Abstract
Barley yellow dwarf viruses (BYDVs, mainly consisting of three strains, GAV, GPV and PAV, in China), barley stripe mosaic virus (BSMV), wheat yellow mosaic virus (WYMV), wheat dwarf virus (WDV) and wheat blue dwarf phytoplasma (WBD) constitute a group of major wheat pathogens that have caused huge yield losses but are scarcely distinguished by their phenotype alone. For the simultaneous detection and discrimination of these seven pathogens in wheat, a multiplex polymerase chain reaction (M-PCR) method was developed in this study through a series of parameter optimizations. Detection and sensitivity tests using samples collected from the field indicate that the M-PCR method can rapidly, simultaneously and relatively effectively detect BYDV-GAV, -GPV, -PAV, BSMV, WYMV, WDV and WBD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat, as one of the most important food crops worldwide, is infected by various kinds of pathogens, including fungi, bacteria, viruses and phytoplasma, which seriously influence the yield and quality of wheat. Since agriculture is a vital part of China’s economy, it is important to develop a method to detect these pathogens quickly, accurately and economically. Compared to fungal and bacterial pathogens, infections caused by viruses and phytoplasma are difficult to distinguish or demonstrate based on their symptoms alone. The major group of viruses and phytoplasma infecting wheat in China consists of wheat yellow mosaic virus (WYMV), barley yellow dwarf virus (BYDV), barley stripe mosaic virus (BSMV), wheat dwarf virus (WDV) and wheat blue dwarf (WBD) phytoplasma, all of which have caused great losses to China’s wheat production.
The group of viruses collectively designated as barley yellow dwarf viruses (BYDVs) belongs to the family Luteoviridae and are important viruses in wheat because of their ability to cause significant diseases and yield losses worldwide. These viruses infect a wide range of species of Gramineae and are transmitted naturally by aphids in a highly specific, circulative, and non-propagative manner [1]. Currently, the viruses causing barley yellow dwarf disease are divided into three groups: BYDV-PAV, BYDV-MAV, and BYDV-PAS in the genus Luteovirus; Cereal yellow dwarf virus-RPV (CYDV-RPV) and CYDV-RPS in the genus Polerovirus; and BYDV-GPV, BYDV-SGV and BYDV-RMV, which have not been assigned to a genus [2, 3]. In these eight isolates of B/CYDVs, only BYDV-PAV, -GPV and -RMV have been found in China [4, 5]. BYDV-PAV and -GPV are both common strains, while BYDV-RMV is a rare isolate in China, which was found only once in Guiyang [6]. Except for these three isolates, another common Chinese strain, BYDV-GAV (named according to Rochow’s system), is serologically related to BYDV-MAV. Although there are no detailed criteria based on nucleotide sequence homology for classification of BYDVs, their high degree of nucleotide and amino acid sequence similarity indicates that BYDV-GAV and BYDV-MAV are very similar [7].
Wheat yellow mosaic virus (WYMV), a soil-borne virus vectored by the fungus-like organism Polymyxa graminis, causes one of the most devastating soil-borne wheat diseases in China, wheat yellow mosaic [8]. Yellow-striped leaves and stunted spring growth are the main symptoms of WYMV infection. However, similar symptoms are also caused by barley stripe mosaic virus (BSMV), the type member of the genus Hordeivirus. BSMV is a seed-borne virus mainly infecting barley, wheat and wild oats [9]. In China, the virus first occurred in Xinjiang Province and subsequently spread throughout China, causing serious yield losses [10].
Wheat blue dwarf disease (WBD), another of the most important diseases of cereal crops, is caused by WBD phytoplasma and is transmitted by a leafhopper vector (Psammotettix striatus L.). This pathogen infects winter wheat in arid and semiarid areas of northwestern China, leading to severe losses [11]. Another leafhopper-transmitted pathogen, wheat dwarf virus (WDV), is a member of the genus Mastrevirus in family Geminiviridae. Its host range includes wheat, barley, oats and many cereal grasses. Typical symptoms of WDV infection include dwarfing, mottling, yellowing and reduction of heading in wheat and barley. Since the first report of a circular single-stranded (ss)-DNA virus, significant economic losses have resulted from infection by and spread of this virus in Europe, Asia and Africa [12].
Earlier detection methods for wheat viruses based on biology [13], serology [14] and electron microscopy, have been developed, but these are only suitable for primary identification of viruses. Recently, molecular methods, mainly including nucleic acid hybridization and polymerase chain reaction (PCR) technology, have been established for detection of viruses. Although hybridization is an economical and effective alternative for detecting and distinguishing different viruses, it is not sensitive enough to detect viruses that are present at low titre. More importantly, most assays using traditional methods and single PCR techniques are only effective for detecting a single virus, and consequently, more time and reagents are needed when multiple viruses are to be detected and identified. The inability of phytoplasmas to be cultured in vitro, their variable titres, and their uneven distribution in plants make their detection very difficult.
Multiplex PCR is a new technique in which several pairs of primers are used together in one PCR, leading to simultaneous amplification of different regions or sizes of DNA fragments [15]. Compared to single PCR, this method decreases the risk of contamination, saves time and reduces the cost. Currently, mixed infections with these seven pathogens are common in the field in northwestern China. A large amount of time and effort is required when using single PCR to detect each of the pathogens that are present in plants infected with a mixture of viruses. In this study, a multiplex PCR system was established and optimized for the simultaneous detection of seven pathogens that infect wheat, including six viruses (BSMV, WDV, WYMV and three strains of BYDVs) and one phytoplasma causing WBD, in wheat leaf tissues collected from three provinces.
Materials and methods
Plant materials
Oat leaf tissue infected with known BYDVs (three strains: PAV, GAV, and GPV) was kindly provided by Dr. Wang, Institute of Plant Protection, Chinese Academy of Agricultural Science. Wheat leaf tissue infected with WBD was stored in our lab. Leaf tissue infected with five other viruses was collected in the field from the provinces of Shaanxi, Gansu and Shanxi, and the viruses were identified in our lab. Test samples were taken from healthy plants or plants infected with single or mixed pathogens in the field. Leaves collected from healthy and infected wheat were stored at −80°C for later use.
Extraction of total nucleic acids and reverse transcription
To detect DNA virus and phytoplasma in field samples, total nucleic acids were extracted using phenol/chloroform. Approximately 500 milligrams of leaf tissue was frozen in liquid nitrogen, ground in an RNase- and DNase-free mortar, and homogenized with 500 μL phenol/chloroform (1:1) and 500 μL extraction buffer in an RNase- and DNase-free 1.5-mL microfuge tube. Total nucleic acids were redissolved in 35 μL of RNase- and DNase-free water. The first-strand cDNAs of five RNA viruses were synthesized using random hexamer as primers and Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The final templates were mixtures of original DNA and first-strand cDNA.
Design and selection of primers
An applicable multiplex reaction needs to include primer pairs targeting each of the different pathogens to be detected. The annealing temperature must be similar for all primer sets, and complementarity between primers must be avoided to prevent the formation of primer dimers, which can significantly impair annealing of primers to the template DNA. Seven sets of primers were designed for simplex and M-PCR amplification of (i) conserved regions within the coat protein (CP) gene of BYDV-PAV and WYMV, (ii) the partial CP genes of BSMV, BYDV-GAV, BYDV-GPV and WDV, and (iii) the ribosomal protein (rp) gene of the WBD phytoplasma. Specific primers for M-PCR were designed using the program Primer 5.0. To facilitate reverse transcription and PCR, all of the primers were designed based on specificity and compatibility. The program DNAMAN was used to select specific primers with similar annealing temperatures in order to ensure the simultaneous detection of seven pathogens in one reaction system. The primer sequences, expected size of amplification products, and the target genes are listed in Table 1. Meanwhile, the primer sequences of BYDV-PAV, -GPV and -GAV were analyzed using Primer Premier 5.0 to determine whether they would be able to amplify the closely related virus RMV.
Multiplex PCR
DNA isolated from materials infected with WBD and WDV was mixed with defined amounts of cDNA synthesized from RNA of BSMV, WYMV and as the M-PCR template. The parameters involved in the M-PCR, including annealing temperature, concentration of Mg2+, polymerase buffer and Taq polymerase, cycle number, and especially the proportion of each specific primer set were optimized in a series of reactions (data not shown). Following optimization, the 12.5-μL PCR mixture included 1 μL of template, 2.5 μL of 25 mM Mg2+ (Promega, MadisonWI), 2.5 μL of a dNTP mixture with each dNTP at 5 mM, 2.5 μL of 10× polymerase buffer (Promega, Madison, WI), and 1.5 μL of 5 U/μL hot-start Taq polymerase (Promega, Madison, WI), and the primers. The samples were amplified using a PTC-100 Peltier Thermal Cycler (Bio-Rad, Hercules, CA), with initial denaturation at 94°C for 3 min, followed by 35 cycles of denaturation at 94°C for 1 min, primer annealing at 53°C for 45 s, and primer extension at 72°C for 1 min, and then final extension at 72°C for 10 min.
Cloning and sequencing of PCR products
In order to confirm the identity of the amplified products, PCR fragments for each of the pathogens were purified from the agarose gels using an H.Q.&.Q. Gel Extraction Kit (U-Gene). These fragments were cloned into the pMD18-T simple vector, introduced into Escherichia coli strain JM109 by transformation and sequenced.
Sensitivity tests of multiplex PCR
Each amplified fragment cloned into the pMD18-T simple vector was adjusted to the same initial concentration and diluted serially tenfold (100 to 106 copies) with deionized water to serve as a template in the optimized multiplex PCR. Seven single PCRs were done using the same template for comparison of amplification efficiency with the M-PCR.
Results
Amplification efficiency and specificity of each primer set
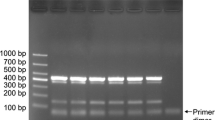
All seven primer sets were added together in equimolar amounts in the M-PCR reaction mixture, and the results indicated that the concentrations of the primers for WBD, WDV and WYMV needed to be increased. Through a series of gradient tests, the ratios of the primer sets were optimized to achieve satisfactory amplification. The amplification efficiency of single pairs of primers in the single PCR procedure was tested under the same conditions that were used for M-PCR. The results indicated that the same templates could be effectively amplified in both reactions (Fig. 1).
Primer specificity was tested by performing a “drop-out” experiment, in which one of the primer pairs at a time was removed to see whether any of the other primer pairs had cross-reacted. Each virus-specific primer set was sequentially eliminated in this experiment (Fig. 2). The third lane shows that good amplification was achieved with all of the primer sets except the WBD primers. Similar results were observed for six other viruses (lanes 4-9) when compared with the multiplex reaction control (lane 10). This demonstrated that the primers used in the multiplex PCR are very specific for their respective virus.
Primer specificity test by a series of multiplex PCR assays. M1, DL2000; M2, Marker IV; lane 2, healthy wheat control; lanes 3-9, a series in which on pair of pathogen-specific primers was sequentially omitted from the M-PCR; lane 10, M-PCR, containing the seven pathogen-specific primer sets and templates, producing a ladder of DNA fragments
Because leaf tissues infected with BYDV-RMV are rarely available in China, a bioinformatics analysis was conducted in place of an experiment to test the cross-amplification of BYDV-PAV, -GPV and -GAV primers (Data shown in Supplementary Online Material). The result indicated that only primer pair GPV1/GPV2 might be able to cross-amplify a 121-bp fragment of an RMV isolate from Montana, USA, but none of the isolates from China. In other words, three primer sets of BYDVs would rarely be able to cross-amplify the fragment of RMV from field samples in China.
Sequence analysis of PCR products
Sequence analysis indicated that, as intended, the amplified products for GAV, GPV, BSMV, PAV, WDV, WYMV and WBD were 274, 342, 503, 600, 735, 878, 1240 bp in length, respectively, which proves the reliability of the M-PCR results.
Detection of seven pathogens in mixed infections in the field
This multiplex PCR detection assay was also tested on field samples collected from the provinces of Shaanxi, Gansu and Shanxi. Test plants were grown in different field and were tested for an array of virus combinations by M-PCR (Fig. 3). The data indicate that most of these field samples were infected by different combinations of these seven pathogens. Field samples from Yangling, Shaanxi, were found to be heavily infected with BYDV-GAV. WBD, BYDV-PAV and WYMV were the main pathogens detected in the plants samples from Hancheng, Shaanxi. The samples from Gansu province were dominantly infected with BYDV-PAV, -GAV and WYMV, while the viruses detected in the samples from Shanxi were mainly different strains of BYDV, especially strain GAV. These samples were also tested by the single PCR method to confirm the M-PCR results, which were generally consistent with those obtained by M-PCR with the exception that one additional virus was detected in samples S2, S5 and s3 (Table 2).
Multiplex PCR detection of field samples. Panel a: Multiplex PCR analysis of field samples from Hancheng and Yangling of Shaanxi province. M1, marker IV; lane 2, healthy wheat control; lanes 3-6, field samples from Hancheng, Shaanxi; lanes 7-9, field samples from Yangling, Shaanxi; lane 10, a positive control M-PCR provides a reference ladder showing all seven amplicons. Panel b: Multiplex PCR analysis of field samples from Gansu province, M1: marker IV; lane 2, healthy wheat control; lanes 3-6, field samples from Gansu; lane 7, a positive control M-PCR providing a reference ladder showing all seven amplicons. Panel c: Multiplex PCR analysis of field samples from Shanxi province. M1, DL 2000; lane 2, healthy wheat control; lanes 3-6, field samples from Shanxi; lane 7, a positive control M-PCR providing a reference ladder showing all seven amplicons
Detection limits of multiplex PCR
A series of sensitivity tests was conducted to determine the detection limits of this M-PCR assay (Fig. 4). Although it is obvious that the amplification efficiency for the different pathogens differed between the single PCR and M-PCR procedures, the ratios of the amount of amplicon produced in each single PCR to that in the corresponding M-PCR were almost identical. The data show that all of the fragments except the rp gene of WBD and the cp gene of BYDV-GAV, were amplified 10 times more efficiently in the single PCR than in the M-PCR, while amplification of rp and GAV-cp was 100 times more efficient.
Comparison of the sensitivity of the single and multiplex PCR procedures. Panel a: Detection limits in each single PCR. Lane 1, marker DS2000; lane 2, negative control with deionized water as template; lanes 3-9, single PCR assays with 10-fold serial dilutions (100 to 106 copies) of recombinant plasmids as templates. The amount of PCR product loaded in each lane was 2.5 μl. Panel b: Detection limits in multiplex PCR. Lane 1: marker DS2000; lane 2, negative control with deionized water as template; lanes 3-9, multiplex PCR with a 10-fold serial dilution (100 to 106 copies) of recombinant plasmids as templates. The amount of PCR product loaded in each lane was 2.5 μl
Discussion
Generally speaking, in the natural environment, a single wheat sample may be infected by combinations of different types of viruses. In this study, a multiplex PCR detection system has been established after several optimizations to detect viruses and a phytoplasma simultaneously in mixed infections in wheat. Several research groups have developed methods for simultaneous detection of several viruses using M-PCR. In the USA, seven wheat viruses [16] were detected by an M-PCR method. However, all of them were RNA viruses, and five of them were pathogens that are not prevalent in China. Although Yue et al. [17] first developed an M-PCR method to detect three RNA viruses and a phytoplasma simultaneously, pathogenic species detected by this method are still restricted to four kinds. Simultaneous detection of a DNA virus, RNA viruses and a prokaryotic pathogen was first achieved in our laboratory. This method has greatly increased detection efficiency and reduced testing costs, and it has been successfully applied to the detection of wheat pathogens present in mixed infections.
Since a multiplex PCR reaction requires multiple specific amplifications in one system, an ideal one is not a simple mixture of the individual PCRs, but instead, a comprehensive analysis of the target product is needed, and repeated tests are required to establish the optimal reaction conditions. Selection of a primer set for each pathogen plays a key role in the M-PCR, not only to make the primers specific, but also to avoid interaction of the primers with each other and try to make the annealing temperature of all primer pairs consistent. Each primer set has been chosen based on sequence alignment to avoid complementarity with any other primers and to minimize the formation of primer dimers. Competition among primers may result in uneven amplifications in the multiplex PCR, with some of the products being barely visible while others are amplified more efficiently. Thus, it is vital to optimize the concentration ratios of the primers for multiplex PCR, as an improper ratio may lead to no amplification or nonspecific amplification of certain target templates. This problem may be overcome by changing the proportions of the primers based on the intensity of the bands in the reaction, increasing the amount of primer for “weak amplifiers” and decreasing it for “strong amplifiers”.
Although M-PCR has the advantage in saving time and cost, its sensitivity appears to be lower than that of a single PCR. Because polymerases buffer, Mg2+, dNTPs and Taq polymerase in the M-PCR are simultaneously consumed by more than one amplification reaction, the efficiency of each reaction was naturally lower than that in the single PCR. However, the 10-fold to 100-fold lower amplification efficiency of the M-PCR compared to the single PCR is acceptable and has little effect on its application for pathogen detection.
Field samples from Shaanxi, Gansu and Shanxi were collected to test the practical applicability of this M-PCR method. Amplification products derived from field samples were cloned and sequenced to ensure that the bands observed corresponded to the pathogens. In one case, a weak extra band appeared between BSMV and GPV when the field sample “S7” from Shaanxi was first tested by the M-PCR method. In order to indentify the extra band, the sample was tested again by M-PCR, but this time the extra band was not observed. Repeated testing of this sample by single and multiple PCR gave uniform results (data shown in Supplemental Online Material). Testing of field samples by this M-PCR method could not be used to perform epidemic analysis of these seven pathogens, while utilization of this M-PCR method could apply to epidemic analysis on the basis of the number of samples and collection sites. In addition, this M-PCR method is potentially applicable for plant quarantine and molecular-assisted breeding.
References
Gray S, Gildow FE (2003) Luteovirus-aphid interactions. Annu Rev Phytopathol 41:539–566
D’Arcy CJ, Domier LL Family Luteoviridae (2005) In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (eds) Virus taxonomy. VIIIth report of international committee on taxonomy of viruses. Elsevier/Academic Press, London, pp 891–900
Zhang WW (2009) The complete nucleotide sequence of the barley yellow dwarf GPV isolate from China shows that it is a new member of the genus Polerovirus. Arch Virol 154:1125–1128
Zhou GH, Cheng ZM, Qian YT, Zhang XC, Rochow WF (1984) Serological identification of Luteoviruses of small grains in China. Plant Dis 68:710–713
Zhou G, Zhang S, Rochow WF (1986) One wheat yellow dwarf virus strain, transmissible by aphid vectors of Schizaphis graminum and Sitobion avenae. Acta Phytopathol Sin 16:17–21
Zhou GH, Zhang SX, Qian YT (1987) Identification and applications of four strains of wheat yellow dwarf virus. Sci Agric Sin 20:7–12
Jin ZB, Wang XF, Chang SJ, Zhou GH (2004) The complete nucleotide sequence and its organization of the genome of Barley yellow dwarf virus-GAV. Sci China Ser C Life Sci 47:175–182
Li D, Yan L, Su N, Han C (1999) The nucleotide sequence of a Chinese isolate of wheat yellow mosaic virus and its comparison with a Japanese isolate. Arch Virol 144:2201–2206
Li GY, Wang GF (1981) Investigation of Barley stripe mosaic virus in spring wheat in Xinjiang. Xinjiang Agric Sci 11:31–32 (in Chinese)
Xie H, Wang ZM, Li WQ, Ni S (1981) Occurrence of Barley stripe mosaic virus in Xinjiang. Acta Phytopathol Sinica 11:11–14 (in Chinese)
Wu YF, Hao XG, Li ZN, Gu PW, An FQ, Xiang JY, Wang HN, Luo ZP, Liu JJ, Xiang Y (2010) Identification of the Phytoplasma Associated with Wheat Blue Dwarf Disease in China. Plant Dis 94(8):977–985
Schubert J, Habekuss A, Kazmaier K, Jeske H (2007) Surveying cereal-infecting geminiviruses in Germany—diagnostics and direct sequencing using rolling circle amplification. Virus Res 127(1):61–70
Rochow WF (1969) Biological properties of four isolates of Barley yellow dwarf virus. Phytopathology 59:1580–1588
French R (1995) Barley yellow dwarf: diagnostic procedures and reagents. In: D’Arcy CJ, Burnett PA (eds) Barley yellow dwarf, 40 years of progress. APS Press, St. Paul, pp 293–305
Casas I, Pozo F, Trallero G, Echevarria JM, Tenorio A (1999) Viral diagnosis of neurological infection by RT multiplex PCR: a search for entero- and herpesviruses in a prospective study. J Med Virol 57(2):145–151
Deb M, Anderson JM (2008) Development of a multiplexed PCR detection method for Barley and Cereal yellow dwarf viruses, Wheat spindle streak virus, Wheat streak mosaic virus and Soil-borne wheat mosaic virus. J Virol Methods 148(1–2):17–24
Yue HN, Wu YF, Li YR, Wei T, Hou W, Wu KK (2008) Simultaneous detection of three wheat virus BSMV, BYDV-PAV, WYMV and WBD phytoplasma by multiplex PCR. Scientia Agricultura Sinica 41:2663–2669 (in Chinese)
Acknowledgments
We thank Mr. Zhao Zhen of NWSUAF for providing wheat virus disease samples collected from the fields, Ms. Yue Hong-ni and Ms. Yang Yang from NWSUAF for editing the manuscript, and Mr. Dai Jin, Mr. Li Zheng-nan, Mr. Song Shuang and Ms. Liu Min-jie of NWSUAF for many suggestions and corrections to the manuscript. We especially thank Professor Baozhong Meng of University of Guelph for revisions and suggestions to the manuscript. This work was supported by a grant from Cultivation of Transgenic Antiviral Wheat Varieties (No. 2009ZX08002-003B).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grant from Cultivation of Transgenic Antiviral Wheat Varieties (No. 2009ZX08002-003B).
Electronic supplementary material
Below is the link to the electronic supplementary material.
705_2012_1294_MOESM2_ESM.tif
Supplementary material 2 (TIFF 3468 kb). Detecting sample S7 by single and multiple PCR methods. M1: Marker DS2000; lane2: The healthy wheat control; lane 3: Detecting S7 by single PCR using the primer set of WBD; lane 4: Detecting S7 by single PCR using the primer set of WYMV; lane 5: Detecting S7 by single PCR using the primer set of WDV; lane 6: Detecting S7 by single PCR using the primer set of PAV; lane 7: Detecting S7 by single PCR using the primer set of BSMV; lane 8: Detecting S7 by single PCR using the primer set of GPV; lane 9: Detecting S7 by single PCR using the primer set of GAV; lane 10: Detecting S7 by M-PCR; lane 11: A positive control M-PCR provides a reference ladder showing all seven amplicons
Rights and permissions
About this article
Cite this article
Tao, Y., Man, J. & Wu, Y. Development of a multiplex polymerase chain reaction for simultaneous detection of wheat viruses and a phytoplasma in China. Arch Virol 157, 1261–1267 (2012). https://doi.org/10.1007/s00705-012-1294-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-012-1294-y