Poult Sci 89:477–483
Jiang T, Liu J, Deng YQ, Xu LJ, Li XF, Han JF, Cao RY, Qin ED, Qin CF (2011) Development and evaluation of a reverse transcription-loop-mediated isothermal amplification assay for rapid detection of enterovirus 71. J Clin Microbiol 49:870–874
Kono Y, Tsukamoto K, Abd Hamid M, Darus A, Lian TC, Sam LS, Yok CN, Di KB, Lim KT, Yamaguchi S, Narita M (2000) Encephalitis and retarded growth of chicks caused by Sitiawan virus, a new isolate belonging to the genus Flavivirus. Am J Trop Med Hyg 63:94–101
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28:e63
Parida M, Horioke K, Ishida H, Dash PK, Saxena P, Jana AM, Islam MA, Inoue S, Hosaka N, Morita K (2005) Rapid detection and differentiation of dengue virus serotypes by a real-time reverse transcription-loop-mediated isothermal amplification assay. J Clin Microbiol 43:2895–2903
Parida M, Sannarangaiah S, Dash PK, Rao PV, Morita K (2008) Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev Med Virol 18:407–421
Richardson J, Molina-Cruz A, Salazar MI, Wt Black (2006) Quantitative analysis of dengue-2 virus RNA during the extrinsic incubation period in individual Aedes aegypti. Am J Trop Med Hyg 74:132–141
Su J, Li S, Hu X, Yu X, Wang Y, Liu P, Lu X, Zhang G, Liu D, Li X, Su W, Lu H, Mok NS, Wang P, Wang M, Tian K, Gao GF (2011) Duck egg-drop syndrome caused by BYD virus, a new Tembusu-related flavivirus. PLoS One 6:e18106
Tang Y, Diao Y, Yu C, Gao X, Chen L, Zhang D (2011) Rapid detection of Tembusu virus by reverse-transcription, loop-mediated isothermal amplification (RT-LAMP). Transbound Emerg Dis 59:208–213
Wang Y, Yuan X, Li Y, Yu K, Yang J, Xu H, Zhang Y, Liao M, Qin Z (2011) Rapid detection of newly isolated Tembusu-related Flavivirus by reverse-transcription loop-mediated isothermal amplification assay. Virol J 8:553
Yan L, Yan P, Zhou J, Teng Q, Li Z (2011) Establishing a TaqMan-based real-time PCR assay for the rapid detection and quantification of the newly emerged duck Tembusu virus. Virol J 8:464
Yan P, Zhao Y, Zhang X, Xu D, Dai X, Teng Q, Yan L, Zhou J, Ji X, Zhang S, Liu G, Zhou Y, Kawaoka Y, Tong G, Li Z (2011) An infectious disease of ducks caused by a newly emerged Tembusu virus strain in mainland China. Virology 417:1–8
Acknowledgments
This work was supported, in part, by the Major Special Program of National Science and Technology of China (No.2009ZX10004-204), and the National Natural Science Foundation of China (No. 30972613, No. 81000722). CFQ was supported by Beijing Nova Program of Science and Technology (No.2010B041).
Conflict of interests
The authors have declared that no competing interests exist.
Author information
Authors and Affiliations
Corresponding author
Additional information
T. Jiang and J. Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jiang, T., Liu, J., Deng, YQ. et al. Development of RT-LAMP and real-time RT-PCR assays for the rapid detection of the new duck Tembusu-like BYD virus. Arch Virol 157, 2273–2280 (2012). https://doi.org/10.1007/s00705-012-1431-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-012-1431-7