Abstract
Long-term persistence of West Nile virus (WNV) infection within vertebrate reservoir hosts is a potential mechanism for overwintering of this (and other) arbovirus(es) at temperate latitudes. The house sparrow (Passer domesticus), an established amplifying host for WNV and other arboviruses, was used as a model to confirm chronicity of WNV infection in passerine birds and to evaluate the feasibility of two overwintering mechanisms: blood-borne infection of arthropod vectors (recrudescence) and oral infection of vertebrate reservoir hosts (ingestion of infected tissues through predation). WNV-inoculated sparrows were monitored for persistent infection for up to 2 years. Infectious virus persisted in tissues through 43 days, but not in sera beyond 6 days. Viral RNA persisted in tissues through 65 days. Chronicity of WNV infection in some tissues, but not blood, supports the predation mechanism of WNV overwintering, but not recrudescence. RNA persistence impacts interpretation and etiologic determination of avian mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Viral persistence, here defined as the continued presence of infectious virus in vertebrate hosts beyond the acute viremic stage, has been documented for most families of arboviruses [18]. This phenomenon may have implications for the maintenance and reinitiation of virus transmission cycles in nature. In temperate regions, extended periods of mosquito inactivity interfere with the continuous transmission of mosquito-borne viruses [30]. Overwintering strategies for these viruses include hibernation of infected adult female mosquitoes, transovarial transmission from female mosquitoes to their offspring, and reintroduction through movement of infectious vectors and/or vertebrate amplifying hosts from warmer climates [29]. Persistently infected vertebrate hosts have not yet been directly linked to an overwintering strategy for any arbovirus.
The overwintering mechanisms of West Nile virus (WNV; family Flavivirus, genus Flaviviridae) are still not fully understood, though WNV has been detected in overwintering mosquitoes in New York, Connecticut, and Utah [1, 22, 27]. However, WNV infection in overwintering mosquitoes is rare in nature [4, 8, 26, 32, 36]. The recovery of infectious WNV from the brain of a hawk in New York in February, a period of mosquito inactivity, raised questions as to potential persistent infection within the hawk, or alternatively, oral transmission to the hawk via consumption of persistently infected prey, probably a rodent or bird [10]. Further evidence of a non-mosquito source of transmission during cold periods in a temperate region, again New York, was the detection of lethal infections among communally roosting crows [6]. The principal mechanism for annual spring emergence and reinitiation of WNV transmission remains unknown.
The house sparrow (Passer domesticus) has been implicated as an important amplifying host in the epizootic cycles of numerous arboviruses in the United States, e.g., eastern equine encephalitis, St. Louis encephalitis (SLEV), and western equine encephalitis viruses (WEEV) [17], and may be important for the maintenance and local spread of WNV. House sparrows are amplifying hosts of WNV and are widespread and abundant throughout North America, where they have demonstrated high seroprevalence rates in numerous regions [12, 14–16]. WNV RNA was detected more than 6 weeks post-infection in tissues from a small sample of experimentally inoculated house sparrows [32]. Therefore, we hypothesized that such infections in house sparrows could be important for overwintering of WNV. To confirm and expand previous observations, we inoculated sparrows with WNV and monitored their infection status for up to 65 days in juveniles, and 2 years in adults.
Materials and methods
Husbandry and initial sampling
Adult house sparrows were collected by mist net in northern Colorado in January and February of 2005. Birds were bled via jugular venipuncture upon arrival to assess WNV serostatus and then housed free-flight in a 12.12 m long (L) × 3.24 m wide (W) × 2.57 m high (H) room provided with sand and water baths, and branches and ropes for perching. Cuttlefish bone, fresh water, and food were available at all times; food consisted of a dry mix with equal parts of millet, milo, cracked corn, cracked sunflower seed, and oats. Dry food was supplemented with live mealworms 1–2 times a week.
These captive sparrows bred in 2007, after which 36 offspring were separated from the flock at approximately 2–4 months of age and housed within cages [0.61 m (L) × 0.38 m (H) × 0.41 m (W) or 0.76 m (L) × 0.43 m(H) × 0.46 m (W); 2–6 birds per cage]. Juveniles were independent and fully developed when separated, and were fed the adult diet plus cooked egg and mealworm pupae ad libitum. Sparrows were acclimated to captivity and/or caged housing for days to weeks prior to experimental inoculation.
Inoculation and sampling of sparrows
All sparrows were bled just prior to WNV inoculation for confirmation of WNV seronegative status. Both seronegative adults (n = 115) and juveniles (n = 35) were needle-inoculated subcutaneously over the chest with between 1,000–4,000 PFU of WNV strain NY99-4132 administered in 0.1 ml BA1 (M199-Hank’s salts, 1% bovine serum albumin, 350 mg/l sodium bicarbonate, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml amphotericin B in 0.05 M Tris, pH 7.6). Adults were inoculated in April 2005, and juveniles in November 2007. Twenty seronegative adults and one seronegative juvenile were kept as non-infected controls. Anti-WNV antibody status was confirmed in all sparrows surviving to 1 month post-inoculation (PI), including non-inoculated control sparrows.
Sparrows exhibiting signs of morbidity, such as lethargy, anorexia, and/or fluffed feathers, were humanely euthanized via sodium pentobarbital overdose administered intravenously. Carcasses of sparrows that died or were euthanized due to morbidity within 8 days of inoculation (n = 11 adults and 8 juveniles) were immediately necropsied or refrigerated and necropsied within 12 h. Tissue and swab samples were also collected opportunistically from adult sparrows that died or were euthanized due to clinical illness (n = 10) between 30 and 354 days PI. At necropsy, oral and cloacal swabs and tissues were collected, including skin (from breast), pectoral muscle, heart, liver, lung, spleen, small intestine, kidney, and cerebrum from all birds, plus breast feathers from juveniles.
The post-inoculation sampling scheme for adult sparrows that survived acute infection (>8 days PI) included collection of oral swabs from 104 adult sparrows at 1 month PI (plus 20 non-inoculated controls), 98 adults (plus 19 non-inoculated controls) at 6 months PI, and 20 previously inoculated birds at each time point of 12, 18, and 24 months PI. In addition, all adults were bled at 1, 6, 12, 18, and 24 months PI to confirm WNV seropositive status. Three adults were sacrificed at each of these time points, followed by necropsy and sample collection as described previously.
The post-inoculation sampling scheme for the 27 juvenile sparrows that survived acute infection plus one negative control was as follows: sera and oral and cloacal swabs were collected every 3 days from 3 to 30 days PI, at which time 14 birds were euthanized (including the negative control). Thereafter, the 14 remaining sparrows were bled and swabbed weekly and euthanized on 65 days PI.
Sample processing and storage
For juvenile sparrows, 0.1 ml of blood was placed into a cryovial containing 0.45 ml of BA1 medium for an approximate 1:10 serum dilution, held at room temperature for approximately 20–30 min for coagulation, and centrifuged at 2,000g for 10 min. A portion of each sample was stored at 4°C and tested within 24 h by Vero cell plaque assay to avoid a freeze-thaw cycle prior to testing, while the remainder was frozen to −80°C for testing by reverse transcriptase-polymerase chain reaction (RT-PCR) within 1 month.
For adult sparrows, blood samples were placed undiluted into serum separator tubes, held at room temperature for approximately 1 h, and centrifuged at 16,000g for 3 min. The sera were stored at −20°C.
Oral and cloacal cavities were sampled by passing cotton-tipped swabs across mucosal surfaces, after which swabs were placed into 1 ml BA1 medium. Swabs from juveniles were aliquotted and stored as for diluted serum samples, while those from adults were stored at −80°C until testing.
Upon necropsy, tissue samples were weighed and placed into cryovials containing 1 ml of BA1 medium with 20% FBS as a 10% tissue suspension (except for spleen, which was at an approximate 5% tissue suspension). A single copper-coated steel 4.5 mm ball bearing (“BB”) was added to each cryovial, and tissue samples were homogenized in a mixer mill (Retsch GmbH, Haan, Germany) for 5 min at 25 cycles/s and homogenates were clarified by centrifugation (16,000g for 3 min). Swab and tissue supernatants from adults were stored at −80°C, while swabs from juveniles were handled as described for live bird samples. Tissue homogenates from juveniles were pooled into three aliquots for testing by RT-PCR as follows: kidney, spleen, small intestine and liver; skin, feather and muscle; and heart, lung and brain. Tissues from all positive pools were tested individually. Tissues collected from juveniles at 30- and 65-days PI (gastrointestinal tract, brain, liver, heart, lung, kidneys, and breast skin) were placed into 10% neutral buffered formalin for 24 h, and then into 70% ethanol until processing for histopathology.
Virus isolation and quantification
Plaque assays were performed to assess and quantify infectious WNV [5]. Briefly, Vero cell monolayers in 6-well plates were inoculated in duplicate with 0.1 ml of sample per well. After 1 h of incubation at 37°C, the cells were overlaid with 3 ml/well of 0.5% agarose (in a yeast extract–lactalbumin overlay medium supplemented with 2,240 mg/l sodium bicarbonate, 292 mg/l l-glutamine, and antibiotics as with BA1). Two days later, cells were overlaid with 0.5% agarose in overlay medium, with 0.004% neutral red dye (Sigma, St. Louis, MO, USA). Viral plaques were counted on the 3rd and 4th days of incubation. Samples tested by plaque assay from juveniles included serially collected oral and cloacal swab and serum samples as well as oral and cloacal swab, heart, kidney, spleen, brain, feathers, skin, skeletal muscle, liver, lung, and intestine collected at necropsy. Samples from adults included oral swabs collected at serial time points as well as oral swab, heart, kidney, spleen, and brain collected at necropsy. Minimum levels of WNV detection by plaque assay were 101.7 log PFU/ml for serum, 100.7 log PFU/swab, and 101.7 PFU/g for tissues. Viral plaques detected by Vero plaque assay were confirmed by reisolation from the original sample, and identified as WNV by VecTest WNV Antigen Assay (VecTest; Medical Analysis Systems, Camarillo, CA, USA) [23] or by RT-PCR.
Serology
Sera collected from all birds just prior to inoculation, as well as from adults and juveniles at approximately 1 month PI and subsequent time points (6, 12, 18 and/or 24 months PI for adults and 65 days PI for juveniles) were assessed for WNV-neutralizing antibodies by plaque-reduction neutralization test (PRNT) [3]. Briefly, sera were heat-inactivated at 56°C for 30 min and then diluted 1:10 in BA1 medium with a challenge dose of approximately 100 PFU of WNV strain NY99-4132, incubated at 37°C for 1 h, and tested immediately by plaque assay as described above. Samples with ≥90% neutralization at a 1:10 dilution were considered anti-WNV antibody positive, while samples with ≤60% neutralization were antibody negative (no neutralization results fell between these criteria of 60–90%).
RT-PCR
Serum, swab, and tissue samples collected from juveniles at ≥9 days PI were tested by RT-PCR. RT-PCR methods for detection of WNV RNA followed those previously described [19], except for use of the Viral RNA Minikit (QIAGEN Inc., Valencia, CA, USA) for RNA extraction and use of the Bio-Rad Icycler IQ™ Real-time Detection system (Bio-Rad, Hercules, CA, USA) for cDNA amplification. A Ct value of 36.5 or less was considered positive for target sequence amplification, while values between of 36.5 and 37.5 were re-tested. Samples were screened with one set of primers specific for the envelope protein of WNV (genome positions were 1160 for forward primer, 1229 for reverse primer, and 1186 for probe) [19].
Immunohistochemistry (IHC)
Tissues (heart, lung, kidney, spleen, intestine, cerebrum, and head skin with feathers removed) from convalescent juveniles were embedded in paraffin, sectioned at 6 μm and stained. Negative and positive control tissues for IHC were obtained from a seronegative juvenile that was not inoculated and from a juvenile that died within the acute phase of infection (4 days PI), respectively. The methodology for IHC was adapted from a published protocol with minor modifications [21]. The primary antibody used was WNV B-956 diluted 1:500 in blocking solution. To minimize non-specific staining, tissues were incubated for 30 min with 0.15 M glycine in phosphate-buffered saline, following the 1% H2O2 incubation step. Endogenous biotin binding was blocked using a kit from DAKO (Carpintaria, CA, USA), and the blocking solution contained 0.2% Tween-80 in addition to Tween-20 and normal serum.
Results
Morbidity and mortality
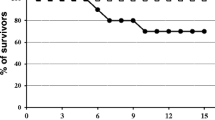
The majority of adult (90%; 104/115) and juvenile (77%; 27/35) sparrows inoculated with WNV survived beyond the acute phase of infection.
Mortality after 8 days PI among inoculated adults occurred on or near 30, 43, 105 (two individuals), 150, 160, 247, 280, 319, and 354 days PI. In nearly all cases, sparrows were found dead; however, one sparrow was euthanized 43 days PI due to open mouth breathing and lethargy; upon necropsy, an extensive space-occupying lesion was evident within the coelomic cavity that histologically revealed a ruptured spleen. Several sparrows had gross evidence of blunt head trauma, another had hemorrhage within the gastrointestinal tract, and several had no gross lesions. The carcasses of sparrows found dead on 160 and 343 days PI were desiccated and did not undergo necropsy or sample collection. None of these convalescent deaths were attributed to WNV infection or appeared in any way to reflect recrudescence of acute infection. No juveniles died past 8 days PI.
West Nile virus shedding, viremia, and detection in tissues among acute and fatal infections
Most juveniles (31/35; 88.6%) had detectable oral shedding 3 days PI with a mean concentration of 103.5 PFU/swab (samples with undetectable levels were considered zero in calculating the mean). Forty percent of juveniles (14/35) had detectable cloacal shedding 3 days PI, with an average of 102.9 PFU/swab. Of the juvenile sparrows that died within 8 days PI, all swabs were positive for infectious WNV, with titers ranging from 102.7–7.0 log PFU/oral swab (includes juveniles and adults) and 102.6–5.7 log PFU/cloacal swab. Oral and cloacal shedding was rare in surviving juveniles after 6 days PI.
The mean peak viremia 3 days PI among juveniles that died within 8 days of inoculation (n = 8) was 109.4 PFU/ml serum, while the mean peak viremia among survivors was 106.0 PFU/ml serum. WNV was not detected in serum beyond 6 days PI.
All tissues from adults that died within 8 days of inoculation (n = 8) tested positive for WNV by plaque assay with the exception of the spleen and brain of one bird tested 7 days PI, and the spleen of another tested 8 days PI. All tissues from juveniles that died within 8 days of virus inoculation were positive except for feathers of one bird. The highest WNV titers in any juvenile sparrow were in spleen (109.8 PFU/g), brain (109.6 PFU/g), kidney (109.2 PFU/g), and liver (109.0 PFU/g). Of birds that died acutely, mean peak titers were generally higher in juveniles than adults (n = 11) (oral swab: 106.2 vs. 105.8 PFU/swab; heart: 107.6 vs. 106.7 PFU/g; kidney: 108.8 vs. 107.5 PFU/g; spleen: 109.1 vs. 106.9 PFU/g; brain: 108.9 vs. 107.3 PFU/g, respectively).
West Nile virus persistent shedding and detection in tissues
At 1 month PI, one of 104 (1.0%) adult sparrows had a low titer of infectious WNV (100.7 PFU/swab) isolated from the oral swab; this same sparrow was euthanized 43 days PI, and WNV was isolated from the spleen (102.7 PFU/g). WNV was not isolated from oral swabs of 98 sparrows sampled at 6 months PI, or from any of 20 sparrows sampled at each 12, 18, and 24 months PI. Similarly, no virus was isolated from swabs or tissues of sparrows that died between 30 and 354 days PI, or from the three birds sacrificed at each time point of 6, 12, 18, or 24 months PI.
Oral and cloacal shedding was detected during the convalescent phase (>8 days PI) in four juveniles and one adult up to 44 days PI (Table 1). Only two juveniles and one adult had detectable oral or cloacal shedding of infectious WNV during this phase, with low titers of 100.7–2.0 PFU/swab.
West Nile virus detection in tissues by RT-PCR occurred from most (12/13; 92.3%) juvenile sparrows euthanized 30 days PI, with skin (10/13; 76.9%), spleen, and kidney (9/13 each; 69.2%) most frequently positive for viral RNA. Low titers of WNV (101.7 PFU/g) were detected by plaque assay in spleen and kidney of two different juveniles euthanized 30 days PI. At 65 days PI, spleen and kidney of one individual and kidney from another were positive by RT-PCR (Table 1).
None of the tissues from juvenile sparrows euthanized at 30 or 65 days PI tested positive by IHC staining, and no lesions were observed by microscopic examination.
Contact transmission from infected to negative control sparrows was not detected, as determined by lack of viral shedding and seroconversion among these control birds at all subsequent sampling time points.
Anti-WNV antibodies
All inoculated sparrows had anti-WNV antibodies by 1 month PI, and antibodies remained detectable at all subsequent sampling time points. No non-inoculated sparrows demonstrated evidence for anti-WNV antibodies during the study.
Discussion
Arboviral persistence has been documented experimentally within a variety of vertebrate hosts, including bats, snakes, primates, and birds [18]. Persistent WNV infections were observed in blood of experimentally inoculated ducks [9] and pigeons at approximately 100 days PI [34]. Relatively high titers of infectious WNV (up to 104.3 PFU/0.5 cm3) were detected in skin of several experimentally inoculated birds at 14–15 days after mosquito inoculation [14]. WNV was detected by RT-PCR in tissues (spleen, kidney, and lung) of experimentally-inoculated passerine and columbid birds at >6 weeks PI [32]. WNV persisted for up to 167 days PI in brains of experimentally inoculated rhesus macaques (Macaca mulatta), though virus lacked cytopathogenicity [28]. Infectious WNV was detected in the urine of experimentally inoculated hamsters (Mesocricetus auratus) for up to 247 days PI, with antigen visible in kidneys by IHC [38]. Finally, persistent WNV infection (35 days PI) was documented in mice that were deficient in CD8+ T cells, but not in wild-type mice [35], suggesting that immunocompromised individuals may be more susceptible to persistent WNV infection. Evidence for persistent infections of avian hosts in nature is limited [10, 20, 39], though avian carcasses have tested positive for WNV RNA during winter periods in Texas and California [32, 37]. Persistent infections of arboviruses in avian tissues have been speculated to relapse, causing potentially infectious viremia for blood-feeding vectors [29].
Persistent WNV infection was detected in several needle-inoculated sparrows in the present study, confirming an earlier observation by Reisen et al. [32] in which 41.5% (34/82) of needle-inoculated individuals of six avian species had detectable WNV RNA at >6 weeks PI. The house sparrow was among these species, of which 33.3% (3/9) of individuals had positive tissues (spleen, lung, and/or kidney), and one of these sparrows had RT-PCR-positive serum. Infectious WNV was recovered from a portion of RNA-positive tissue samples; tissue samples were passaged through C6/36 Aedes albopictus (Skuse) cell culture prior to Vero cell plaque assay to maximize infectious virus recovery. Our results are in accordance with those of Reisen et al. [32] and support the notion that detection of viral RNA at chronic time points following initial infection is not uncommon, especially in kidney, while the detection of circulating WNV or RNA in serum appears much less frequent. More rigorous detection methods such as those employed by Reisen et al. and others (e.g., cocultivation of kidney tissue) [32, 38] increase the likelihood of recovering infectious virus. On the other hand, RT-PCR is a common method used in diagnostics and surveillance for acute infections. However, as with acute infections, chronic infections in birds may result in the detection of WNV RNA.
The potential health effects of low levels of WNV in vertebrate tissues remain unknown. The observation of low levels of WNV or viral RNA in a tissue or swab sample may not provide an accurate diagnosis in a diseased bird. A bird with viral RNA in tissues or swabs may have died from a primary cause unrelated to persistent WNV infection. Such carcasses would not reflect current WNV transmission, an important consideration for surveillance [32]. In Colorado, low levels of infectious WNV were detected in avian carcasses of species not commonly believed to suffer WNV-associated morbidity or mortality, such as rock pigeons (Columba livia) and mourning doves (Zenaida macroura) [11, 23].
Overwintering of WNV continues to be an enigma. Survival of infected mosquitoes is one means, but persistently infected vertebrate hosts may also lead to viral maintenance, although the occurrence of the latter in nature has not been shown. Persistent WNV infection may lead to relapse and viral recrudescence in birds due to intrinsic factors within the bird (e.g., reproduction, molt, migration) or extrinsic factors in the environment (e.g., poor food or habitat availability, adverse environmental conditions). Recrudescence from persistent infection is likely associated with decay of humoral immunity below a certain protection threshold, allowing recurrence of viremia to titers infectious to vectors [29–31]. Whereas we found evidence for persistent infection in tissues through detection of RNA, we were unable to detect relapse of viremia; therefore, our data do not support the recrudescence theory. While antibodies to WNV are protective and long-lasting in some birds [25], their presence does not exclude the possibility of relapse of WNV infection within tissues [7, 33]. In addition, the potential for viral recrudescence following immunosuppression was studied by inoculating cyclophosphamide-treated house finches (Carpodacus mexicanus) with either WEEV or SLEV. Immunosuppression did not lead to relapses in infectious viremia [33]. Finally, persistent WNV in prey animals, including birds, may lead to oral transmission to predatory birds and other animals during times of interrupted mosquito activity [2, 10, 13, 14, 24]. Our data support the predation theory of overwintering through the detection of WNV–RNA-positive tissues beyond the acute phase of infection. Efforts should continue to characterize the occurrence of persistent viral infections in birds and other vertebrates and to understand the significance of this phenomenon in nature. Whether consumption of prey animals with persistent WNV RNA in tissues can lead to transmission and active infection in predators warrants additional investigation.
References
Anderson JF, Main AJ (2006) Importance of vertical and horizontal transmission of West Nile virus by Culex pipiens in the Northeastern United States. J Infect Dis 194:1577–1579
Austgen LE, Bowen R, Bunning ML, Davis BS, Mitchell CJ, Chang G-JJ (2004) Experimental infection of cats and dogs with West Nile virus. Emerg Infect Dis 10:82–86
Beaty BJ, Calisher CH, Shope RE (1995) Arboviruses. In: Lennette EH, Lennette DA, Lennette ET (eds) Diagnostic procedures for viral, rickettsial, and chlamydial infections, 7th edn. American Public Health Association, Washington, pp 189–212
Bolling BG, Moore CG, Anderson SL, Blair CD, Beaty BJ (2007) Entomological studies along the Colorado front range during a period of intense West Nile virus activity. J Am Mosq Control Assoc 23:37–46
Bunning ML, Bowen RA, Cropp CB, Sullivan KG, Davis BS, Komar N, Godsey MS, Baker D, Hettler DL, Holmes DA, Biggerstaff BJ, Mitchell CJ (2002) Experimental infection of horses with West Nile virus. Emerg Infect Dis 8:380–386
Dawson JR, Stone WB, Ebel GD, Young DS, Galinski DS, Pensabene JP, Franke MA, Eidson M, Kramer LD (2007) Crow deaths caused by West Nile virus during winter. Emerg Infect Dis 13:1912–1914
Diamond MS, Shrestha B, Mehlhop E, Sitati E, Engle M (2003) Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol 16:259–278
Farajollahi A, Crans WJ, Bryant P, Wolf B, Burkhalter KL, Godsey MS, Aspen SE, Nasci RS (2005) Detection of West Nile viral RNA from an overwintering pool of Culex pipiens pipiens (Diptera: Culicidae) in New Jersey, 2003. J Med Entomol 42:490–494
Fedorova TN, Stavskiy AV (1972) Latent infection of wild ducks with Omsk hemorrhagic fever and West Nile viruses. In: Chumakov MP (ed) Aktualnye problemy virusologii i profilaktiki virusnykh zabolevaniy. Institute of Poliomyelitis Virus and Encephalitis AMN SSSR, Moscow, p 226 (in Russian)
Garmendia AE, Van Kruiningen HJ, French RA, Anderson JF, Andreadis TG, Kumar A, West B (2000) Recovery and identification of West Nile virus from a hawk in winter. J Clin Microbiol 38:3110–3111
Gerhold RW, Tate CM, Gibbs SE, Mead DG, Allison AB, Fischer JR (2007) Necropsy findings and arbovirus surveillance in mourning doves from the southeastern United States. J Wildl Dis 43:129–135
Godsey MS, Blackmore MS, Panella NA, Burkhalter K, Gottfried K, Halsey LA, Rutledge R, Langevin SA, Gates R, Lamonte KM, Lambert A, Lanciotti RS, Blackmore CGM, Loyless T, Stark L, Oliveri R, Conti L, Komar N (2005) West Nile virus epizootiology in the southeastern United States, 2001. Vector Borne Zoonotic Dis 5:82–89
Klenk K, Snow J, Morgan K, Bowen R, Stephens M, Foster F, Gordy P, Beckett S, Komar N, Gubler D, Bunning M (2004) Alligators as West Nile virus amplifiers. Emerg Infect Dis 10:2150–2155
Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M (2003) Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis 9:311–322
Komar N, Panella NA, Burns JE, Dusza SW, Mascarenhas TM, Talbot TO (2001) Serologic evidence for West Nile virus infection in birds in the New York City vicinity during an outbreak in 1999. Emerg Infect Dis 7:621–625
Komar N, Panella NA, Langevin SA, Brault AC, Amador M, Edwards E, Owen JC (2005) Avian hosts for West Nile virus in St. Tammany Parish, Louisiana, 2002. Am J Trop Med Hyg 73:1031–1037
Kruszewicz AG (1995) The epizootic role of the house sparrow (Passer domesticus) and the tree sparrow (Passer montanus). Literature Review. In: Pinowski J, Kavanagh BP, Pinowska B (eds) Nestling mortality of granivorous birds due to microorganisms and toxic substances: Synthesis. Polish Scientific Publishers, Warsaw, pp 339–351
Kuno G (2001) Persistence of arboviruses and antiviral antibodies in vertebrate hosts: its occurrence and impacts. Rev Med Virol 11:165–190
Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT (2000) Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol 38:4066–4071
Lopes H, Redig P, Glaser A, Armien A, Wünschmann A (2007) Clinical findings, lesions, and viral antigen distribution in great gray owls (Strix nebulosa) and barred owls (Strix varia) with spontaneous West Nile virus infection. Avian Dis 51:140–145
Miura TA, Travanty EA, Oko L, Bielefeldt-Ohmann H, Weiss S, Beauchemin N, Holmes KV (2008) The spike glycoprotein of murine coronavirus MHV-JHM mediates receptor-independent infection and spread in the central nervous system of Ceacam1a −/− mice. J Virol 82:755–763
Nasci RS, Savage HM, White DJ, Miller JR, Cropp BC, Godsey MS, Kerst AJ, Bennett P, Gottfried K, Lanciotti RS (2001) West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg Infect Dis 7:1–3
Nemeth NM, Beckett S, Edwards E, Klenk K, Komar N (2007) Avian mortality surveillance for West Nile virus in Colorado. Am J Trop Med Hyg 76:431–437
Nemeth N, Gould D, Bowen R, Komar N (2006) Natural and experimental West Nile virus infection in five raptor species. J Wildl Dis 42:1–13
Nemeth NM, Kratz GE, Bates R, Scherpelz JA, Bowen RA, Komar N (2008) Naturally induced humoral immunity to West Nile virus infection in raptors. Ecohealth 5:298–304
Peiris JSM, Amerasinghe FP (1994) West Nile fever. In: Beran GW, Steele JH (eds) Handbook of zoonoses, section B. viral, 2nd edn. CRC Press, Boca Raton, pp 139–148
Phillips RA, Christensen K (2006) Field-caught Culex erythrothorax larvae found naturally infected with West Nile virus in Grand County, Utah. J Mosq Control Assoc 22:561–562
Pogodina VV, Frolova MP, Malenko GV, Fokina GI, Koreshkova GV, Kiseleva LL, Bochkova NG, Ralph NM (1983) Study on West Nile virus persistence in monkeys. Arch Virol 75:71–86
Reeves WC (1990) Overwintering of arboviruses. In: Reeves WC (ed) Epidemiology and control of mosquito-borne arboviruses in California, 1943–1987. California Mosquito and Vector Control Association, Sacramento, pp 357–382
Reisen WK, Chiles RE, Green EN, Fang Y, Mahmood F (2003) Previous infection protects house finches from re-infection with St. Louis encephalitis virus. J Med Entomol 40:300–305
Reisen WK, Chiles RE, Martinez V, Fang Y, Green E, Clark S (2004) Effect of dose on house finch infection with Western equine encephalomyelitis and St. Louis encephalitis viruses. J Med Entomol 41:978–981
Reisen WK, Fang Y, Lothrop HD, Martinez VM, Wilson J, O’Connor P, Carney R, Cahoon-Young B, Shafii M, Brault A (2006) Overwintering of West Nile virus in southern California. J Med Entomol 43:344–355
Reisen WK, Kramer LD, Chiles RE, Green E-GN, Martinez VM (2001) Encephalitis virus persistence in California birds: preliminary studies with house finches. J Med Entomol 38:393–399
Semenov BF, Chunikhin SP, Karmysheva VI, Iakovleva NI (1973) Study of chronic forms of arbovirus infections in birds. 1. Experiments with West Nile, Sindbis, Bhandja and Sicilian mosquito fever viruses. Vestn Akad Med Nauk SSSR 28:79–83 (in Russian)
Shrestha B, Diamond MS (2004) Role of CD8+ T cells in control of West Nile virus infection. J Virol 78:8312–8321
Taylor FM, Work TH, Hurlbut HS, Rizk F (1956) A study of the ecology of West Nile virus in Egypt. Am J Trop Med Hyg 5:579–620
Tesh RB, Parsons R, Siirin M, Randle Y, Sargent C, Guzman H, Wuithiranyagool T, Higgs S, Vanlandingham DL, Bala AA, Haas K, Zerinque B (2004) Year-round West Nile virus activity, Gulf Coast region, Texas and Louisiana. Emerg Infect Dis 10:1649–1652
Tesh RB, Siirin M, Guzman H, Travassos da Rosa APA, Wu X, Duan T, Lei H, Nunes MR, Xiao S-Y (2005) Persistent West Nile virus infection in the golden hamster: studies on its mechanism and possible implications for other flavivirus infections. J Infect Dis 192:287–295
Yaremych SA, Warner RE, Mankin PC, Brawn JD, Raim A, Novak R (2004) West Nile virus and high death rate in American crows. Emerg Infect Dis 10:709–711
Acknowledgments
We are grateful to P. Gordy, A. Bosco-Lauth, K. Jones, P. Oesterle, N. Roberts and others for logistical support. K. Burkhalter provided advice on RT-PCR and R. McLean provided editorial comments. This research was funded by National Institutes of Health contract N01-AI25489, Emerging Infectious Viral Disease Unit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nemeth, N., Young, G., Ndaluka, C. et al. Persistent West Nile virus infection in the house sparrow (Passer domesticus). Arch Virol 154, 783–789 (2009). https://doi.org/10.1007/s00705-009-0369-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-009-0369-x