Abstract
The core protein of hepatitis C virus (HCV) has been implicated in HCV-induced liver pathogenesis. Previous data have shown that the HCV core protein has pleiotropic functions, including transcriptional regulation of a number of cellular genes, although the mechanism of gene regulation remains unclear. Wnt/β-catenin signaling is also involved in hepatocellular carcinoma (HCC) tumorigenesis. To elucidate the molecular mechanism of HCV pathogenesis, we examined whether HCV core protein activates Wnt/β-catenin signaling in the hepatoma cell line SMMC-7721. The effects of core protein on Wnt/β-catenin signaling cascades were investigated by luciferase reporter gene assay, immunofluorescence, western blot and RT-PCR analysis. Here, we demonstrate that HCV core protein plays an essential role in activating β-catenin/Tcf-4-dependent transcriptional activity and increases active β-catenin expression and nuclear accumulation in SMMC-7721 cells. An RT-PCR assay indicated that core protein upregulates gene expression of canonical Wnt ligands, such as Wnt2, Wnt3, Wnt3a, Wnt8b, Wnt10a, Wnt10b, frizzled receptors Fzd1, 2, 5, 6, 7, 9, and LRP5/6 co-receptors. However, Wnt antagonists SFRP3, 5 and Dkk1 were moderately repressed. Furthermore, ectopic expression of core protein markedly promoted cell proliferation. The soluble Fzd molecule FrzB or the β-catenin inhibitor siBC efficiently blocked cell growth stimulation by the core gene. Our present findings demonstrate that the HCV core protein activates canonical Wnt signaling through tight regulation of several important molecules upstream of β-catenin and presumably results in promotion of cell proliferation in the SMMC-7721 cell line. Taken together, these data suggested that the core protein may be directly involved in Wnt/β-catenin-mediated liver pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatitis C virus (HCV) infection is a public health problem worldwide. It is estimated that up to 175 million individuals are infected HCV, and two-thirds of cases become chronic hepatitis, liver cirrhosis, even hepatocellular carcinoma (HCC) [1, 2]. However, the molecular events that lead to chronic liver diseases during HCV infection are poorly understood [3]. HCV belongs to the genus Hepacivirus, family Flaviviridae, and has a positive-sense, single-stranded RNA genome of 9.6 kb. Its genome encodes a single polyprotein precursor containing approximately 3010 amino acids, which is processed by host and viral proteases into 10 mature proteins: the structural proteins (Core, E1, E2/p7), and the nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A, NS5B) [4]. Among them, HCV core protein, a multifunctional protein, has not only been shown to be involved in RNA replication, viral particle assembly and release [5–7], but it also interferes with the host’s physiological defense and metabolism by interacting with various cellular proteins, such as NF-κB, pRb, p53, TGF-β and DEAD box protein [8–12]. In addition, evidence exists that the core protein regulates diverse cell signaling pathways involved in HCV-related pathogenesis. For instance, core protein may lead to cellular transformation and insulin resistance by interacting with STAT3 and MAPK cascades [13, 14].
Wnt signaling is divided into two pathways: the canonical pathway and a noncanonical pathway, which is closely associated with many facets of human biology, such as embryogenesis, organogenesis, and tissue homeostasis [15, 16]. Most studies have been focused on canonical Wnt/β-catenin signaling, as it is involved in regulating key physical and pathological processes including cellular proliferation, differentiation and transformation [17]. In its inactivated state, β-catenin, the main protein of this signaling cascade, is phosphorylated at its serine-threonine residues by binding the adenomas polyposis coli (APC)/Axin/glycogen synthase kinase-3β (GSK-3β) complex and then degraded by proteosome. In its activated state, Wnts bind to frizzled (Fzd) receptors and LDL-related protein (LRP) 5/6 co-receptors, which prevents GSK-3β from carrying out β-catenin phosphorylation via hyperphophorylation of disheveled (Dvl). β-catenin is released from the APC/Axin/GSK-3β complex and then translocates into the nucleus and associates with T cell factor (Tcf)/lympocyte enhancer factor (Lef), which results in controlling the transcription of downstream genes such as c-myc [18], cyclin D1 [19], and survivin [20]. Wnt antagonists, as negative regulators, inhibit canonical Wnt/β-catenin signaling and are divided into two categories: soluble Fzd-related proteins (SFRPs), which compete with Wnt ligands for binding to Fzd receptors, and Dickkopf proteins (Dkks), which prevent formation of the Fzd-Wnt-LRP5/6 complex in response to Wnt [21].
Recent studies have indicated that approximately 26% of mutations in β-catenin have been found in hepatocellular carcinoma (HCC) associated with HCV infection [22]. Mutations in Axin [23], APC deletion [24], GSK-3β inactivation [25], dephosphorylation of β-catenin [26] and upregulation of frizzled 7 [27] are involved in activation of Wnt/β-catenin signaling. Furthermore, Fukutomi and coworkers recently showed, using microarray analysis, that HCV-core-transfected Huh7 cells upregulated Wnt-1 and WISP-2 transcription [28], and these cells demonstrated increased proliferation, DNA synthesis and cell cycle progression. In this context, we hypothesized that core protein is involved in the oncogenic transformation process, possibly through activation of Wnt/β-catenin signaling.
As expected, our present findings have demonstrated that the HCV core protein activates canonical Wnt signaling through tight regulation of molecules upstream of β-catenin, consequently resulting in acceleration of cell proliferation, and this activity may play an important role in HCV pathogenesis.
Materials and methods
Cell culture and chemicals
HEK293 cells and the human hepatoma cell line SMMC-7721 [29, 30] were cultured in complete Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS, Hyclone), 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2. Unless indicated otherwise, all chemicals were purchased from Sigma-Aldrich.
Construction of recombinant adenoviruses expressing HCV core, Wnt3A, FrzB and siBC
In order to generate adenoviral vectors expressing HCV core protein, the full-length gene for the HCV core protein (genotype 1a) was amplified by PCR from plasmid HFL (provided by Dr. Charles M. Rice of Rockefeller University, USA) and cloned into the shuttle vector pAdTrack-TO4 (provided by Dr. T.-C He of the University of Chicago, USA) and subsequently used to generate the adenoviral recombinant AdCore. Adenoviruses were produced and amplified in HEK293 cells. For construction of the adenoviral vectors expressing Wnt3A, the coding region of mouse Wnt3A (kindly provided by Roel Nusse of Stanford University) was amplified by PCR and subcloned into pAdTrack-CMV, resulting in pAdTrack-Wnt3A. These shuttle vectors were used to generate recombinant adenovirus AdWnt3A as described previously [31, 32]. Adenoviruses expressing the soluble Fzd molecule FrzB and β-catenin inhibitor siBC were generated previously using the AdEasy system [34]. AdCore, AdFrzB and AdWnt3A also express GFP as a marker for monitoring infection efficiency. An analogous adenovirus expressing only GFP (AdGFP) was used as a control [33].
Luciferase assay
Cells were seeded in 25-cm2 flasks and transfected with 2 μg of β-catenin/Tcf4-responsive luciferase reporter pTOP-Luc [34] per flask using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. At 16 h after transfection, cells were replated to 24-well plates and infected with AdGFP, AdCore or AdWnt3A. At 24 h postinfection, cells were lysed, and cell lysates were collected for luciferase assays using Promega’s Luciferase Assay Kit. Each assay was performed in triplicate.
RNA isolation and RT-PCR analysis
Total RNAs were extracted from cultured cells using TRIzol Reagent (Invitrogen) according to the manufacturer’s protocol, and all RNA samples were treated with DNase to exclude DNA contamination. Total RNA was used to generate cDNA templates by reverse transcription reaction with random hexamer and MMLV RT (Promega). The first-strand cDNA products were further diluted 5- to 10-fold and used as PCR templates. The primer sequences used to identify Wnt, Wnt receptor and Wnt antagonist gene expression are listed in Table 1. Endogenous GAPDH expression was used as a control.
Western blotting and antibodies
Whole-cell extracts of exponentially growing cells were prepared in lysis buffer (Promega) containing the complete cocktail of proteases inhibitors (Roche), and protein concentrations were determined using the BCA protein assay reagent (Pierce). Immunoblot analysis for β-catenin (Santa Cruz, sc-7199), active β-catenin (Millipore, 05-665) and HCV Core (Abcam, ab2740) were performed with 50-70 μg of proteins run on 10% SDS polyacrylamide gels and transferred to PVDF membranes (Millipore). Secondary antibodies coupled to HRP were purchased from Jackson ImmunoResearch Laboratories. Detection was performed with SuperSignal West Pico Chemiluminescencent Substrate Kits (Pierce). Equal protein loading was verified by western blot assay with GAPDH antibody.
Immunofluorescence
SMMC-7721 cells infected with AdCore, AdWnt3A or AdGFP control were fixed with 4% formaldehyde, permeabilized with 0.3% Triton X-100 and incubated with β-catenin (Santa Cruz, sc-7199) overnight. After washing, cells were incubated with Alexa Fluor 594-labeled secondary antibody (Invitrogen) for 1 h. Cells were counterstained with DAPI to label nuclei. The presence of β-catenin was visualized under a confocal fluorescent microscope.
MTS proliferation assay
MTS assays were performed with a Cell Titer 96 AQ One Solution Cell Proliferation Assay (Promega) at the indicted time points. Twenty μl of MTS reagent was added to each well of a 96-well assay plate containing 0.5×104 cells in culture medium, and the plate was incubated for 2 h at 37°C in 5% CO2. The absorbance at 490 nm was recorded every 24 h in triplicate until day 4 by using a microplate reader (Synergy HT Multi-mode Microplate Reader, Bio-Tek). Each assay was repeated at least three times.
Crystal violet staining
The staining procedure was carried out as described previously [35]. Briefly, cells were fixed for 20 min in 10% buffered formalin and then stained with 0.5% crystal violet solution at room temperature for 30 min with gentle shaking. The plates were washed by submersion in flowing tap water. Microplates were allowed to air dry and incubated with 0.2% Triton X-100 for 30 min at room temperature with gentle shaking to dissolve the dye. Then, 100 μl of sample from each well was transferred into a fresh 96-well microplate, and the OD at 570 nm was measured in a microplate reader (Synergy HT Multi-mode Microplate Reader, Bio-Tek).
Statistical analysis
For the luciferase reporter assay, MTS and quantitative cell viability assay, data are shown as mean ± SDs. Student’s t-test was used for quantitative analysis of crystal violet staining. The significance level was defined as p<0.05.
Results
HCV core protein activates Wnt/β-catenin signaling in hepatoma cell line SMMC-7721
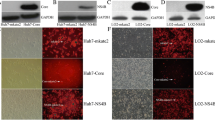
Previous data have shown that when the canonical Wnt pathway is in an active state, β-catenin accumulates in the cytoplasm, is transferred to the nucleus and then binds to transcriptional factor T-cell factor (Tcf4) to drive activation of specific target genes, including c-Myc [18], cyclin D1 [19] and WISP-2 [36], which have been shown to be important for carcinogenesis. Therefore, we asked whether HCV core protein plays an important role in activating canonical Wnt/β-catenin signaling in hepatoma cells lines. Since the endogenous β-catenin/TCF activity was maintained at a basal level in SMMC-7721 cells (Fig. 1C blank control), we chose this cell line to perform Tcf-4-dependent transcriptional activity assays. First of all, we confirmed effective infection of SMMC-7721 cells with AdCore, AdWnt3A or AdGFP control (Fig. 1A) and the expression of HCV core protein or Wnt3a in SMMC-7721 cells by RT-PCR (Fig. 1B, panel a) and western blot assay (Fig. 1B panel b). Then, we detected β-catenin/Tcf4-dependent transcriptional activity by luciferase reporter gene assay. The reporter plasmid pTOP-Luc construct contains six repeats of the Tcf-4 sequence recognized by β-catenin. As illustrated in Fig. 1C, compared with the GFP control, Tcf-4-dependent transcriptional activity was increased 3-4-fold by overexpression of HCV core protein alone. In other words, ectopic expression of HCV core protein resulted in activation of β-catenin/Tcf-4-dependent transcriptional activity in SMMC-7721 cells.
HCV core protein activates Wnt/β-catenin signaling in hepatoma cell line SMMC-7721. (A) Effective infection of SMMC-7721 cells with AdGFP, AdWnt3A or AdCore. SMMC-7721 cells were infected with AdGFP, AdWnt3A or AdCore, and the infection efficiency was examined under a fluorescence microscope. BF: blank field. Magnification, ×200. (B) Expression of HCV core or Wnt3A in SMMC-7721 cells. Cells were infected with AdGFP control, AdWnt3A or AdCore for 24 h. Total RNA was isolated for RT-PCR analysis using primers specific for HCV core or the Wnt3A gene (panel a), and protein expression was determined by western blotting using anti-core antibody (Abcam) (panel b). Endogenous GAPDH expression was used as a control. (C) HCV core induces β-catenin/Tcf4-mediated transactivation. SMMC-7721 cells were transfected with Tcf4/LEF1 reporter pTOP-Luc and infected with AdGFP, AdCore and AdWnt3A. At 24 h postinfection, cells were collected for luciferase assays. Error bars show standard deviation for at least three independent experiments performed in triplicate. Data are present as mean ± S.D. *P<0.05 (vs GFP). (D) Active β-catenin levels are increased in core-expressing cells. SMMC-7721 cells were infected with AdGFP control, AdCore, or AdWnt3A for 24 h, and then, a total cell lysate was prepared and immunoblotted with anti-β-catenin antibody (Santa Cruz Biotechnology) or anti-active-β-catenin antibody (Millipore). GAPDH was used as a loading control. Experiments were performed in triplicate, and representative results are shown. (E) Quantitative analysis of the western blotting results in D. The signal intensities were determined by ImageJ software. Fold change was calculated by dividing signal intensities of active β-catenin with total β-catenin. Data are present as mean ± S.D. *P<0.05 (vs GFP)
The hallmark of canonical Wnt signaling activation is cytoplasmic accumulation and nuclear translocation of stabilized β-catenin protein. To examine active and total β-catenin expression in hepatoma cells, we measured the levels of these proteins by immunoblot assay in core-expressing cells. As shown in Fig. 1D, both the active form of β-catenin, which is dephosphorylated at Ser 37 or Thr41 [37], and total β-catenin accumulated in core-expressing cells. Quantitative analysis showed that the expression level of active β-catenin increased approximately 1.5-fold in AdCore-infected cells compared to GFP control cells (Fig. 1E). We then examined the subcellular localization of β-catenin using confocal immunofluorescent microscopy. As expected, stabilization of cytosolic β-catenin was significantly enhanced and some of it was translocated to the nucleus in AdCore-infected cells (Fig. 2, panels D to F) but not in GFP control cells (Fig. 2, panels A to C). These results indicated that the Tcf-4-dependent transcriptional activity observed in core-expressing cells was due to cytosolic accumulation and consequently nuclear translocation of β-catenin.
Cytosolic accumulation and nuclear translocation of β-catenin induced by HCV core. SMMC-7721 cells were infected with AdGFP (panels A to C), AdCore (panels D to F) or AdWnt3A (panels G to I) for 24 h. Cells were fixed and stained with an anti-β-catenin antibody and a fluorescence-labeled secondary antibody. Cells were counterstained with 4’, 6-diamidino-2-phenylindole (DAPI) to label nuclei. The presence of β-catenin was visualized under a confocal immunofluorescence microscope. Nuclear translocation of β-catenin is indicated by arrows
Collectively, these results indicate that HCV core protein plays an important role in activation of the Wnt/β-catenin pathway in hepatoma cell line SMMC-7721.
HCV core protein regulates the level of expression of Wnt ligands, Fzd receptors and Wnt antagonists in SMMC-7721 cells
Although activation of the canonical Wnt/β-catenin pathway by core protein was observed, the underlying molecular mechanism was not clear. We postulated that the core protein activates the β-catenin pathway, possibly by modulating its upstream genes and expression of antagonists. To test this, semi-quantitative PCR analysis was used to examine the expression profile of Wnt ligands, Fzd receptors and Wnt antagonists in core-expressing cells. We found that, compared with the GFP control, the expression level of Wnt3, 3a, and 10b were moderately increased in SMMC-7721 cells infected with AdCore, and expression of Wnt2, 8b and 10a was slightly increased in these cells. Among the eight canonical Wnt ligands, neither Wnt1 nor Wnt8a was readily detectable (Fig. 3A).
HCV core protein regulates the expression level of Wnt ligands, Fzd receptors and Wnt antagonists in SMMC-7721 cells. Wnt ligands (A), Frizzled receptors (B), LRP5/6 co-receptors (C) and Wnt antagonists (D) involved in canonical Wnt signaling were tested for expression by RT-PCR assay. Cells were infected with AdGFP control, AdWnt3A or AdCore for 24 h and used for RT-PCR analysis. All samples were normalized with GAPDH as shown in E. PCR results were confirmed in three sets of independent experiments
Wnt proteins execute their diverse biological functions through binding to their receptors Fzd and co-receptors LRP5/6. We next studied the expression pattern of Wnt receptors and co-receptors using a semi-quantitative PCR assay in the SMMC-7721 cell line. As shown in Fig. 3B and C, the expression levels of Fzd1, 2, 5, 6, 7, 9 and the two co-receptors, LRP5 and 6, were significantly upregulated after ectopic expression of core protein, whereas no obvious change in Fzd3, 4 and 10 expression was observed. It is interesting to note that Fzd8 expression was downregulated in core-expressing cells. These results indicated that core protein activates Wnt/β-catenin signaling, possibly through upregulation of both canonical Wnt ligands (Wnt2, 3, 3a, 8b, 10a, 10b) and their corresponding Frizzled receptors (Fzd1, 2, 5, 6, 7, 9) as well as co-receptors LRP5/6, and then executes their biological functions.
We next sought to investigate whether Wnt antagonists are implicated in regulating canonical Wnt signaling activity in core-expressing cells. RT-PCR analysis was again used to examine the expression levels of five known SFRPs and three Dkk molecules [38]. Not surprisingly, the results demonstrated that the expression level of SFRP3, 5 and Dkk1 were significantly decreased in the presence of core protein (Fig. 3D). These data, together with the upregulation of several of important Wnt ligands and Fzd receptors by core protein, suggested that core activates the canonical Wnt pathway through tight regulation of molecules upstream of β-catenin.
Wnt antagonists partially inhibit cell growth stimulation by the core gene
Previous data have demonstrated that increased β-catenin translocation to the nucleus usually correlates with an increase in cell proliferation. Since our study indicated that core protein enhanced β-catenin accumulation and nuclear translocation, we presumed that core protein may promote cell proliferation through activation of the Wnt/β-catenin pathway. To test the notion, SMMC-7721 cells were infected with AdCore, AdWnt3A or AdGFP, and the cell proliferation rate was measured by MTS assay. Consistent with our hypothesis, when core protein was expressed in SMMC-7721 cells, the cell proliferation rate was significantly increased (Fig. 4A).
Core-stimulated cell proliferation is partially blocked by Wnt antagonists. (A) HCV core promotes cell proliferation. SMMC-7721 cells were infected with an optimal titer of AdGFP control, AdWnt3A or AdCore and then plated into a 96-well plate at 0.5×104/well. The absorbance at 490 nm was recorded in triplicate every 24 h. Data are present as mean ± S.D. *P<0.05 (Core vs GFP at the corresponding time points). (B) Crystal violet cell viability assay of core-expressing cells. SMMC-7721 cells were infected with AdGFP control, AdWnt3A or AdCore and then plated at low density in a 6-well plate. The cells were stained with crystal violet at 1 week after plating. Experiments were performed in triplicate, and representative results are shown. (C) Quantitative analysis of cell viability in B. Cell viability was quantitatively assessed using a colorimetric assay (see Materials and methods), and differences in colorimetric values were subjected to statistical analysis. *P<0.05 (vs GFP). (D) Core-stimulated cell proliferation is partially blocked by Wnt antagonists. SMMC-7721 cells were infected with an optimal titer of AdCore plus AdGFP, AdsiBC or AdFrzB, and then plated and recorded as in A (triplicate experiments). *P<0.05 (vs GFP+Core) (E) Crystal violet cell viability assay. SMMC-7721 cells were treated as in D and stained at 1 week after plating (triplicate experiments). (F) Quantitative analysis of cell viability in E. *P<0.05 (vs GFP+Core)
Using the crystal violet staining method, we explored cell proliferation further in core-expressing cells. As shown in Fig. 4B, overexpression of core protein or Wnt3a led to a marked increase in SMMC-7721 cell proliferation. Further quantitative analysis demonstrated the colorimetric value in core-expressing cells is approximately twofold of that in the GFP control (Fig. 4C). These findings strongly suggested that ectopic expression of core protein confers on cells the ability to accelerate cell proliferation. To further understand the role of the canonical Wnt pathway in core-dependent cell growth stimulation, SMMC-7721 cells were coinfected with AdCore plus β-catenin inhibitor AdsiBC or the soluble Fzd molecule AdFrzB [34]. MTS analysis indicated that overexpression of siBC or FrzB efficiently blocked cell growth stimulated by the core gene (Fig. 4D). The similar inhibitory effects of siBC or FrzB on core-stimulated cell proliferation were also observed by using crystal violet staining (Fig. 4E and F). Taken together, these data strongly suggest that ectopic expression of core protein observably increases cell proliferation through activation of the Wnt/β-catenin-dependent pathway.
Discussion
Over the last several years, ample evidence has made it clear that HCV core protein is involved in HCV-associated diseases, such as chronic hepatitis, hepatic steatosis, and HCC, through interaction with diverse cellular signaling pathways. On the other hand, Wnt/β-catenin signaling closely correlates with HCC development and progression. To investigate the molecular mechanism of HCV-induced pathogenesis, we explored the potential involvement of the HCV core protein in activation of the Wnt/β-catenin pathway. Our study demonstrated that HCV core protein alone can elevate Tcf-4-dependent transcriptional activity and then induce active β-catenin accumulation and partial nuclear translocation in SMMC-7721 cells. These findings are supported by earlier reports in which, in contrast to the high level of nuclear β-catenin staining, strong cytoplasmic or whole-mount cell staining was seen much more commonly in some tumor types, such as gastrointestinal stromal tumors [39], liposarcoma [40], malignant fibrous histiocytoma and osteosarcoma [41].
We also found that not only Wnt3, Fzd6 and Fzd7 were moderately increased, but also Wnt2, 3a, 8b, 10a, 10b and Fzd1, 2, 5, 9 as well as two co-receptors, LRP5 and 6, were upregulated in SMMC-7721 cells infected with AdCore. As positive modulators, all of these important upstream molecules eventually lead to activation of β-catenin signaling by repressing the effect of GSK-3β on phosphorylation of β-catenin [42], whereas the expression of Wnt1 and Wnt8a is almost undetectable in SMMC-7721 cells. The reason for this result is that a distinct expression pattern of Wnt ligands is observed in HCC cells [15]. In SMMC-7721 cells, Wnt1 and Wnt8a may not be involved in activation of canonical Wnt/β-catenin signaling. More interestingly, one class of Frizzled receptors, Fzd8, was even downregulated in core-expressing cells. These findings may have several implications. First, previous data showed that, unlike the defined canonical (Fzd1, Fzd5, Fzd7 and Fzd9) and noncanonical Fzds (Fzd2, Fzd3, Fzd4 and Fzd6), Fzd8 and Fzd10 are classified as an atypical Fzd subgroup [15]. Responsiveness to the Wnt signaling pathway in HCC cells is determined by the highly complex interaction of Wnts and Fzds receptors and complicated crosstalk among downstream cellular events, of which only some are medicated by canonical Wnt pathways. The expression pattern of Fzd8 in core-expressing cells indicates that this type of Frizzled receptor may not be involved in the activation of the canonical Wnt pathway. Secondly, it has been reported that the balance of ubiquitylation and deubiquitylation of Fzds determines the cellular responsiveness to Wnt signaling, both in mammalian cells and in Drosophila [43]. Thus, the ubiquitylation status of Fzd8 may determine its recycling to the plasma membrane and finally control its expression level. Further studies should be done to explore the molecular mechanism behind core-induced downregulation of Fzd8.
Meanwhile, we confirmed that the expression level of co-receptors LRP5 and 6 was clearly enhanced, as Dkk1-promoted degradation of LRP5/6 [44] was obviously repressed by core protein. In addition, previous data have shown that other Wnt antagonists-sFRPs genes are inactivated by promoter methylation in the course of activation of the Wnt/β-catenin pathway in HCC [45]. Likewise, in our study the expression of sFRP3, sFRP35 and Dkk1 decreased significantly or even became undetectable in the presence of core protein. These observations indicate that the core protein activates the canonical Wnt pathway through tight regulation of molecules upstream of β-catenin, including Wnt ligands, frizzled receptors, co-receptors and mRNA expression of their antagonists.
Aberrant activation of β-catenin signaling results in abnormal cellular functions, such as enhanced cell growth and malignant cell transformation. The importance of aberrant Wnt signaling in colorectal cancer is clear. However, aberrant Wnt signaling may play a role in many other types of malignancies, even those in which the classical mutations associated with the pathway (i.e., APC truncations, β-catenin mutations) are not present. In human breast cancer, there are many reports of inactivation of negative regulators of the Wnt signaling pathway. For example, Frizzled-related protein 1 (FRP1/ FRZB), a secreted Wnt inhibitor, is frequently deleted in human breast cancers [46]. Similarly, there are numerous studies that have documented the amplification or overexpression of positive regulators of components of this pathway. Upregulation of Dvl has been reported in ductal breast cancers [47]. In HCC, mutation of the proto-oncogene β-catenin, upregulation of frizzled-7 and dephosphorylation of β-catenin are frequently observed [48]. Here, we demonstrate that ectopic expression of the HCV core leads to a marked increase of SMMC-7721 cells proliferation. On the other hand, overexpression of the β-catenin inhibitor siBC or the soluble Fzd molecule FrzB suppresses core-dependent cell growth, suggesting that HCV core promotes cell proliferation through the Wnt/β-catenin-dependent pathway. There are two ways in which core protein might enhance cell growth. First, nuclear accumulation of β-catenin could form a transcriptional complex with Tcf and activate downstream target genes, such as c-Myc, Cyclin D1 and WISP-2, which dictate cell growth and cell-cycle progression. Terris and colleagues have clearly demonstrated Wnt-1-mediated β-catenin/Tcf-4 transcription-enhanced cell proliferation and reduced apoptosis in human HCC cell lines [49]. Second, core protein has been shown to decrease the amount of the cyclin-dependent inhibitor p21WAF1 through interaction with the p53 protein [50, 51]. This may result in an unbalance between cell proliferation and apoptosis, ultimately contributing to malignant cellular transformation.
In general, our results strongly suggest that in SMMC-7721 cells, HCV core protein activates canonical Wnt/β-catenin signaling through tight regulation of several upstream molecules of this signaling pathway, which leads to an increase in cell growth rate. These observations offer a new insight into the pathogenesis of HCV-related diseases and might aid in the development of a potent therapeutic strategy.
Change history
04 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00705-021-05275-9
References
Alter HJ (1995) To C or not to C: these are the questions. Blood 85:1681–1695
WHO (1997) Hepatitis C: global prevalence. Wkly Epidemiol Rec 72:341–348
Georgel P, Schuster C, Zeisel MB, Stoll-Keller F, Berg T, Bahram S, Baumert TF (2010) Virus-host interactions in hepatitis C virus infection: implications for molecular pathogenesis and antiviral strategies. Trends Mol Med 16(6):277–286
Drazan KE (2000) Molecular biology of hepatitis-C infection. Liver Transpl 6:396–406
Kang SM, Choi JK, Kim SJ, Kim JH, Ahn DG, Oh JW (2009) Regulation of hepatitis C virus replication by the core protein through its interaction with viral RNA polymerase. Biochem Biophys Res Commun 386:55–59
Masaki T, Suzuki R, Murakami K, Aizaki H, Ishii K, Murayama A, Date T, Matsuura Y, Miyamura T, Wakita T, Suzuki T (2008) Interaction of hepatitis C virus nonstructural protein 5A with core protein is critical for the production of infectious virus particles. J Virol 82:7964–7976
Majeau N, Fromentin R, Savard C, Duval M, Tremblay MJ, Leclerc D (2009) Palmitoylation of hepatitis C virus core protein is important for virion production. J Biol Chem 284(49):33915–33925
Joo M, Hahn YS, Kwon M, Sadikot RT, Blackwell TS, Christman JW (2005) Hepatitis C virus core protein suppresses NF-kappaB activation and cyclooxygenase-2 expression by direct interaction with IkappaB kinase beta. J Virol 79(12):7648–7657
Cho J, Baek W, Yang S, Chang J, Sung YC, Suh M (2001) HCV core protein modulates Rb pathway through pRb down-regulation and E2F-1 up-regulation. Biochim Biophys Acta 1538(1):59–66
Kao CF, Chen SY, Chen JY, Wu Lee YH (2004) Modulation of p53 transcription regulatory activity and post-translational modification by hepatitis C virus core protein. Oncogene 23(14):2472–2483
Battaglia S, Benzoubir N, Nobilet S, Charneau P, Samuel D, Zignego AL, Atfi A, Bre′chot C, Bourgeade MF (2009) Liver cancer-derived hepatitis C virus core proteins shift TGF-Beta responses from tumor suppression to epithelial-mesenchymal transition. PLoS One 4:e4355
Ariumi Y, Kuroki M, Abe K, Dansako H, Ikeda M, Wakita T, Kato N (2007) DDX3 DEAD-box RNA helicase is required for hepatitis C virus RNA replication. J Virol 81(24):13922–13926
Yoshida T, Hanada T, Tokuhisa T, Kosai K, Sata M, Kohara M, Yoshimura A (2002) Activation of STAT3 by the hepatitis C virus core protein leads to cellular transformation. J Exp Med 196:641–654
Banerjee S, Saito K, Goughoulte MA, Meyer K, Ray RB, Rayet R (2008) Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream Akt/protein kinase b signaling pathway for insulin resistance. J Virol 82:2606–2612
Yuzugullu H, Benhaj K, Ozturk N, Senturk S, Celik E, Toylu A, Tasdemir N, Yilmaz M, Erdal E, Akcali KC, Atabey N, Ozturket M (2009) Canonical Wnt signaling is antagonized by noncanonical Wnt5a in hepatocellular carcinoma cells. Mol Cancer 8:90
Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127:469–480
Herbst A, Kolligs FT (2007) Wnt signaling as a therapeutic target for cancer. Methods Mol Biol 361:63–91
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512
Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398(6726):422–426
Zhang T, Otevrel T, Gao Z, Gao Z, Ehrlich SM, Fields JZ, Boman BM (2001) Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res 61(24):8664–8667
Bi Y, Huang JY, He Y, Zhu GH, Su YX, He BC, Luo JY, Wang Y, Kang Q, Luo Q, Chen L, Zuo GW, Jiang W, Liu B, Shi Q, Tang M, Zhang BQ, Weng YG, Huang AL, Zhou L, Feng T, Luu HH, Haydon RC, He TC, Tang N (2009) Wnt Antagonist SFRP3 inhibits the differentiation of mouse hepatic progenitor cells. J Cell Biochem 108:295–303
de la Coste A, Romagnol B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, Perret C (1998) Somatic mutations of the β-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA 95:8847–8851
Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujitam M, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, Nakamura Y (2000) AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Gen 24:245–250
Oda H, Imai Y, Nakatsuru Y, Hata J, Ishikawa T (1996) Somatic mutations of the APC gene in sporadic hepatoblastomas. Cancer Res 56:3320–3323
Ban KC, Singh H, Krishnan R, Seow HF (2003) GSK-3β phosphorylation and alteration of β-catenin in hepatocellular carcinoma. Cancer Lett 199:201–208
Miyoshi Y, Iwao K, Nagasawa Y, Aihara T, Sasaki Y, Imaoka S, Murata M, Shimano T, Nakamura Y (1998) Activation of the β-catenin gene in primary hepatocellular carcinomas by somatic alterations involving exon 3. Cancer Res 58:2524–2527
Merle P, de la Monte S, Kim M, Herrmann M, Tanaka S, Von Dem Bussche A, Kem MC, Trepo C, Wands JR (2004) Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology 127:1110–1122
Fukutomi T, Zhou Y, Kawai S, Eguchi H, Wands JR, Li J (2005) Hepatitis C virus core protein stimulates hepatocyte growth: correlation with upregulation of Wnt1 expression. Hepatology 41:1096–1105
Hu YW, Liu CY, Du CM, Zhang J, Wu WQ, Gu ZL (2009) Induction of apoptosis in human hepatocarcinoma SMMC-7721 cells in vitro by flavonoids from Astragalus complanatus. J Ethnopharmacol 123(2):293–301
Li S, Dong P, Wang J, Zhang J, Gu J, Wu X, Wu W, Fei X, Zhang Z, Wang Y, Quan Z, Liu Y (2010) Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via a ROS/JNK-dependent mitochondrial pathway. Cancer Lett 298(2):222–230
Luo JY, Deng ZL, Luo XJ, Tang N, Song WX, Chen J, Sharff AK, Luu HH, Haydon RC, Kinzler WK, Vogelstein B, He TC (2007) A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc 2(5):1236–1247
Si W, Kang Q, Luu HH, Park JK, Luo Q, Song WX, Jiang W, Luo X, Li X, Yin H, Montag AG, Haydon RC, He TC (2006) CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Mol Cell Biol 26(8):2955–2964
He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B (1998) A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 95(5):2509–2514
Tang N, Song WX, Luo J, Luo X, Chen J, Sharff KA, Bi Y, He BC, Huang JY, Zhu GH, Su YX, Jiang W, Tang M, He Y, Wang Y, Chen L, Zuo GW, Shen J, Pan X, Reid RR, Luu HH, Haydon RC, He TC (2009) BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. J Cell Mol Med 13(8B):2448–2464
Castro-Garza J, Barrios-García HB, Cruz-Vega DE, Said-Fernández S, Carranza-Rosales P, Molina-Torres CA, Vera-Cabrera L (2007) Use of a colorimetric assay to measure differences in cytotoxicity of Mycobacterium tuberculosis strains. J Med Microbiol 56(Pt 6):733–737
Longo KA, Kennell JA, Ochocinska MJ, Ross SE, Wright WS, MacDougald OA (2002) Wnt signaling protects 3T3-L1 preadipocytes from apoptosis through induction of insulin-like growth factors. J Biol Chem 277(41):38239–38244
Hou XN, Tan Y, Li ML, Dey SK, Das SK (2004) Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol 18(12):3035–3049
Chen L, Wang K, Shao Y, Huang J, Li X, Shan J, Wu D, Zheng JJ (2008) Structural insight into the mechanisms of Wnt signaling antagonism by Dkk. J Biol Chem 283(34):23364–23370
Montgomery E, Torbenson MS, Kaushal M, Fisher C, Abraham SC (2002) Beta-catenin immunohistochemistry separates mesenteric fibromatosis from gastrointestinal stromal tumor and sclerosing mesenteritis. Am J Surg Pathol 26(10):1296–1301
Sakamoto A, Oda Y, Adachi T, Saito T, Tamiya S, Iwamoto Y, Tsuneyoshi M (2002) Beta-catenin accumulation and gene mutation in exon 3 in dedifferentiated liposarcoma and malignant fibrous histiocytoma. Arch Pathol Lab Med 126(9):1071–1078
Haydon RC, Deyrup A, Ishikawa A, Heck R, Jiang W, Zhou L, Feng T, King D, Cheng H, Breyer B, Peabody T, Simon MA, Montag AG, He TC (2002) Cytoplasmic and/or nuclear accumulation of the beta-catenin protein is a frequent event in human osteosarcoma. Int J Cancer 102(4):338–342
MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26
Mukai A, Yamamoto-Hino M, Awano W, Watanabe W, Komada M, Goto S (2010) Balanced ubiquitylation and deubiquitylation of Frizzled regulate cellular responsiveness to Wg/Wnt. EMBO J 29:2114–2125
Monga SPS (2009) Role of Wnt/β-catenin signaling in liver metabolism and cancer. Int J Biochem Cell Biol 3084–3093
Li YH, Lu WY, King TD, Liu CC, Bijur GN, Bu GJ (2010) Dkk1 stabilizes Wnt co-receptor LRP6: implication for Wnt ligand-induced LRP6 down-regulation. PLoS One e11014
Ugolini F, Adélaïde J, Charafe-Jauffret E, Ngugen C, Jacquemier J, Jordan B, Birnbaun D, Pebusque MJ (1999) Differential expression assay of chromosome arm 8p genes identifies frizzled-related (FRP1/FRZB) and fibroblast Growth factor receptor 1 (FGFR1) as candidate breast cancer genes. Oncogene 18:1903–1910
Nagahata T, Shimada T, Harada A, Naga H, Onda M, Yokoyama S, Shiba T, Jin E, Kawanami O, Emi M (2003) Amplification, up-regulation and over-expression of DVL-1, the human counterpart of the Drosophila disheveled gene, in primary breast cancers. Cancer Sci 94:515–518
Merle P, Kim M, Herrmann M, Gupte A, Lefrançois L, Califano S, Trépo C, Tanaka S, Vitvitski L, de la Monte S, Wands JR (2005) Oncogenic role of the frizzled-7/beta-catenin pathway in hepatocellular carcinoma. J Hepatol 43(5):854–862
Terris B, Pineau P, Bregeaud L, Valla D, Belghiti J, Tiollais P, Degott C, Dejean A (1999) Close correlation between beta-catenin gene alterations and nuclear accumulation of the protein in human hepatocellular carcinomas. Oncogene 18(47):6583–6588
Yamanaka T, Kodama T, Doi T (2002) Subcellular localization of HCV core protein regulates its ability for p53 activation and p21 suppression. Biochem Biophys Res Commun 294:528–534
Zakut R, Givol D (1995) The tumor suppression function of p21Waf is contained in its N-terminal half (‘half-WAF’). Oncogene 11:393–395
Acknowledgments
We thank Dr. Jianming Hu of The Pennsylvania State University College of Medicine, USA, for critical reading of the manuscript, Dr. Charles M. Rice of Rockefeller University, USA, for kind provision of plasmid HFL, Dr. T.-C He of University of Chicago, USA, for kind provision of plasmid pTop-luc and adenoviruses expressing Wnt3A, FrzB and siBC. This work was supported by research grants from China National Natural Science Foundation (#30972586, NT), Natural Science Foundation Project of CQ CSTC (2009BA5036, NT), Natural Science Foundation Project of CQMU (NT) and Major National S&T program (2008ZX10001-016, ALH).
Author information
Authors and Affiliations
Corresponding authors
Additional information
J. Liu and Z. Wang contributed equally to this study.
Rights and permissions
About this article
Cite this article
Liu, J., Wang, Z., Tang, J. et al. Hepatitis C virus core protein activates Wnt/β-catenin signaling through multiple regulation of upstream molecules in the SMMC-7721 cell line. Arch Virol 156, 1013–1023 (2011). https://doi.org/10.1007/s00705-011-0943-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-011-0943-x