Abstract
To delineate the impact of non-motor markers (REM sleep behavior disorder (RBD), orthostatic hypotension (OH), cardiac sympathetic denervation, hyposmia) on neuronal injury in early-stage Parkinson’s disease (PD), we measured the plasma neurofilament light chain (NFL) level of PD patients and evaluated its relationship with these markers. The study population comprised a cohort of 77 patients with PD and 54 controls. OH was assessed using 5-min head-up tilt-table test. Other clinical parameters such as RBD, Unified Parkinson’s Disease Rating Scale (UPDRS), cognition, Cross‐Cultural Smell Identification Test (CCSIT), white matter hyperintensity (WMH), cardiac metaiodobenzylguanidine (MIBG) and striatal dopamine transporter (DAT) uptake were assessed. Plasma NFL levels were measured using Simoa platform. During mean 24.8 months of follow-up, 70 patients remained PD, 5 patients converted to Parkinson-plus syndrome (P + converter), and 2 were lost to follow-up. NFL level did not differ between PD and control groups (age-adjusted means 10.40 pg/mL vs 9.51 pg/mL, p = 0.151), but PD patients with OH (median 15.31 pg/mL) had higher levels compared with those without OH (median 9.2 pg/mL, p = 0.008), as well as the control group (median 9.7 pg/mL, p = 0.002). P + converter group had the highest plasma NFL level (38.17 pg/mL, p < 0.001). In a multiple regression analysis, OH, age, and disease duration independently correlated with plasma NFL level. This finding adds biomarker-based evidence for poor clinical outcomes associated with OH in patients with PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The neurofilament light chain (NFL) is gaining increasing attention as a disease progression biomarker in Parkinson’s disease (PD). NFL, a protein subunit of the neurofilaments that constitute the neuronal cytoskeleton, is abundant in large myelinated axons and is released into the interstitial space upon axonal injury or degeneration (Parnetti et al. 2019). Recent studies have shown that serum or cerebrospinal fluid (CSF) levels of NFL are correlated with cognitive decline and motor severity in patients with PD, confirming the potential of NFL as a marker of disease progression (Backstrom et al. 2020; Lin et al. 2019; Mollenhauer et al. 2020).
PD is a neurodegenerative disorder with heterogeneous phenotypes. Many studies have reported poor clinical outcomes associated with the non-motor markers, such as orthostatic hypotension (OH), REM sleep behavior disorder (RBD), and lower cardiac MIBG uptake. OH strongly correlated with reduced survival and increased risk of dementia (De Pablo-Fernandez et al. 2017; Goldstein et al. 2015; Pilotto et al. 2019); RBD increased the risk of dementia, synergistically acting with OH (De Pablo-Fernandez et al. 2019; Fereshtehnejad et al. 2017); lower cardiac MIBG uptake correlated with the prevalence and severity of non-motor symptoms, such as RBD, cognitive impairment, and OH (Kim et al. 2017).
In this study, using plasma NFL as a disease progression marker, we aim to investigate its correlation with these non-motor markers, while controlling for age, motor severity, cognitive function, white matter hyperintensities, and striatal dopamine transporter uptake.
Methods
Study participants
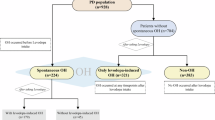
The study population comprised a cohort of 77 patients with PD and 54 control subjects, from the Parkinson Center at Ajou University Hospital, a tertiary referral center in Suwon, South Korea. The subjects were enrolled between October 2018 and October 2019. The diagnosis of PD was based on the clinical diagnostic criteria of the UK Parkinson’s Disease Society Brain Bank (Hughes et al. 1992). All patients were examined by movement disorder specialists and were excluded if they showed signs of atypical parkinsonian syndromes. Only patients aged 40–85 years and with disease duration ≤ 3 years were enrolled. All patients were scheduled for regular follow-up in the outpatient clinic every 2–3 months for at least 1 year, and those lost to follow-up were excluded in the analyses.
The control group consisted of the spouses of patients or other patients with minor neurological conditions such as headache, dizziness, or non-parkinsonian tremors. All the participants in the control group were examined by movement disorder specialists (DGP and JHY) for any signs of parkinsonism, and went through screening for OH, RBD, and hyposmia.
For both groups, we excluded those who had either abnormal focal neurological signs (other than tremor and parkinsonism) or cognitive dysfunction (Mini-Mental State Exam [MMSE] score less than 24). We also excluded those with medical conditions including diabetes mellitus, renal insufficiency, cancer, and symptomatic vascular disease.
Standard protocol approvals, registrations, and patient consent
Written informed consent was obtained from all participants, and the study was approved by the Institutional Review Board of Ajou University Hospital.
Assessment of OH
All subjects in the study underwent a head-up tilt-table test. After resting in the supine position for at least 15 min, systolic and diastolic blood pressure (BP) and heart rate were measured at baseline and then repeatedly measured at 1, 3, and 5 min while maintaining a 60° head-up tilt position. According to the consensus statement, OH was defined as a sustained reduction in systolic BP of ≥ 20 mmHg or in diastolic BP of ≥ 10 mmHg within 3 min of standing (Freeman et al. 2011). In the case of BP drop after 3 min of standing, subjects were designated as having delayed OH and marked separately. Those with OH severe enough to satisfy the autonomic failure criteria for MSA in the initial tests were excluded from the study.
Assessment of probable RBD
The presence of RBD was assessed using a RBD screening questionnaire (RBDSQ) (Stiasny-Kolster et al. 2007), and those with a score of 5 or higher were classified as probable RBD (pRBD). The possibility of secondary RBD due to alcohol or medications was ruled out by careful history taking. Participants in the control group were also screened with RBDSQ, and only those with a score below 5 were enrolled.
Assessment of motor and cognitive functions
Participants’ motor and cognitive functions were assessed at the time of enrollment. The motor subscale of the Unified Parkinson’s Disease Rating Scale (mUPDRS) was used to assess the severity of motor symptoms in the PD group. Scores during off-state were used in the analysis. Cognitive function was assessed using the Korean version of the Montreal Cognitive Assessment (MoCA) (Kang et al. 2009).
Assessment of white matter hyperintensities (WMH)
All patients underwent brain MRI. All T2 and FLAIR images were reviewed, and WMH scores were graded using the Fazekas scale by DGP and JHY, who were blinded to the diagnosis and clinical profile (Fazekas et al. 1987). If the score was discordant between raters, the final score was determined by consensus. Periventricular and deep WMH were graded on a scale of 0–3. The sum of the periventricular and deep WMH scores (range 0–6) was used in the analyses.
123I-MIBG myocardial scintigraphy and quantification of dopamine transporter (DAT) uptake
All participants in the PD group underwent cardiac 123I-MIBG scintigraphy. After intravenous injection of 123I-labeled MIBG, planar images of the chest region were acquired at 15 min (early) and 180 min (delayed). To evaluate the heart-to-mediastinum (H/M) ratio, 5 × 5-pixel regions of interest were drawn manually over the left ventricle and upper mediastinum on the anterior planar image. The H/M count ratios of MIBG uptake, defined as the average counts/pixel in the myocardium divided by that in the upper mediastinum, were calculated at 15 min (early H/M) and 180 min (delayed H/M). The delayed H/M ratio was used in the analysis.
All participants in the PD group underwent 18F-FP-CIT (N-3–fluoropropyl-2-β-carboxymethoxy-3-β-(4-iodophenyl) nortropane) positron emission tomography (PET); scanning was initiated at 90 min after intravenous injection of 18F-FP-CIT. 18F-FP-CIT uptake in the brain was quantified using PMOD Neuro tool (PNEURO, version 3.7, PMOD Technologies, Zürich, Switzerland), as described previously (Choi et al. 2016). The automatically segmented MRI scans and respective PET images were co-registered and then spatially normalized according to the standard Montreal Neurological Institute T1 template. An automated anatomic labeling atlas was subsequently applied. Regional uptake values in the bilateral caudate nucleus and putamen were divided by those in the cerebellar cortex uptake, to provide regional standardized uptake value ratios (SUVRs). The regional SUVRs of the bilateral caudate nucleus and putamen in each patient were used in the analyses.
Smell identification test
Olfaction was assessed using the Cross‐Cultural smell Identification Test (CCSIT), a widely used test of odor identification involving a scratch-and-sniff test of 12 microencapsulated odorants with a forced choice of four alternatives per item; a high score indicates a good olfactory performance. Hyposmia was determined using age- and sex-specific thresholds described previously (Doty et al. 1996).
Measurement of the plasma NFL level
We collected 28 mL venous blood from each participant at enrollment. All sampling took place during the morning (10:00–12:00), and the samples were centrifuged within 2 h of collection. Plasma aliquots were stored in microcentrifuge tubes at − 80 °C. The samples were transferred to the Simoa platform using an NFL assay kit (Quantrix; Lexington, MA), and NFL levels were measured blinded to the clinical diagnosis.
Statistical analysis
Numerical variables are expressed as means (standard deviations) or medians [interquartile range (IQR)] after verifying a normal distribution using Shapiro–Wilk’s method. Categorical variables are expressed as numbers with the percentage. The Kruskal–Wallis test was used for comparisons among multiple groups. For post hoc analysis, the Conover–Iman test was used with Bonferroni correction. The χ2 test was used to compare categorical variables. The plasma NFL level was compared between the PD and control groups using one-way analysis of covariance (ANCOVA) while controlling for age. Estimated marginal means were used to calculate age-adjusted means for each group. To achieve the normality of the residuals, we log-transformed the plasma NFL data for ANCOVA and linear regression analysis. The forward and backward stepwise Akaike’s information criterion method was used to select the variables for multiple regression analyses. Residual analysis showed no violation of basic assumptions for linear models. A p value < 0.05 was considered to indicate statistical significance. Simple correlations between two continuous variables were analyzed using Spearman’s method or simple linear regression. The sensitivity and specificity for differentiating between groups were assessed using a receiver operating characteristic (ROC) analysis. All statistical analyses were performed using R software (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient demographic and clinical characteristics
At enrollment, 77 patients with PD and 54 controls were included in the study, and blood samples were obtained. Two patients in the PD group were lost to follow-up and were excluded from the analyses. Among the remaining 75 PD patients, 5 (4 with MSA, 1 with dementia with Lewy bodies (DLB)) converted to Parkinson-plus syndrome (P + Converter) during follow-up. As a result, the study participants comprised three groups: PD group (n = 70), control group (n = 54), and P + converter group (n = 5).
Among the 70 patients in the PD group, 13 had OH in the initial assessment, among which none exhibited delayed OH. These patients were classified as PD with OH (PDOH+ group), and the rest as PD without OH (PDOH− group). Over at least 1 year of follow-up (mean 24.8 months), patients in the PDOH+ group remained levodopa responsive and showed no signs of atypical parkinsonian symptoms other than OH. In contrast, patients in the P + converter group did not exhibit OH at the initial assessments and were clinically indistinguishable from PD at enrollment; however, during the follow-up period, they started to show severe autonomic dysfunction or cognitive decline (Supplementary table).
Table 1 shows the demographic and clinical characteristics of the study participants. There was a significant difference in age among the groups (p = 0.041), but pairwise comparisons with Bonferroni correction were insignificant. The patients in PDOH+ group were more likely to have pRBD and lower cardiac MIBG uptake, when compared with those in PDOH− group. P + converter group had higher cardiac MIBG uptake compared to PDOH+ group.
Comparison of the plasma NFL level among groups
There was a significant difference in the plasma NFL level among groups (p < 0.0001, Fig. 1). P + converter group had the highest plasma NFL level (median 38.2 pg/mL), which differed significantly with the control (p < 0.001) and PDOH− group (p < 0.001). There was no significant difference between the control and PD group as a whole (age-adjusted means 9.51 pg/mL vs 10.40 pg/mL, p = 0.151), but PDOH+ had higher plasma NFL level compared to the control (median 15.4 pg/mL vs 9.7 pg/mL, p = 0.008).
PDOH+ also had higher plasma NFL level when compared with PDOH− group (median 9.2 pg/mL, p = 0.002). The difference remained significant even after the adjustment for age, disease duration, antihypertensive medications, MoCA score, education years, and WMH score (β = 0.266, p = 0.011).
Correlations between plasma NFL level and other clinical features of PD
Within PD group (both PDOH+ and PDOH−), we investigated the impact of pRBD, cardiac MIBG uptake, and other clinical/imaging markers on the NFL level. There was no difference in plasma NFL level according to presence or absence of pRBD. The delayed H/M ratio on cardiac MIBG scintigraphy negatively correlated with the log2NFL (R = − 0.36, p = 0.003), but the significance was lost after age adjustment (Table 2).
After age adjustments, log2NFL correlated with neither striatal DAT uptake nor WMH score (Table 2). Disease duration, MoCA score, and off-state mUPDRS score also did not correlate with log2NFL. There was no significant difference in the plasma NFL level between the patients with or without olfactory dysfunction.
Stepwise multiple regression analysis
Stepwise multiple regression analysis was performed to select variables among age, sex, disease duration, OH, pRBD, hyposmia, MoCA, years of education, mUPDRS, cardiac MIBG uptake, striatal DAT uptake, and WMH score. The final model included age, disease duration, OH, pRBD, motor UPDRS, cardiac MIBG uptake, striatal DAT uptake, and WMH score (Table 2). The R2 value for the model was 0.56, and the adjusted R2 value was 0.48 (p < 0.0001) with an Akaike’s information criterion value of 78.6. In this model, age, disease duration, and OH showed independent positive correlations with log2NFL.
ROC analysis
We conducted ROC analysis of NFL in differentiating P + converters from PD group as a whole (Fig. 2A) and from PDOH+ group (Fig. 2B). For differentiating P + converters from PD, we found very high ROC area under the curve of 0.997 (CI 0.989–1, p < 0.001). Although PDOH+ patients were those with higher levels of NFL level among PD group, the differentiation of P + converters from PDOH+ still resulted in high ROC area under the curve of 0.985 (CI 0.942–1, p = 0.002).
Discussion
Among patients with PD of less than 3 years’ duration, plasma NFL levels were significantly higher in those patients with PD and OH and in those who converted to MSA or DLB during over 1 year of follow-up.
Previous studies have shown mixed results regarding differences in the NFL level between PD and control groups (Hansson et al. 2017; Lin et al. 2019; Marques et al. 2019; Sampedro et al. 2020). A meta-analysis showed no significant difference in the CSF NFL level between PD and control groups (Wang et al. 2019). However, a recently published longitudinal study suggested that despite no difference in the NFL level between PD and control groups at baseline, the difference becomes more apparent with increasing disease duration (Mollenhauer et al. 2020). This may be consistent with our results, as our study included only early-stage patients with a disease duration ≤ 3 years.
During follow-up, five patients converted to Parkinson-plus syndrome (four patients with MSA and one with DLB). These patients had a higher level of plasma NFL than the control and PD groups, suggesting that the plasma NFL level has value in discriminating between PD and atypical parkinsonian syndrome during the early stage when clinically indistinguishable. This finding is in line with previous studies showing higher NFL levels in patients who were later diagnosed with atypical parkinsonian syndrome (Hansson et al. 2017; Marques et al. 2019; Mollenhauer et al. 2020). Blood NFL level could be a useful screening tool in future clinical trials for PD, and may help in discriminating PD from other parkinsonian disorders, especially in the setting where the presence of OH complicates differential diagnosis.
To the best of our knowledge, this is the first study to evaluate the impact of OH on the NFL level in PD patients. OH independently correlated with plasma NFL level, along with age and disease duration. In the patients with PD, OH has previously been considered to suggest poor prognosis, as these patients showed earlier cognitive decline, postural instability and falls, and even a lower survival rate (Centi et al. 2017; Pilotto et al. 2019; Udow et al. 2016). Despite the existing evidence, it is still unclear whether the association between OH and a poor clinical outcome is due to a diffuse pathological burden particularly in those with OH (“diffuse pathology” theory), or due to hypoperfusion injury caused by OH (“cerebral hypoperfusion” theory) (Udow et al. 2016).
A recent report using mediation analysis suggested that WMH fully mediates the effect of diastolic OH on future cognitive decline in patients with PD (Dadar et al. 2020). Centi et al. reported that the cognitive test scores of PD patients with OH were lower when performed standing than when performed lying down, which also supports the cerebral hypoperfusion theory (Centi et al. 2017). Our result seems to support the “diffuse pathology” theory, as the plasma NFL level was higher in patients with OH independent of the WMH score. However, this might also be explained by the ability of the plasma NFL level, rather than the WMH score, to predict cortical injury, as the plasma NFL level may be a more sensitive marker of neuronal injury compared with WMH. In support of this, a recent study that measured cortical thickness and intracortical mean diffusivity in patients with PD showed that the serum NFL level correlated with cortical macro- and microstructural damage (Sampedro et al. 2020).
There are complex inter-relationships between OH, RBD, and cardiac sympathetic denervation in PD. RBD, along with OH, suggests more diffuse brainstem pathology, and there have been previous reports suggesting the association between OH and RBD (Pilotto et al. 2019). Cardiac sympathetic denervation measured by MIBG uptake has been associated with non-motor markers such as OH, RBD, or cognitive decline (Choi et al. 2017; Kim et al. 2016). Our results also show that PDOH+ group more commonly has pRBD and lower cardiac MIBG uptake than PDOH− group. However, our results indicate that only OH, not RBD or cardiac MIBG uptake, is associated with increased plasma NFL level. Several explanations can be inferred. This again may imply that cerebral hypoperfusion due to OH, not diffuse brainstem pathology, is more responsible for neuronal injury, or RBD or cardiac sympathetic denervation can present from prodromal stage of PD (Sakakibara et al. 2014; Taki et al. 2004), and when the diagnosis is established, the variation between subjects could be too slight to reflect disease severity.
Several limitations should be acknowledged. First, the sample size was relatively small, especially in the PDOH+ group (n = 13) and P + converter group (n = 5). This may have caused the non-significant differences in the MoCA or WMH score between groups and limit the interpretation of the ROC analyses. Second, we used consensus criteria for the definition of OH, which included decreases in both systolic and diastolic blood pressure. There is growing evidence that diastolic OH or supine hypertension is more relevant to target organ damage (Kaufmann and Palma 2020; Palma et al. 2020), but because of the small number of PD patients with OH in our study, we could not subdivide the patients further. Third, there is potential concern regarding the diagnosis of PDOH+ patients because diagnostic accuracy could be low during the early stages of PD. However, we followed all the patients regularly for more than a year (mean 24.8 months), and those who showed signs of atypical parkinsonism were re-allocated to the P + group. Fourth, we did not perform detailed cognitive testing in all patients, although specific cognitive domains (e.g., frontal/executive function or memory) could correlate with the NFL level (Mollenhauer et al. 2020). Finally, it would have been better to quantify the WMH burden instead of the visual WMH scoring using the Fazekas scale.
In conclusion, OH was independently associated with a higher plasma NFL level in early-stage PD patients. This finding adds biomarker-based evidence for poor clinical outcomes associated with OH in the patients with PD.
Availability of data and material
Anonymized data presented in this article will be shared by request from any qualified investigator. Requests should be made to Jung Han Yoon (jhyoon@gmail.com).
Code availability
Not applicable.
Change history
22 October 2021
Jae Whan Kim is a co-first author
References
Backstrom D, Linder J, Jakobson Mo S, Riklund K, Zetterberg H, Blennow K, Forsgren L, Lenfeldt N (2020) NfL as a biomarker for neurodegeneration and survival in Parkinson disease. Neurology 95(7):e827–e838. https://doi.org/10.1212/WNL.0000000000010084
Centi J, Freeman R, Gibbons CH, Neargarder S, Canova AO, Cronin-Golomb A (2017) Effects of orthostatic hypotension on cognition in Parkinson disease. Neurology 88(1):17–24. https://doi.org/10.1212/wnl.0000000000003452
Choi WH, Um YH, Jung WS, Kim SH (2016) Automated quantification of amyloid positron emission tomography: a comparison of PMOD and MIMneuro. Ann Nucl Med 30(10):682–689. https://doi.org/10.1007/s12149-016-1115-6
Choi MH, Yoon JH, Yong SW (2017) Cardiac sympathetic denervation and dementia in de novo Parkinson’s disease: a 7-year follow-up study. J Neurol Sci 381:291–295. https://doi.org/10.1016/j.jns.2017.09.010
Dadar M, Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB, Collins DL (2020) White matter hyperintensities mediate impact of dysautonomia on cognition in Parkinson’s disease. Mov Disord Clin Pract 7(6):639–647. https://doi.org/10.1002/mdc3.13003
De Pablo-Fernandez E, Tur C, Revesz T, Lees AJ, Holton JL, Warner TT (2017) Association of autonomic dysfunction with disease progression and survival in Parkinson disease. JAMA Neurol 74(8):970–976. https://doi.org/10.1001/jamaneurol.2017.1125
De Pablo-Fernandez E, Lees AJ, Holton JL, Warner TT (2019) Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. JAMA Neurol 76(4):470–479. https://doi.org/10.1001/jamaneurol.2018.4377
Doty RL, Marcus A, Lee WW (1996) Development of the 12-item Cross-cultural smell identification test (CC-SIT). Laryngoscope 106(3 Pt 1):353–356. https://doi.org/10.1097/00005537-199603000-00021
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149(2):351–356. https://doi.org/10.2214/ajr.149.2.351
Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB (2017) Clinical criteria for subtyping Parkinson’s disease: biomarkers and longitudinal progression. Brain 140(7):1959–1976. https://doi.org/10.1093/brain/awx118
Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG (2011) Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 21(2):69–72. https://doi.org/10.1007/s10286-011-0119-5
Goldstein DS, Holmes C, Sharabi Y, Wu T (2015) Survival in synucleinopathies: a prospective cohort study. Neurology 85(18):1554–1561. https://doi.org/10.1212/WNL.0000000000002086
Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, Norgren N, Linder J, Forsgren L, Constantinescu R, Zetterberg H, Blennow K (2017) Blood-based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology 88(10):930–937. https://doi.org/10.1212/wnl.0000000000003680
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184. https://doi.org/10.1136/jnnp.55.3.181
Kang Y, Park J, Yu K, Lee B (2009) A reliability, validity, and normative study of the Korean-Montreal Cognitive Assessment (K-MoCA) as an instrument for screening of Vascular Cognitive Impairment (VCI). Korean J Clin Psychol 28(2):549–562
Kaufmann H, Palma JA (2020) White matter hyperintensities in the synucleinopathies: orthostatic hypotension, supine hypertension, or both? Mov Disord Clin Pract 7(6):595–598. https://doi.org/10.1002/mdc3.13000
Kim JS, Park HE, Oh YS, Lee SH, Park JW, Son BC, Lee KS (2016) Orthostatic hypotension and cardiac sympathetic denervation in Parkinson disease patients with REM sleep behavioral disorder. J Neurol Sci 362:59–63. https://doi.org/10.1016/j.jns.2016.01.020
Kim JS, Park HE, Park IS, Oh YS, Ryu DW, Song IU, Jung YA, Yoo IR, Choi HS, Lee PH, Lee KS (2017) Normal ‘heart’ in Parkinson’s disease: is this a distinct clinical phenotype? Eur J Neurol 24(2):349–356. https://doi.org/10.1111/ene.13206
Lin CH, Li CH, Yang KC, Lin FJ, Wu CC, Chieh JJ, Chiu MJ (2019) Blood NfL: a biomarker for disease severity and progression in Parkinson disease. Neurology 93(11):e1104–e1111. https://doi.org/10.1212/wnl.0000000000008088
Marques TM, van Rumund A, Oeckl P, Kuiperij HB, Esselink RAJ, Bloem BR, Otto M, Verbeek MM (2019) Serum NFL discriminates Parkinson disease from atypical parkinsonisms. Neurology 92(13):e1479–e1486. https://doi.org/10.1212/wnl.0000000000007179
Mollenhauer B, Dakna M, Kruse N, Galasko D, Foroud T, Zetterberg H, Schade S, Gera RG, Wang W, Gao F, Frasier M, Chahine LM, Coffey CS, Singleton AB, Simuni T, Weintraub D, Seibyl J, Toga AW, Tanner CM, Kieburtz K, Marek K, Siderowf A, Cedarbaum JM, Hutten SJ, Trenkwalder C, Graham D (2020) Validation of serum neurofilament light chain as a biomarker of Parkinson’s disease progression. Mov Disord off J Mov Disord Soc. https://doi.org/10.1002/mds.28206
Palma JA, Redel-Traub G, Porciuncula A, Samaniego-Toro D, Millar Vernetti P, Lui YW, Norcliffe-Kaufmann L, Kaufmann H (2020) The impact of supine hypertension on target organ damage and survival in patients with synucleinopathies and neurogenic orthostatic hypotension. Parkinsonism Relat Disord 75:97–104. https://doi.org/10.1016/j.parkreldis.2020.04.011
Parnetti L, Gaetani L, Eusebi P, Paciotti S, Hansson O, El-Agnaf O, Mollenhauer B, Blennow K, Calabresi P (2019) CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol 18(6):573–586. https://doi.org/10.1016/s1474-4422(19)30024-9
Pilotto A, Romagnolo A, Tuazon JA, Vizcarra JA, Marsili L, Zibetti M, Rosso M, Rodriguez-Porcel F, Borroni B, Rizzetti MC, Rossi C, Vizcarra-Escobar D, Molano JR, Lopiano L, Ceravolo R, Masellis M, Espay AJ, Padovani A, Merola A (2019) Orthostatic hypotension and REM sleep behaviour disorder: impact on clinical outcomes in α-synucleinopathies. J Neurol Neurosurg Psychiatry 90(11):1257–1263. https://doi.org/10.1136/jnnp-2019-320846
Sakakibara R, Tateno F, Kishi M, Tsuyusaki Y, Terada H, Inaoka T (2014) MIBG myocardial scintigraphy in pre-motor Parkinson’s disease: a review. Parkinsonism Relat Disord 20(3):267–273. https://doi.org/10.1016/j.parkreldis.2013.11.001
Sampedro F, Perez-Gonzalez R, Martinez-Horta S, Marin-Lahoz J, Pagonabarraga J, Kulisevsky J (2020) Serum neurofilament light chain levels reflect cortical neurodegeneration in de novo Parkinson’s disease. Parkinsonism Relat Disord 74:43–49. https://doi.org/10.1016/j.parkreldis.2020.04.009
Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH (2007) The REM sleep behavior disorder screening questionnaire–a new diagnostic instrument. Mov Disord off J Mov Disord Soc 22(16):2386–2393. https://doi.org/10.1002/mds.21740
Taki J, Yoshita M, Yamada M, Tonami N (2004) Significance of 123I-MIBG scintigraphy as a pathophysiological indicator in the assessment of Parkinson’s disease and related disorders: it can be a specific marker for Lewy body disease. Ann Nucl Med 18(6):453–461. https://doi.org/10.1007/bf02984560
Udow SJ, Robertson AD, MacIntosh BJ, Espay AJ, Rowe JB, Lang AE, Masellis M (2016) ‘Under pressure’: is there a link between orthostatic hypotension and cognitive impairment in α-synucleinopathies? J Neurol Neurosurg Psychiatry 87(12):1311–1321. https://doi.org/10.1136/jnnp-2016-314123
Wang SY, Chen W, Xu W, Li JQ, Hou XH, Ou YN, Yu JT, Tan L (2019) Neurofilament light chain in cerebrospinal fluid and blood as a biomarker for neurodegenerative diseases: a systematic review and meta-analysis. J Alzheimer’s Dis JAD 72(4):1353–1361. https://doi.org/10.3233/jad-190615
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (no. NRF-2017R1C1B5018378 (J.H.Y), NRF-2018M3A9E8023859 (J.H.Y)) and the intramural research fund of Ajou University of medical center.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (no. NRF-2017R1C1B5018378 (JHY), NRF-2018M3A9E8023859 (JHY)) and the intramural research fund of Ajou University medical center.
Author information
Authors and Affiliations
Contributions
DGP: formal analysis; investigation; visualization; writing—original draft. JWK: investigation; methodology; resources. Y-SA: investigation; methodology; resources. JC: conceptualization; data curation; supervision; writing—review and editing. JHY: conceptualization; funding acquisition; supervision; writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki, and approved by the Institutional Review Board of Ajou University Hospital (AJIRB-BMR-SMP-18-555).
Consent to participate
Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jae Whan Kim is a co-first author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, D.G., Kim, J.W., An, YS. et al. Plasma neurofilament light chain level and orthostatic hypotension in early Parkinson’s disease. J Neural Transm 128, 1853–1861 (2021). https://doi.org/10.1007/s00702-021-02423-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-021-02423-y