Abstract
Here, we show that heat shock cognate protein 70 (Hsc70) in shrimp cells can inhibit apoptosis induced by white spot syndrome virus (WSSV) infection. Caspase-3 protease activity of hemocytes increased significantly, correlating with a reduction in endogenous Hsc70 late in WSSV infection. Hsc70 dsRNA caused a significant increase in caspase-3 activity in the hemocytes of non-infected shrimp and WSSV-infected shrimp. We propose that upregulation of Hsc70 expression early in WSSV infection may also be used to prevent premature apoptotic cell death, and the precipitous downregulation of Hsc70 late in WSSV infection may lead to the timed induction of apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Molecular chaperones from the family of 70 kDa heat shock proteins (Hsp70s) are conserved among many different kinds of organisms. The constitutively expressed members of this family, heat shock cognate proteins (Hsc70s), have been shown to be involved in protein folding in the cytoplasm, protein import into the endoplasmic reticulum, mitochondria and chloroplasts, and trafficking of receptors and coated vesicles [2, 7, 14]. In the case of animal viruses, interactions with Hsc70 appear to be involved in cell entry, virion assembly and disassembly, cell transformation, and DNA replication [6, 15, 21].
In addition, Hsp70 systems are essential for survival under stress conditions. Therefore, the induction of Hsp70/Hsc70 protein production may serve an additional purpose for the virus, namely, the prevention or delay of host cell death until the progeny is ready to leave. It is therefore, not surprising that interference with apoptotic signal transduction pathways in both directions—inhibition and activation—is a common phenomenon accompanying viral infection [9, 11]. It was shown previously that embryonic proinsulin increases the level of the Hsc70 and reduces the incidence of apoptosis in the neurulating chick embryo [4]. Hsc70 is also directly involved in cell survival during neurulation, as demonstrated by the fact that specific downregulation of endogenous Hsc70 by antisense oligodeoxynucleotide interference provokes an increase in apoptosis both in vitro and in vivo. In parallel, activation of caspase-3 is increased after Hsc70 antisense oligodeoxynucleotide treatment [13].
The shrimp Hsc70 gene has been cloned [10], but whether it is recruited by white spot syndrome virus (WSSV) or how this recruitment is performed is unknown. WSSV, the type member of the genus Whispovirus in the family Nimaviridae, is one of the most devastating pathogens of shrimp and other species of crustacean [18]. Since first appearing in the 1990s in Taiwan, WSSV has quickly spread to the whole world and caused severe damage to the shrimp industry. Our previous studies have indicated that interaction between VP28, one of the major structural proteins of WSSV [20], and Hsc70 can facilitate virus spread. It has also been shown that VP28 is directly involved in the systemic infection of the shrimp by WSSV [16]. Because of the diverse functions of Hsp70 proteins and their increased expression and association with viral proteins during viral infection, it can be speculated that Hsc70 plays an important role in certain aspects of viral infection as a cellular chaperone [8].
In this study, we show that caspase-3 protease activity in hemocytes increases with reduction of endogenous Hsc70 late in WSSV infection. Hsc70 dsRNA was found to cause a significant increase in caspase-3 activity in hemocytes of non-infected shrimp and WSSV-infected shrimp.
WSSV production
The WSSV isolate originated from penaeid shrimp (Penaeus monodon) and proliferated in an alternate host, the crayfish (Procambarus clarkii). WSSV-infected tissue (10 g) from penaeid shrimp (except hepatopancreas) was homogenized in 200 ml TN buffer (20 mM Tris–HCl, 400 mM NaCl, pH 7.4). After centrifugation at 3,000×g for 5 min, the supernatant was filtered using a nylon net (400 mesh) and injected (at a dilution of 1:100 in 0.9% NaCl) intramuscularly into healthy crayfish in the lateral area of the fourth abdominal segment. Each crayfish was about 7–10 cm in length and 20–30 g in weight. After 5–6 days, the dead and moribund crayfish were collected, and virus was purified as described [19].
Hemolymph preparation and primary shrimp cell culture
Hemolymph was taken from the heart using a sterile 1 ml syringe, preloaded with 100 μl anticoagulant (500 U/ml heparin sodium salt). The amount of hemolymph collected ranged from 200 to 500 μl, depending on the size of the shrimp. The hemolymph was centrifuged immediately at 600×g for 5 min (4°C) to separate the hemocytes from the plasma. Lymphoid organs were obtained from fresh adult P. monodon (100 g) and then washed. The lymphoid organs were minced into fragments as small as possible, which were the filtered and transferred to the wells of a 24-well plate, and 1 ml of the culture medium containing 2 × L-15 (Invitrogen) supplemented with 15% FCS, 1% glucose, 5 g/l NaCl, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 5 μg/ml gentamicin was added to each well. The plate was then sealed and incubated at 28°C until 70–80% confluent monolayers were formed. These cells were then ready for use.
dsRNA synthesis
Double-stranded RNA was synthesized in vitro using a Megascript kit (Ambion), following the manufacturer’s instructions. For the synthesis of dsRNA from Hsc70 and EGFP, PCR primers containing the T7 RNA-polymerase-binding site (sense and antisense) were used separately in a single PCR to generate a transcription template for both strands of the dsRNA. The sequence used for Hsc70 was the last 759 nucleotides of the Hsc70 open reading frame (GenBank, AF474375), while the entire gene sequence was used for the synthesis of dsRNA from EGFP. The sequences corresponding to the hsc70 and egfp gene were verified by sequence analysis. Hsc70: 5′-GAATTAATACGACTCACTATAGGGAGACTGGGTATCGAGACTGCCGGCG-3′ and 5′-GAATTAATACGACTCACTATAGGGAGATTAATCGACTTCCTCGATGGTG-3′; EGFP: 5′-GAATTAATACGACTCACTATAGGGAGAATGGTGAGCAAG GGCGAGGAGC-3′ and 5′-GAATTAATACGACTCACTATAGGGAGATTA CTTGTACAGCTCGTCCATG-3′. The products from a 100 μl PCR reaction were purified using a High Pure PCR Product Purification Kit (Roche Molecular Biochemicals) and then dissolved in TE buffer. One microgram of PCR purified DNA template was used to perform the RNA synthesis reaction in a 50 μl volume with T7 RNA polymerase and then removed by treatment with RNase-free DNase. Other than the gene-specific sequences, the dsRNA contained no unrelated sequences. The concentration of the purified dsRNAs dissolved in TE buffer was determined using a spectrophotometer. Fifteen microgram of each dsRNA was injected into shrimp 4 h prior to injection with WSSV. Fifteen microgram dsRNA was added to the primary shrimp lymphoid cells just before electroporation. Cells were electroporated in 0.4 cm-gap cuvettes using a Bio-Rad Gene Pulser set at 1.5 kV and 25 μF, with two pulses delivered 10 s apart, and the cells were immediately transferred to fresh medium.
Hoechst 33342 staining and DNA fragment extraction
Shrimp primary cells were collected and washed with PBS and then fixed in fresh Carnoy’s fixative solution (methanol/acetic acid, 3:1 by volume). After staining with Hoechst 33342 (0.5 μg/ml in PBS) for 30 min, representative photomicrographs were taken with a Leica DM/LM microscope under UV illumination (380 nm).
DNA oligonucleosomes were extracted for 2 h at 37°C from shrimp primary cells in a 10 mM Tris (pH 8.0), 1 mM EDTA–1% sodium dodecylsulfate (SDS) buffer containing 70 μg proteinase K/ml, and NaCl (final concentration, 1 M) was added. The extracts were treated with phenol–chloroform and precipitated with ethanol, and the resuspended DNA was analyzed by agarose gel electrophoresis.
Caspase activity assay
Caspase-3/7 activity was measured using an Apo-ONE® Homogeneous Caspase-3/7 Assay kit (Promega). The Apo-ONE® Caspase-3/7 Reagent contains a profluoresent caspase-3/7 consensus substrate, bis-(N-CBZ-l-aspartyl-l-valyl-aspartic acid amide) rhodamine 110 (Z-DEVD-R110) and an optimized bifunctional cell lysis/activity buffer, which efficiently lyses cultured cells and supports optimal caspase-3/7 enzymatic activity. The substrate and buffer were combined at a ratio of 1:100 and added directly to equal volume of hemocytes from infected shrimp or non-infected shrimp at 0, 12, 24, 48, 72, and 120 h.p.i. in a 96-well plate and then mixed and incubated for 1 h. Caspase-3/7 enzymatic activity was determined by monitoring the rate of liberation of rhodamine 110 using an excitation wavelength of 485 nm and an emission wavelength of 520 nm, using an enzyme-linked immunosorbent assay reader (BIO-TEK). The amount of fluorescent product generated was representatives of the amount of active caspase-3/7 present in hemocytes at the indicated time.
Immunoblotting
Healthy crayfish were purchased from a market, and were maintained for 2 days before they were randomly divided into two groups. One group was infected by injection, while the other group was not infected. Hemocytes were prepared from the non-infected and infected crayfish at different times (0, 2, 4, 6, 12, 24, 48, and 72 h.p.i.) and lysed under nondenaturing condition with phosphate-buffered saline buffer for 15 min on ice. Hemocyte lysates were homogenized by passing twice through a 26-gauge needle and centrifuged at 12,000×g and 4°C for 10 min. The pellet proteins were separated on a SDS-PAGE gel and then transferred to a PVDF membrane. The transferred proteins were detected using an anti-Hsc70 monoclonal antibody (StressGen Biotechnologies, Victoria, Canada).
Immunofluorescense analysis
WSSV-infected primary lymphoid cells were collected at 24 and 48 h.p.i. and cultured on cover slips. The cover slips were washed two times with PBS, and the cells were fixed with 3.7% formaldehyde for 10 min, followed by a brief permeabilization with 0.5% Triton X-100 in PBS. The cells were blocked for 30 min in PBS containing 3% normal serum and then incubated for 2 h with primary anti-Hsc70 antibody (diluted 1:200 in 1% normal serum in PBS). The cover slips were washed three times with PBS and then incubated for 1 h with goat anti-rat IgG secondary antibodies conjugated with FITC (Santa Cruz; diluted 1:400 in 1% normal donkey serum in PBS). Samples were washed four times, and the results were analyzed using a laser scanning confocal microscope MRC 1000 (BioRad).
Silencing of endogenous Hsc70 induces primary cell apoptosis in shrimp
To demonstrate a direct role for Hsc70 in prevention of developmental cell death, shrimp primary lymphoid cells were transfected with dsRNA. As expected, Hsc70 expression in shrimp primary cultures was effectively silenced, and shrimp Hsc70 dsRNA increased the incidence of apoptosis seen in primary lymphoid cells. This effect was confirmed and quantified in primary cells. Compared with cells that were treated with EGFP dsRNA or without dsRNA, the cells that were transfected with Hsc70 dsRNA or actinomycin D showed obvious characteristics of apoptosis at 24 h (Fig. 1I). Hoechst 33342 staining of shrimp primary cells showed that Hsc70 dsRNA could induce primary cell apoptosis, and condensed chromatin of treated cells appeared as intense blue spots in the nucleus, as was also seen with actinomycin D treatment (Fig. 1II). DNA extracted from control cells treated without dsRNA (Fig. 1III, lane 1) or primary cells treated with EGFP dsRNA (Fig. 1III, lane 2) was intact, but all of the DNA of cells treated with Hsc70 dsRNA and treated with actinomycin D had been degraded into a 180–220 bp ladder (Fig. 1III, lanes 3, 4, 5). These results support a direct involvement of endogenous Hsc70 in prevention of primary cell death.
Reduction of endogenous Hsc70 in shrimp primary cells induces apoptosis. I Effect of Hsc70 dsRNA on morphology of shrimp primary cells. A In the control group with no dsRNA, no significant characteristics of apoptosis appeared in the primary cells. B After treating with Hsc70 dsRNA for 24 h, the number of primary cells decreased, and the cells exhibited obvious characteristic of apoptosis. C Cells were transfected with EGFP dsRNA for 24 h, and no obvious apoptosis characteristic could be observed. D After treating with actinomycin D for 24 h, the number of primary cells decreased, and the cells exhibited obvious characteristic of apoptosis. II Hoechst 33342 staining of shrimp primary cells after Hsc70 dsRNA treatment. A Cells that were not treated with dsRNA were used as control. The center of the nucleus of control shrimp primary cells was diffusely stained pale blue. B Cells were treated with EGFP dsRNA for 24 h. No obvious chromatin condensation could be observed. C Cells were transfected with Hsc70 dsRNA for 24 h. The intense blue spots within the nucleus indicate chromatin condensation. D Cells were treated with actinomycin D for 24 h. The intense blue spots within the nucleus indicate chromatin condensation. The preparations were viewed with a Leica DM/LM microscope under UV illumination (380 nm). III DNA fragmentation analysis of shrimp primary cells after Hsc70 dsRNA treatment. Lane 1 cells with no dsRNA harvested at 24 h; lane 2 cells transfected with EGFP dsRNA at 24 h; lane 3 cells transfected with Hsc70 dsRNA at 24 h; lane 4 cells harvested after addition of actinomycin D for 24 h; lane 5 cells harvested after treatment with actinomycin D for 48 h
Caspase-3 activity and Hsc70 expression levels increase at different stages when cells are infected by WSSV
It has been shown that WSSV infection can trigger apoptosis in the lymphoid organ cells of P. monodon [1]. To address the issue of possible association with caspase-3 activation in the induction of cell apoptosis, the hemocyte lysates from non-infected and infected shrimp were tested for caspase-3/7-like proteases activity. As can be easily deduced from Fig. 2, the caspase-3/7-like protease activity was clearly induced by 24 h postinfection and reached the highest level at 48 h postinfection in the hemocyte lysates from WSSV-infected shrimp. A rapid recovery to a low level at 120 h post infection was noted. By contrast, no significant caspase-3-like protease activity could be detected in the non-infected shrimp (Fig. 2a).
a Caspase-3/7-like protease activity in hemocytes obtained from non-infected and WSSV-infected shrimp. Hemocyte lysate supernatants were prepared at 2, 4, 6, 12, 24, 48, 72 and 120 h postinfection and assayed for DEVD cleavage activity using Z-DEVD-R110 as substrate. DEVD-R110 hydrolysis produced from hemocytes extracts was monitored over a 30 min period at 37°C and quantified in relative fluorescent units. Vertical bars represent standard deviations of averages from three independent measurements. b Relative expression of hemocytic Hsc70 in non-infected and infected shrimp at different times (0, 2, 4, 6, 12, 24, 48, and 72 h postinfection). The hemocytes were incubated with the appropriate primary anti-Hsc70 monoclonal antibody, and Hsc70 expression in shrimp hemocytes was observed by flow cytometry using fluorescence of fluorescein isothiocyanate (FITC)-conjugated goat anti-rat IgG secondary antibodies. Values are expressed as means ± SEM (n = 5)
This result also revealed that the highest level of caspase-3-like protease activity could be measured at 48 h postinfection, whereas at the same time, the lowest level of Hsc70 expression was observed in shrimp hemocytes during WSSV infection (Fig. 2b). The Hsc70 protein level in the non-infected or infected shrimp hemocytes was detected with the appropriate primary monoclonal antibody to Hsc70 by flow cytometry. The fluorescence of Hsc70 increased at an early stage and reached its highest level at 15 h postinfection, and it decreased suddenly at 36 and 48 h post infection. This indicates that the highest level of apoptosis and the lowest level of Hsc70 expression occurred at the same time in the hemocyte lysates from the WSSV-infected shrimp. More interestingly, at the highest level of Hsc70 expression in the hemocyte lysates from the WSSV-infected shrimp, the lowest level of caspase-3 was detected.
Caspase-3-like protease activity increases following Hsc70 dsRNA treatment in infected shrimp hemocytes
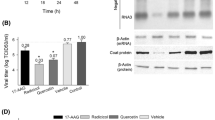
In order to evaluate the possible association of downstream effector caspase-3 with Hsc70 during WSSV infection in shrimp hemocytes, an attempt to silence Hsc70 with RNA interference in shrimp was carried out. Four hours after shrimp were injected with 15 μg Hsc70 dsRNA, or EGFP dsRNA as a negative control, they were injected intramuscularly with WSSV. There was no obvious difference in Hsc70 expression in hemocytes of infected shrimp, non-infected shrimp injected with EGFP dsRNA, and mock-injected shrimp (Fig. 3a). In vivid contrast, Hsc70 dsRNA molecules induced a statistically significant decrease in Hsc70 expression in WSSV-infected hemocytes and non-infected hemocytes (Fig. 3a).
Caspase-3-like protease activity increases following Hsc70 dsRNA treatment in infected shrimp hemocytes. a Time course of Hsc70 expression in hemocytes during dsRNA treatment of non-infected or infected shrimp. Individual shrimp were injected with 5 μg of dsRNA (Hsc70 dsRNA, EGFP dsRNA and no dsRNA) as indicated, and Hsc70 expression in the hemocytes of the non-infected or infected shrimp at 2, 12, 24, 48 and 72 h after injection of dsRNA was analyzed by immunoblotting. Expression of shrimp actin is shown as a reference. The ratios indicated at the bottom of the lanes were determined by densitometry scanning of the membranes after immunoblotting. b, c, Caspase-3/7-like protease activity in hemocytes obtained from non-infected and WSSV-infected shrimp after Hsc70 dsRNA treatment. Hemocyte lysate supernatants were prepared at 2, 4, 6, 12, 24, 48, 72 and 120 h after injection of dsRNA (Hsc70 dsRNA, EGFP dsRNA and no dsRNA) and assayed for DEVD cleavage activity using Z-DEVD-R110 as substrate. DEVD-R110 hydrolysis in hemocyte extracts was monitored over a 30 min period at 37°C and quantified in relative fluorescent units. Vertical bars represent standard deviations of averages from three independent determinations
To examine possible interference in shrimp hemocytes, we demonstrated the presence of activated caspase-3 in shrimp hemocytes after treatment with Hsc70 dsRNA (Fig. 3b, c). Compared with control treatments of EGFP dsRNA or no dsRNA, the hemocytes from both infected and non-infected shrimp were found to contain activated caspase-3 after treatment with dsRNA Hsc70 (Fig. 3b, c). Moreover, together with the gradual decrease in Hsc70 expression of the hemocytes of both infected and non-infected shrimp treated with Hsc70 dsRNA, caspase-3 activity increased continuously over time (Fig. 3b, c).
In eukaryotic cells, Hsp70-family chaperones not only continuously survey the folding status of proteins as part of their quality control function, which is especially important under stress conditions, they are also involved in the regulation of fundamental cellular processes such as the cell cycle and apoptosis. The functional interaction of viruses with these chaperones therefore contributes to reprogramming the host cell, specifically to allow re-entry into the cell cycle and to avoid premature apoptosis [3].
It has been shown that Hsp70 plays an important role in cell apoptosis. Hsp70 interferes with the signal transduction pathways leading to programmed cell death at several levels [5, 12]. Rubio et al. [13] previously showed that the levels of Hsc70 expression are low in apoptotic cells, and they showed that blocking Hsc70 protein expression in chicken embryos using antisense oligonucleotides increased the levels of apoptosis. These observations pose interesting questions regarding the mechanism of Hsc70 in WSSV infection: are only substrate binding by Hsc70 and its chaperone activity involved? If not, what other role does Hsc70 have in WSSV infection?
Up to now, direct genetic evidence for the function and mechanism of Hsc70 in WSSV infection has not been available. To explore the role of Hsc70 in WSSV infection further and to determine the direct relationship between Hsc70 activity and cell survival in shrimp hemocytes, we silenced Hsc70 protein expression in non-infected shrimp, infected shrimp and lymphoid primary cells using RNA interference and subsequently analyzed the level of apoptosis in the time course of the experiment. As expected, Hsc70 dsRNA increased the level of cell apoptosis. This experiment provided the first evidence for a direct causal link between Hsc70 activity and cell survival in shrimp hemocytes. These results also suggest that endogenous Hsc70 is directly involved in prevention of primary cell death.
It has been demonstrated that cell apoptosis is the major symptom induced in shrimp infected by WSSV [17]. Thus, we proposed that there is interplay between expression of Hsc70 and cell apoptosis levels in infected shrimp hemocytes. At the early stage of infection, Hsc70 appeared to be stimulated by WSSV infection to inhibit cell apoptosis induced by WSSV infection. Conversely, at the late stage of WSSV infection, it was down-regulated and reduced to below its constitutive expression level by unidentified factors or co-chaperones, resulting in the absence of apoptosis inhibition activity.
The role of Hsc70 in WSSV infection was further analyzed at the level of apoptosis (taking the activated caspase-3 activity as the criterion). To our surprise, we found that the lowest level of expression of Hsc70 expression occurred concurrently with the highest activity of cell apoptosis (48 h postinfection). The highest expression level of Hsc70, however, accompanied almost undetectable changes in the level of cell apoptosis. This increased or decreased expression of Hsc70 at different stages of infection may represent a strategy to induce chaperone proteins for use at different times of the virus life cycle.
This study provides the first evidence for a direct causal link between Hsc70 activity and cell survival in the shrimp system. Although, it is still unclear precisely how Hsc70 protects cells from apoptosis at the early stage and promotes cells to undergo apoptosis at the late stage of virus infection, our study provides an important clue that knockdown of Hsc70 causes an increase in the level of activated caspase-3, indicating that one of its functions might be to control the activity of this key protein of the apoptotic pathway. Although much more work needs to be done, these early results clearly confirm that there is more to Hsc70 than its role as a chaperone.
References
Anggraeni MS, Owens L (2000) The haemocytic origin of lymphoid organ spheroid cells in the penaeid prawn Penaeus monodon. Dis Aquat Organ 40:85–92
Cripe TP, Delos SE, Estes PA, Garcea RL (1995) In vivo and in vitro association of hsc70 with polyomavirus capsid proteins. J Virol 69:7807–7813
D’Onofrio C, Puglianiello A, Amici C, Faraoni I, Lanzilli G, Bonmassar E (1995) HSP70 production and inhibition of cell proliferation in Molt-4 T-cells after cell-to-cell transmission of HTLV-I: effect of PGA1. Leuk Res 19:345–356
De la Rosa EJ, Vega-Nunez E, Morales AV, Serna J, Rubio E, de Pablo F (1998) Modulation of the chaperone heat shock cognate 70 by embryonic (pro) insulin correlates with prevention of apoptosis. Proc Natl Acad Sci USA 95:9950–9955
Fazakerley JK, Allsopp TE (2001) Programmed cell death in virus infections of the nervous system. Curr Top Microbiol Immunol 253:95–119
Florin L, Becker KA, Sapp C, Lambert C, Sirma H, Muller M, Streeck RE, Sapp M (2004) Nuclear translocation of papillomavirus minor capsid protein L2 requires Hsc70. J Virol 78:5546–5553
Guerrero CA, Bouyssounade D, Zarate S, Isa P, Lopez T, Espinosa R, Romero P, Mendez E, Lopez S, Arias CF (2002) Heat shock cognate protein 70 is involved in rotavirus cell entry. J Virol 76:4096–4102
Hohfeld J (1998) Regulation of the heat shock conjugate Hsc70 in the mammalian cell: the characterization of the anti-apoptotic protein BAG-1 provides novel insights. Biol Chem 379:269–274
Li CY, Lee JS, Ko YG, Kim JI, Seo JS (2000) Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem 275:25665–25671
Lo WY, Liu KF, Liao IC, Song YL (2004) Cloning and molecular characterization of heat shock cognate 70 from tiger shrimp (Penaeus monodon). Cell Stress Chaperones 9:332–343
Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B (2000) The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol 20:7146–7159
Muthumani K, Hwang DS, Desai BM, Zhang D, Dayes N, Green DR, Weiner DB (2002) HIV-1 Vpr induces apoptosis through caspase 9 in T cells and peripheral blood mononuclear cells. J Biol Chem 277:37820–37831
Rubio E, Valenciano AI, Segundo C, Sanchez N, de Pablo F, De la Rosa EJ (2002) Programmed cell death in the neurulating embryo is prevented by the chaperone heat shock cognate 70. Eur J Neurosci 15:1646–1654
Sagara Y, Ishida C, Inoue Y, Shiraki H, Maeda Y (1998) 71-kilodalton heat shock cognate protein acts as a cellular receptor for syncytium formation induced by human T-cell lymphotropic virus type 1. J Virol 72:535–541
Saphire AC, Guan T, Schirmer EC, Nemerow GR, Gerace L (2000) Nuclear import of adenovirus DNA in vitro involves the nuclear protein import pathway and hsc70. J Biol Chem 275:4298–4304
van Hulten MC, Witteveldt J, Snippe M, Vlak JM (2001) White spot syndrome virus envelope protein VP28 is involved in the systemic infection of shrimp. Virology 285:228–233
Wongprasert K, Khanobdee K, Glunukarn SS, Meeratana P, Withyachumnarnkul B (2003) Time-course and levels of apoptosis in various tissues of black tiger shrimp Penaeus monodon infected with white-spot syndrome virus. Dis Aquat Organ 55:3–10
Wongteerasupaya C, Vickers JE, Sriurairatana S, Tassanakajon A, Nash GL, Akarajamom A, Boonsaeng V, Panyim S, Withyachumnarnkul B, Flegel TW (1995) A non-occluded, systemic baculovirus that occurs in cells of ectodermal and mesodermal origin and causes high mortality in the black tiger prawn Penaeus monodon. Dis Aquat organ, 21:69–77
Xie X, Li H, Xu L, Yang F (2005) A simple and efficient method for purification of intact white spot syndrome virus (WSSV) viral particles. Virus Res 108:63–67
Yang F, He J, Lin X, Li Q, Pan D, Zhang X, Xu X (2001) Complete genome sequence of the shrimp white spot bacilliform virus. J Virol 75:11811–11820
Zarate S, Cuadras MA, Espinosa R, Romero P, Juarez KO, Camacho-Nuez M, Arias CF, Lopez S (2003) Interaction of rotaviruses with Hsc70 during cell entry is mediated by VP5. J Virol 77:7254–7260
Acknowledgments
This work was supported by grants from the National Nature Science Foundation of China (30800852, 30972276) and the National 863 Project of China (2006AA09Z445).
Author information
Authors and Affiliations
Corresponding author
Additional information
Feng Yan and Ding Xia contributed equally to the article.
Rights and permissions
About this article
Cite this article
Yan, F., Xia, D., Hu, J. et al. Heat shock cognate protein 70 gene is required for prevention of apoptosis induced by WSSV infection. Arch Virol 155, 1077–1083 (2010). https://doi.org/10.1007/s00705-010-0686-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-010-0686-0