Abstract
The nucleocapsid protein of the European genotype of porcine reproductive and respiratory syndrome virus (type 1, PRRSV-1) exhibited extensive size polymorphism (124–130 amino acids), correlating with phylogenetic grouping of ORF7 as well as ORF5 nucleotide sequences, thereby validating ORF7 size as an independent PRRSV-1 subtype marker. Based on new sequence information from the Russian Federation, we propose division of European genotype PRRSV-1 into 3 subtypes: a pan-European subtype 1 and East European subtypes 2 and 3, with nucleocapsid protein sizes of 128, 125 and 124 amino acids, respectively. The genetic differences between European genotype PRRSV subtypes affected diagnostic RT-PCR primer binding sites. Using Escherichia coli-expressed ORF7 protein, we confirmed that even the relatively closely related PRRSV subtypes 2 and 3 were antigenically different. Finally, the isoelectric point (pI) correlated with the nucleocapsid protein size for European genotype PRRSV subtypes, suggesting subtype-specific compensatory structural changes associated with subtype-specific ORF7 sizes. Thus, the new ORF7-based subtype division of PRRSV-1 proposed here is biologically meaningful and practically relevant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Porcine reproductive and respiratory syndrome virus (PRRSV) was recognized as a clinical entity in North America and Europe by the end of the 1980s, but later studies have suggested the existence of the virus in the pig population since 1979 [3]. The PRRSV that emerged in North America had limited genetic relationship to the PRRSV that emerged in Europe. Thus, PRRSV is recognized as consisting of 2 genotypes: European (EU genotype, type 1, PRRSV-1) and North American (NA genotype, type 2, PRRSV-2) [20]. The two PRRSV genotypes produce the same disease symptoms but are antigenically very different, and there is poor protection against cross-infection in pigs [27, 28].
To explain the near-cotemporal emergence of different PRRSV genotypes on two continents, a mechanism involving global triggering factors has been suggested, such as rapid global spread of related porcine pathogens acting as helper viruses [22]. Yet, the reason for the virtually simultaneous emergence of widely differing PRRSV genotypes on two continents remains a puzzle. Also, because the diversity of PRRSV-1 significantly exceeds that of PRRSV-2 [8, 22], it is possible that PRRSV evolution history or emergence mechanisms differed between Europe and North America [22].
Adding to the emergence puzzle, recent work has shown that East European countries appear to harbour European genotype viruses of exceptional diversity [21, 22]. Intriguingly, a sharp demarcation exists between West and East European variants of PRRSV-1 at the eastern border of Poland [22]. As the spread of PRRSV is predominantly associated with movement of infected pigs or semen, the striking geographical diversity pattern of PRRSV-1 in Europe was suggested to be due to the unique trade patterns during the cold war period [22].
Because ancestral virus populations would be expected to exhibit the highest diversity, comparing the diversity of PRRSV-1 between Eastern and Western Europe may provide clues to the emergence of PRRSV. Also, not only is there limited cross-protection between the genotypes of PRRSV [27, 28], but diversity within PRRSV-1 as well as within PRRSV-2 has consistently been shown to impact vaccine efficacy [12, 14]. Additionally, it is presently unknown how the very high level of sequence diversity within PRRSV-1 affects diagnostic, serological and RT-PCR assays [26]. As a complicating factor in addressing the issues above, and in contrast to PRRSV-2, the diversity of PRRSV-1 appears sufficiently high to warrant definition of subtypes [22]. However, too few sequences were available from Eastern Europe for subtype definition criteria to be proposed [21, 22]. Therefore, we have, in the present study, examined PRRSV-1 sequence diversity in European and Asian regions of the Russian Federation. Based on the new data, combined with all previously reported sequences of PRRSV-1, we propose criteria for division of PRRSV-1 in three main subtypes.
Materials and methods
Farm and sampling information
Samples were obtained from 37 farms in the Russian Federation, from 1996 to 2006 (Supplementary Table S1, available online). From 10 farms, repeated sampling was done at different time points. Samples consisted of serum or lung tissue from aborted foetuses or clinically ill growing pigs. The 37 farms represented 27 administrative units from 10 economic regions of the Russian Federation where pig production is concentrated (Fig. 1). The farms were large, with reproductive herds of 15,000–120,000 sows, and the majority of the farms experienced respiratory problems in weaned pigs at the time of sampling. All farms used replacement stock of Russian origin. The ND and BT farms also used Estonian stock, and the ZV farm also used Danish and Canadian replacement stock. The KR, BLG and NV farms also used Belarusian, English and Polish replacement stock, respectively.
Location of the Russian farms from which samples were obtained. Top panel, Russian Federation outlined in bold. Bottom panel, detail of Russian Federation west of Ural Mountains. Economic regions of the Russian Federation: Northern (N), North Western (NW), Kaliningrad (K), Central (C), Volga-Viatka (V-V), Urals (U), Central Black Earth (CBE), Volga (V), Northern Caucasus (NC), West Siberian (WS) and Far Eastern (FE). Farm locations east and west of the Urals are indicated in the top and bottom panels, respectively. PRRSV-1 subtype 1 is marked “*”, subtype 2 “+”, subtype 3 “#”, ungrouped “x”. Sequence numbers correspond to Supplementary Table S1, available online. West of the Polish eastern border (top panel: PL, Poland), only subtype 1 of PRRSV-1 has so far been reported
With the exception of the BLG farm, where Porcilis PRRS (Intervet) vaccine was used, all farms used Russian modified live as well as inactivated vaccines produced by the Federal Centre for Animal Health (Vladimir) and by NARVAC (Moscow). The sequences reported in the present work do not represent vaccine viruses (A. V. Scherbakov, personal communication). Additionally, samples from 5 Belarusian farms (1999–2001) were obtained.
RT-PCR and sequencing
Total RNA was extracted directly from clinical samples, and nested RT-PCR was used to amplify complete the ORF5 and ORF7. The following primers were used for ORF7 single-tube RT-PCR: First round, ORF7For 5′ TACATTCTGGCCCCTGCCCA 3′ (14,405–14,424) and ORF7Rev 5′ TTAATTTCGGTCRCATGGTTC 3′ (15,080–15,100). Second round, ORF7NFor 5′ GCTGTTAAACGRGGAGTGGT 3′ (14,564–14,583) and ORF7NRev 5′ TCGCCCTAATTGAATAGGTGACT 3′ (15,028–15,050).
The following primers were used for ORF5 single-tube RT-PCR: First round, ORF5For 5′ CCGTCTGTGATGAGRTGGGC 3′ (13,435–13,454) and ORF5Rev 5′ GGAYACTTTTAGGGCRTATATCAT 3′ (14,171–14,194). Second round, ORF5NFor 5′ TGGGCYACAACCATTGCTTG 3′ (13,450–13,469) and ORF5NRev 5′ CACAGGTGTATATGTKATGCTAAA 3′ (14,147–14,170). Numbers in parentheses refer to the Lelystad virus sequence (GenBank M96262).
The ORF7 primer-binding sites are completely conserved between the EU genotype and NA genotype prototype PRRSV sequences (Lelystad, M96262, and VR2332, AY150564).
Purified, non-cloned PCR amplicons were cycle sequenced on both strands, using the nested PCR primers and a radioactivity-based method (CycleReader DNA Sequencing Kit, Fermentas).
Phylogenetic analysis and pI calculation
The obtained sequences were compared to a reference set selected from GenBank to represent the full range of genetic diversity and geographic locations of PRRSV-1, comprising sequences from Europe, Asia and North America (Supplementary Table S1, available online). The PRRSV-2 prototype VR2332 was used as out-group in phylogenetic analysis. Sequence alignment and neighbor-joining-based phylogenetic tree construction were done using Clustal W and CLC Gene Free Workbench software, with bootstrap values based on 1,000 analysis replicates (v4.0.1, CLC bio A/S, Aarhus, Denmark).
The isoelectric point (pI) was calculated for deduced ORF7 (nucleocapsid) protein sequences using EditSeq (Lasergene, DNAStar) software.
ORF7 (nucleocapsid) protein expression and Western blotting
ORF7 RT-PCR products were cloned in pET 14b (Novagen), and N-terminally His-tagged ORF7 protein was expressed in E. coli, essentially as described [5]. Inclusion bodies were purified using BugBuster reagent, essentially according to the manufacturer’s recommendations (Novagen) [24]. Inclusion body protein was solubilised in 8 M urea, 10 mM 2-mercaptoethanol, 100 mM Tris, pH 8. Protein content was normalized based on SDS-PAGE and Coomassie staining. Fully denaturing and reducing Western blotting of ORF7 protein with sera from naturally infected swine was done using the NuPAGE reagent suite (Invitrogen), and detection was with HRP-conjugated secondary antibody and ECLAdvance chemiluminescent substrate (GE Healthcare), using a CCD camera for membrane imaging (LAS3000, FujiFilm).
Results
PRRSV-1 diversity in the Russian Federation exceeds the diversity in Western Europe
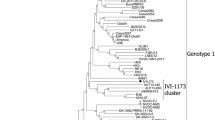
This study provided 22 new sequences of ORF5 and 57 new sequences of ORF7 of PRRSV strains from Russia and Belarus from 1996 to 2006 (Fig. 1). All of the obtained ORF5 and ORF7 sequences were clearly of European genotype, i.e. PRRSV-1 (Supplementary Table S1, available online). In an ORF7 phylogeny, most of the new Russian sequences exhibited clustering essentially conforming to the previously suggested subtypes 1, 2 and 3 of PRRSV-1 (Fig. 2a) [22]. The Russian sequences were located in all three subtypes: 24 sequences from 18 farms in subtype 1, 24 sequences from 19 farms in subtype 2 and 1 sequence in subtype 3. Interestingly, while reference PRRSV-1 sequences from Western and Central Europe, Asia and North America formed a tight group within subtype 1 (Fig. 2a, dotted circle within subtype 1), the Russian subtype 1 sequences were more diverse (Fig. 2a, Russian subtype 1 sequences are all outside the dotted circle). Finally, three Russian sequences from the VL farm formed an independent cluster between subtypes 2 and 3 (Fig. 2a, 85–87). These sequences had an ORF7 size of 387 nt and a 3 nt insertion similar to subtype 2 sequences (Fig. 3, compare VL-3 and VR sequences). However, in the VL sequences, the 3-nt insertion was located at residues 35–37, whereas it was located at residues 141–143 in the subtype 2 sequences (Fig. 3).
Phylogenetic trees of new Russian and reference PRRSV-1 sequences. a ORF7 tree, b ORF5 tree. Solid circles indicate PRRSV-1 subtypes 1, 2 and 3. The dotted circle indicates subtype 1 PRRSV-1 sequences previously reported from Central and Western Europe. PRRSV-1 subtype 1 sequences outside the dotted circle are all from Russia. For comparison to the shown PRRSV-1 (European genotype) sequences, the most diverse PRRSV-2 (American genotype) ORF7 sequences currently available exhibit only 11% nucleotide divergence, which corresponds to the diversity within PRRSV-1 subtype 3. PRRSV-1 subtypes 2 and 3 have only been reported from Eastern Europe. Likewise, all the ungrouped sequences are from Eastern Europe. Branch numbers correspond to Supplementary Table S1, available online
PRRSV-1 ORF7 sequence diversity affects binding sites for diagnostic RT-PCR primers. The alignment shows selected ORF7 sequences of maximally diverse PRRSV-1 strains. Boxes correspond to two forward (right arrows) and one reverse (left arrow) diagnostic PCR primer positions [6]
In the PRRSV-1 ORF7 phylogeny, there was concordance between the size of ORF7, and the phylogenetic grouping (Fig. 2a and Supplementary Table S1, available online). Thus, subtype 1 contained viruses with ORF7 size of 387 nt, subtype 2 contained viruses with ORF7 size of 378 nt, and subtype 3 contained viruses with ORF7 size of 375 nt (Fig. 2a). Only 4 of 57 (7%) sequences were ungrouped (Fig. 2a). To evaluate whether ORF7 size was a marker for PRRSV-1 subtypes (as opposed to the ORF7 phylogeny being confounded by different sequence length), we compared ORF5 and ORF7 phylogenies (Fig. 2a, b). The bootstrap support was lower for ORF5 than for ORF7 trees, as might be expected due to the lower conservation of ORF5. Also, more sequences exhibited intermediate grouping in the ORF5 than in the ORF7 phylogeny (Fig. 2b; for example, sequences 56, 44, 54, 71, 89, 90, 92, 43, 61, 93, 45). This has previously been observed for PRRSV-1, and was in some cases shown to be due to recombination [8]. Nevertheless, the grouping seen in ORF7 phylogeny was essentially recapitulated in the ORF5 phylogeny (Fig. 2a, b).
Evolution of PRRSV-1 strains on farms over time
On 13 farms, repeated sampling was done over time (Supplementary Table S1, available online). In the majority of farms, very closely related viruses from the same PRRSV-1 subtype were found over time (up to 11 years in the case of the NV farm, Supplementary Table S1, available online, sequences 67–69). Thus, the great diversity of PRRSV-1 (Fig. 2a) was likely founded in the pre-emergence diversity of the ancestral virus population, as opposed to representing very rapid post-emergence evolution [9].
On one Belarusian and two Russian farms, evidence for co-circulation as well as succession of different PRRSV-1 subtypes was found (Supplementary Table S1, available online, Soz, KH and TM farms, sequences 13–15, 52–54 and 79–80, respectively). This indicated that cross-protection between PRRSV-1 subtypes may be limited.
Correlation between PRRSV-1 subtype and geographic location
Only very limited sequence information was obtained from Siberian and Far Eastern Russian regions in this study, but of 7 sequences from east of the Urals, one was subtype 1 and six were subtype 2 (Fig. 1a, numbers 51, 52–54 and 67–69). Intriguingly, subtype 3 was only found in Belarus, with the exception of a single sequence, which originated from Kursk in the Central Black Earth region of Russia (Fig. 1b, number 57).
Implications of genetic diversity for RT-PCR diagnostics
The ORF7 of PRRSV-1 has been assumed to be well conserved and is consequently the target of many diagnostic RT-PCR assays [6, 26]. In a selection of the most diverse PRRSV-1 ORF7 sequences currently available, mutations were frequently observed in diagnostic RT-PCR primer sites, frequently affecting 3′ end and central primer regions thought relevant for primer performance (Fig. 3). Up to 4 nucleotide mutations were frequently observed in RT-PCR primer-binding sites of 21–22 nt length (Fig. 3).
Antigenic differences between PRRSV-1 subtypes 2 and 3
For ORF7 protein expression, we selected the Vas and Zap farms, representing subtypes 2 and 3 of PRRSV-1 (Supplementary Table S1, available online, numbers 16 and 22). ORF7 protein was expressed at high level in E. coli, and was easily purified in inclusion body form by simple detergent extraction (data not shown) [24]. In preliminary experiments, sera from naturally infected pigs from the Vas and Zap farms were examined for reactivity preference towards recombinant Vas and Zap ORF7 proteins by fully denaturing and reducing Western blotting. Eight of the 10 sera from the Vas farm reacted more strongly with Vas than with Zap antigen. Six of the 9 sera from the Zap farm reacted more strongly with Zap than with Vas antigen (not shown). Generally, sera reacted between 2 and 4-fold stronger with the cognate antigen (Fig. 4 and not shown). Thus, the natural sequence variability of PRRSV-1 ORF7 protein was associated with antigenic differences in naturally infected pigs. Interestingly, sera from pigs on the Zap farm had higher cross-reactivity against Vas ORF7 protein than did Vas sera against Zap antigen (Fig. 4 and not shown). Such asymmetrical cross-reactivity patterns have previously been described for PRRSV-1 [17]. Interestingly, of the 11 genetic differences between Vas and Zap ORF7 protein (10 amino acid changes and 1 insertion/deletion, Fig. 4), only 2 mapped to epitope sites defined by murine monoclonal antibodies (Fig. 4). This suggested that porcine and murine B-cells differ in epitope recognition in the ORF7 protein, as previously observed for the ORF4 protein [17].
Subtype-specificity of antibody responses in pigs naturally infected with PRRSV-1 subtype 2 and subtype 3 viruses. ORF7 protein from PRRSV-1 subtypes 2 and 3 was expressed in E. coli and used for Western blot analysis with sera from naturally infected pigs. The alignment shows deduced ORF7 protein sequence (see Supplementary Table S1, available online, number 16 and 22 for GenBank accession numbers of the Vas and Zap sequences). Dot identity to top (Vas). Dash insertion/deletion. The E. coli-expressed proteins have N-terminal His tags, not shown in the alignment. Boxes labelled A-D correspond to antigenic sites identified by murine monoclonal antibody mapping [15]. A Western blot was done with graded ORF7 protein levels from 1 (highest antigen level loaded in lane) to 1/8 (8-fold lower antigen level loaded in lane), as shown in the figure. The sera were from naturally infected piglets, 2–3 months of age, and were ORF7 RT-PCR positive. Probing with HisTag antibody blot confirmed equal loading of antigen (bottom panel). Serum from PRRSV-free pigs was negative in the Western blot assay (not shown)
Discussion
In previous papers examining the molecular phylogeny of the European genotype of PRRSV, i.e. PRRSV-1, we have continually had to increase our estimate of the diversity of this PRRSV genotype in European countries [7, 8, 16, 21, 22]. Recently, we suggested that the diversity of PRRSV-1 was sufficient to warrant subdivision into at least 3 subtypes [22]. However, the new subtypes 2 and 3 were defined by sequences from only 6 and 7 farms, respectively, in Belarus and Lithuania [21, 22]. Also, the established subtype 1 was exclusively defined by West European sequences [21, 22].
The new PRRSV-1 ORF7 sequences from the Russian Federation grouped in subtype 1 (24 sequences, 18 farms), subtype 2 (24 sequences, 19 farms) and subtype 3 (1 sequence). Thus, the new Russian sequences supported the previously proposed subtypes 1, 2 and 3 of PRRSV-1 (Fig. 2a).
Intriguingly, in previous studies as well as this one, we observed PRRSV-1 sequences that did not group in subtypes 1, 2 or 3. Without exception, such highly diverse sequences were from Eastern Europe—in this case, two Belarusian farms and one Russian farm (Fig. 2a, 7, 10, 85, 86, 87). Thus, we continue to consider it a very worthwhile undertaking to sample PRRSV diversity east of Poland to establish whether additional subtypes of PRRSV-1 may exist.
Following subtype definition, a logical next step is to estimate the subtype prevalence. This is not trivial, as the diversity of PRRSV-1 is sufficiently large to affect binding sites for diagnostic RT-PCR primers (Fig 3). The binding sites for our ORF7 RT-PCR primers are completely conserved between the European and American genotypes of PRRSV, i.e. PRRSV-1 and PRRSV-2 (see “Materials and methods”). While we have not examined the sensitivity of our ORF7 PCR primers against different PRRSV-1 subtypes, we believe studies with conserved ORF7 primers provide the best data set for subtype prevalence estimation [8, 21, 22]. Thus, based on combined data from this work and our previous studies, and without regard to geographical location and geographical sampling bias, PRRSV-1 subtype 1 is a majority subtype (78% of total 200 sequences), while 2 and 3 are minority subtypes (16.5 and 11.5% of total 200 sequences) (T. Stadejek, personal communication).
Geographical location greatly impacts the prevalence of PRRSV-1 subtypes. PRRSV-1 subtypes 2 and 3 have only been reported from Eastern Europe [21, 22], and PRRSV-1 subtype 1 accounts for all previously reported PRRSV-1 sequences from Western Europe [1, 8, 13, 21, 22, 25]. Interestingly, we found that subtype 1 is also frequent in Eastern Europe and Russia (Fig. 1). This raises the question of how subtype 1 has spread in Europe. On one hand, the lack of penetration of subtypes 2 and 3 into Western Europe (Fig. 1) mirrors the predominant historical trade patterns in breeding animals and semen, supporting that subtype 1 spread from Western to Eastern Europe. On the other hand, subtype 1 appears to exhibit a higher diversity in Russia than in Western Europe (Fig. 2a, West European subtype 1 sequences indicated by a dotted circle), supporting an East European origin also of subtype 1 [23]. Further sequence studies in Western and Eastern Europe with RT-PCR primers known to detect all subtypes may resolve this issue.
The new sequences from pigs from the Russian Federation made it possible to more confidently describe the defining characteristics of PRRSV-1 subtypes 1, 2 and 3. The nucleocapsid-encoding ORF7 is ideal for PRRSV-1 subtype definition. First, ORF7 is used by most laboratories for RT-PCR and serological diagnostics. Second, ORF7 of PRRSV-1 exhibits very striking length polymorphism, which correlates with phylogenetic grouping, and therefore appears to be an independent subtype marker (Supplementary Table S1, available online; Table 1; Fig. 2). However, for definition of subtypes to be practically relevant, subtypes should differ in aspects of, for example, virus biology, disease control, or diagnostics. As mentioned above, PRRSV-1 subtypes 1, 2 and 3 exhibit strikingly different geographical distributions in Europe, likely laid down by historical cold-war trade patterns (Fig. 1). The observed lack of penetration of subtypes 2 and 3 into Western Europe (Fig. 1) [21, 22], and stability of subtypes on farms over time (Supplementary Table S1, available online), suggests a temporal stability of the ORF7 subtype definition, which seems very attractive for practical purposes. As regards disease control, even within subtype 1, there is limited cross-protection against reinfection [12], and accordingly, we in some cases observed co-circulation or succession of different PRRSV-1 subtypes on farms over time (Supplementary Table S1, available online, Soz, KH and TM farms, sequences 13–15, 52–54 and 79–80). As regards diagnostics, the PRRSV-1 subtype shows differences in target-binding sites for diagnostic RT-PCR primers (Fig. 3). Finally, even between the relatively closely related subtypes 2 and 3, sera from naturally infected pigs exhibit a subtype preference (Fig. 4). Thus, ORF7-based subtype division of PRRSV-1 is not only possible (Fig. 2a; Table 1) but appears biologically meaningful.
We have been able to find only one example of viral nucleocapsid protein plasticity comparable to PRRSV-1 [18, 19]. In contrast, the length of the PRRSV-2 nucleocapsid protein is essentially invariable (Table 1). There are only three examples of PRRSV-2 ORF7 size polymorphisms, representing less than 1% of the total of approximately 235 PRRSV-2 ORF7 sequences in GenBank: A Canadian strain exhibiting an extra codon at positions 127–129 [10], a Mexican strain having a codon insertion at positions 40–42 [2], and a Chinese strain exhibiting a codon deletion at positions 314–316 (EF552431). However, in contrast to PRRSV-1, the PRRSV-2 ORF7 length variants were not phylogenetically informative.
Influenza HA tags added to the N or C terminus of the PRRSV-1 ORF7 protein were not stable in the context of an infectious clone of Lelystad virus [11]. Thus, the length variability in ORF7 amongst PRRSV-1 subtypes (Fig. 4; Table 1) would be expected to be coupled to compensatory changes in ORF7 and possibly also at other sites in the PRRSV genome. Encapsidation of the viral genome is one key nucleocapsid protein function, and this involves interactions between positive charges in the nucleocapsid and negative charges in the RNA genome [4]. The C terminus of the nucleocapsid protein accounts for the majority of the length plasticity but has no strongly charged residues and is thought to be important for nucleocapsid protein dimer and oligomer formation [4]. In contrast, the N terminus of the nucleocapsid protein contains positively charged residues involved in RNA interactions [4] but is minimally affected by insertions/deletions across PRRSV-1 subtypes. Thus, we reasoned that charge characteristics might shed light on whether ORF7 length plasticity was associated with compensatory functional changes. In fact, the predicted isoelectric point correlated with nucleocapsid protein size for PRRSV-1 subtypes 1, 2 and 3 (Table 1; Fig. 5a), suggesting that ORF7 length plasticity was indeed coupled with compensatory changes in structural nucleocapsid characteristics. Interestingly, subtype 3, while being the closest to PRRSV-2 ORF7 protein length (124 vs. 123 amino acids), differed the most from PRRSV-2 in pI value (Table 1; Fig. 5a). To explore this further, we included other arteriviruses in the pI/size analysis (Fig. 5b). Interestingly, all 7 arteriviral data points could be connected by 2 near-parallel lines with high coefficients of correlation, and PRRSV-1 and PRRSV-2 were on separate lines (Fig. 5b). Thus, it appears that the arteriviral group of greatly differing nucleocapsid sizes may represent two independent evolutionary trajectories with the same underlying mechanisms governing size/charge constraints (Fig. 5b).
Correlation between PRRSV-1 nucleocapsid protein length and putative compensatory functional changes. a The ORF7 protein length and isoelectric point data from Table 1 were plotted. Bars indicate 1 SD. b For comparison to PRRSV-1 and PRRSV-2, the nucleocapsid proteins of the arteriviruses lactate dehydrogenase elevating virus (LDV, one sequence), equine arteritis virus (EAV, 14 sequences), and simian hemorrhagic fever virus (SHFV, one sequences) were used (GenBank accession numbers available on request). LDV, EAV and SHFV have nucleocapsid proteins of 115, 110 and 111 amino acids, with pI of 10.62, 11.83 and 11.11, respectively
References
Balka G, Hornyak A, Balint A, Kiss I, Kecskemeti S, Bakonyi T, Rusvai M (2008) Genetic diversity of porcine reproductive and respiratory syndrome virus strains circulating in Hungarian swine herds. Vet Microbiol 127:128–135
Batista L, Pijoan C, Lwamba H, Johnson CR, Murtaugh MP (2004) Genetic diversity and possible avenues of dissemination of porcine reproductive and respiratory syndrome virus in two geographic regions of Mexico. J Swine Health Prod 12:170–175
Carman S, Sanford SE, Dea S (1995) Assessment of seropositivity to porcine reproductive and respiratory syndrome (PRRS) virus in swine herds in Ontario—1978 to 1982. Can Vet J 36:776–777
Doan DN, Dokland T (2003) Structure of the nucleocapsid protein of porcine reproductive and respiratory syndrome virus. Structure 11:1445–1451
Drew TW (1996) Studies on the genome and proteins of porcine reproductive and respiratory syndrome virus. PhD Thesis, The Open University, London
Fetzer C, Pesch S, Ohlinger VF (2006) High risk of false positive results in a widely used diagnostic test for detection of the porcine reproductive and respiratory syndrome virus (PRRSV). Vet Microbiol 115:21–31
Forsberg R, Oleksiewicz MB, Petersen AM, Hein J, Bøtner A, Storgaard T (2001) A molecular clock dates the common ancestor of European-type porcine reproductive and respiratory syndrome virus at more than 10 years before the emergence of disease. Virology 289:174–179
Forsberg R, Storgaard T, Nielsen HS, Oleksiewicz MB, Cordioli P, Sala G, Hein J, Bøtner A (2002) The genetic diversity of European type PRRSV is similar to that of the North American type but is geographically skewed within Europe. Virology 299:38–47
Forsberg R (2005) Divergence time of porcine reproductive and respiratory syndrome virus subtypes. Mol Biol Evol 22:2131–2134
Gagnon CA, Dea S (1998) Differentiation between porcine reproductive and respiratory syndrome virus isolates by restriction fragment length polymorphism of their ORFs 6 and 7 genes. Can J Vet Res 62:110–116
Groot Bramel-Verheije MH, Rottier PJ, Meulenberg JJ (2000) Expression of a foreign epitope by porcine reproductive and respiratory syndrome virus. Virology 278:380–389
Labarque G, Van Gucht S, Van Reeth K, Nauwynck H, Pensaert M (2003) Respiratory tract protection upon challenge of pigs vaccinated with attenuated porcine reproductive and respiratory syndrome virus vaccines. Vet Microbiol 95:187–197
LeGall A, Legeay O, Bourhy H, Arnauld C, Albina E, Jestin A (1998) Molecular variation in the nucleoprotein gene (ORF7) of the porcine reproductive and respiratory syndrome virus (PRRSV). Virus Res 54:9–21
Mengeling WL, Lager KM, Vorwald AC, Koehler KJ (2003) Strain specificity of the immune response of pigs following vaccination with various strains of porcine reproductive and respiratory syndrome virus. Vet Microbiol 93:13–24
Meulenberg JJM, van Nieuwstadt AP, vanEssenZandbergen A, BosdeRuijter JNA, Langeveld JPM, Meloen RH (1998) Localization and fine mapping of antigenic sites on the nucleocapsid protein N of porcine reproductive and respiratory syndrome virus with monoclonal antibodies. Virology 252:106–114
Oleksiewicz MB, Bøtner A, Toft P, Grubbe T, Nielsen J, Kamstrup S, Storgaard T (2000) Emergence of porcine reproductive and respiratory syndrome virus deletion mutants: correlation with the porcine antibody response to a hypervariable site in the orf 3 structural glycoprotein. Virology 267:135–140
Oleksiewicz MB, Bøtner A, Toft P, Normann P, Storgaard T (2001) Epitope mapping porcine reproductive and respiratory syndrome virus by phage display: the nsp2 fragment of the replicase polyprotein contains a cluster of B-cell epitopes. J Virol 75:3277–3290
Pappu SS, Pappu HR, Lastra R, Niblett CL (1994) Variability in the length of the amino terminal sequence contributes to the capsid protein diversity among dasheen mosaic potyvirus isolates. Arch Virol 136:407–413
Pappu SS, Pappu HR, Rybicki EP, Niblett CL (1994) Unusual amino-terminal sequence repeat characterizes the capsid protein of dasheen mosaic potyvirus. J Gen Virol 75(Pt 1):239–242
Snijder EJ, Brinton MA, Faaberg K, Godeny EK, Gorbalenya AE, MacLachlan NJ, Mengeling WL, Plagemann GW (2005) Family Arteriviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (eds) Virus taxonomy: 8th report of the International Committee on Taxonomy of Viruses, Elsevier/Academic Press
Stadejek T, Stankevicius A, Storgaard T, Oleksiewicz MB, Belak S, Drew TW, Pejsak Z (2002) Identification of radically different variants of porcine reproductive and respiratory syndrome virus in Eastern Europe: towards a common ancestor for European and American viruses. J Gen Virol 83:1861–1873
Stadejek T, Oleksiewicz MB, Potapchuk D, Podgorska K (2006) Porcine reproductive and respiratory syndrome virus strains of exceptional diversity in eastern Europe support the definition of new genetic subtypes. J Gen Virol 87:1835–1841
Stadejek T, Oleksiewicz M, Stankevicius A, Potapchuk D, Scherbakov A (2007) Molecular epidemiology of EU-genotype PRRSV in Europe: clues to PRRSV emergence, and implications for disease control. In: 5th international symposium on emerging and re-emerging pig diseases. Krakow, Poland, pp 135–136
Stadejek T, Oleksiewicz MB (2007) Indirect ELISA with recombinant nucleocapsid protein for genotype-specific PRRSV serology: a simple and cost-effective antigen purification scheme. In: 5th international symposium on emerging and re-emerging pig diseases. Krakow, Poland, p 189
Suarez P, Zardoya R, Martin MJ, Prieto C, Dopazo J, Solana A, Castro JM (1996) Phylogenetic relationships of European strains of porcine reproductive and respiratory syndrome virus (PRRSV) inferred from DNA sequences of putative ORF–5 and ORF–7 genes. Virus Res 42:159–165
Truyen U, Wilhelm S, Genzow M, Schagemann G (2006) Porcine reproductive and respiratory syndrome virus (PRRSV): a ring test performed in Germany to assess RT-PCR detection methods. J Vet Med B 53:68–74
van Woensel PA, Liefkens K, Demaret S (1998) European serotype PRRSV vaccine protects against European serotype challenge whereas an American serotype vaccine does not. Adv Exp Med Biol 440:713–718
van Woensel PAM, Liefkens K, Demaret S (1998) Effect on viraemia of an American and a European serotype PRRSV vaccine after challenge with European wild-type strains of the virus. Vet Rec 142:510–512
Author information
Authors and Affiliations
Corresponding author
Additional information
T. Stadejek, M. B. Oleksiewicz and A. V. Scherbakov have contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Stadejek, T., Oleksiewicz, M.B., Scherbakov, A.V. et al. Definition of subtypes in the European genotype of porcine reproductive and respiratory syndrome virus: nucleocapsid characteristics and geographical distribution in Europe. Arch Virol 153, 1479–1488 (2008). https://doi.org/10.1007/s00705-008-0146-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-008-0146-2