Abstract
Normal cognition is an established selection criteria for subthalamic (STN) deep brain stimulation (DBS) in Parkinson’s disease (PD), while concern has been raised as to aggravated cognitive decline in PD patients following STN-DBS. The present longterm study investigates cognitive status in all patients (n = 104) suffering from PD, who were treated via continuous bilateral STN-DBS between 1997 and 2006 in a single institution. Preoperative neuropsychological results were available in 79/104 of the patients. Thirty-seven of these patients were additionally assessed after 6.3 ± 2.2 years (range 3.6–10.5 years) postsurgery via neuropsychological and motor test batteries, classifying cognitive conditions according to established criteria. At DBS-surgery patients, available for longterm follow-up (n = 37; mean age 67.6 ± 6.9 years, mean disease duration 11.3 ± 4.1 years), showed no (24.3%; 9/37) or mild preoperative cognitive impairment (MCI, 75.7%; 28/37). Postoperatively (mean disease duration: 17.1 ± 5.1 years), 19% of the patients (7/37) had no cognitive impairment, while 41% of the patients presented with either MCI or dementia (15/37, respectively). Preoperative MCI correlated with conversion to dementia by trend. Overall, STN-DBS-treated patients deteriorated by 1.6/140 points/year in the Mattis dementia rating scale. Disease duration, but not age, at DBS-surgery negatively correlated with postoperative cognitive decline and positively correlated with conversion to dementia. This observational, “real-life” study provides longterm results of cognitive decline in STN-DBS-treated patients with presurgical MCI possibly predicting the conversion to dementia. Although, the present data is lacking a control group of medically treated PD patients, comparison with other studies on cognition and PD do not support a disease-modifying effect of STN-DBS on cognitive domains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subthalamic (STN) deep brain stimulation (DBS) is an effective treatment option in Parkinson disease (PD). Conflicting reports point to a potential detrimental effect of STN-DBS on cognition (Daniels et al. 2010; Smeding et al. 2006; Volkmann et al. 2010; Weaver et al. 2009; Williams et al. 2010; Witt et al. 2008; York et al. 2008; Zangaglia et al. 2009, 2012; Odekerken et al. 2016; Combs et al. 2015). Concomitantly, the natural course of PD has been shown to be associated with the development of dementia of different degrees in 17.4% after 5 years following diagnosis (Pedersen et al. 2017) and 83% following 20 years after disease onset (Hely et al. 2008). Furthermore, an incidence rate of 10% for dementia in PD patients (Aarsland et al. 2001; Marder et al. 1995) and a prevalence of 30% following 8 years of disease onset was reported in a systematic review (Aarsland et al. 2005). In contrast, a longitudinal study showed a minor degree of dementia in PD of 13% following 7 years (Balzer-Geldsetzer et al. 2011).
Numerous predictors at baseline for dementia in PD have been identified, including age, disease duration, mild cognitive impairment (MCI), orthostatic hypotension, REM sleep behaviour disorder (RBD), motor impairment, male sex, hallucinations, and axial symptoms (Aarsland et al. 2001, 2003; Marder et al. 1995; Anang et al. 2014, 2017; Hely et al. 2008; Domellof et al. 2015; Cereda et al. 2016).
Motor and nonmotor fluctuations appear after 5–10 years following onset of PD. STN-DBS has been shown to improve quality of life in PD patients, ameliorating motor fluctuations or therapy-refractory tremor (Deuschl et al. 2006; Schuepbach et al. 2013). Growing knowledge about cognitive and psychiatric effects of STN-DBS is essential to adequately advise patients suffering from disabling PD with and without cognitive dysfunction, potentially eligible for surgery.
Methods
Between 1997 and 2006 all patients (n = 104) suffering from PD at University Hospital Berlin Charité, who were treated via continuous bilateral STN-DBS were included after 6.3 ± 2.2 years (range 3.6–10.5 years) surgery. Inclusion criteria were the diagnosis of PD following the United Kingdom Parkinson’s Disease Society Brain Bank Criteria, bilateral implantation of STN-electrodes, written informed consent for neuropsychological investigation according to the declaration of Helsinki and permission of local ethics commission. Exclusion criteria comprised atypical PD, implantation of unilateral electrode, pallidal or thalamic target localisation, explantation of neurostimulation system and missing presurgical neuropsychological data.
All postsurgical available patients were reassessed by neuropsychological and motor test batteries with identical presurgical tests.
Investigation of motor function was performed by Unified Parkinson Disease Rating Scale, motor subscore (UPDRSm) and Hoehn and Yahr Stage (Fahn et al. 1987; Hoehn and Yahr 1967) in the best on-condition of stimulation and medication.
The assessment of global cognition using modified Mattis Dementia Rating Scale (mMDRS) (Mattis 1988) and the five cognitive domains (i.e., memory, executive function, language, attention, working memory) via Rey Auditory Verbal Learning Test (RAVLT), semantic and phonemic fluency, Stroop-Test, Trail Making Test (TMT), Digit span [for detailed description of neuropsychological tests see (Lezak 1995)] was performed postsurgically in all available patients. Due to missing presurgical data of the visual memory part (4 points), the maximal MDRS score comprised 140 points in all patients (= mMDRS).
Cognitive conditions of each patient were classified as no or mild cognitive impairment or dementia according to level-1 criteria of movement disorders society (Emre et al. 2007; Litvan et al. 2012) in pre- and postsurgical states.
Impairment of each cognitive tests was defined as performance below threshold 16th percentile or more than 1 standard deviation compared to normative age-matched data presurgery or intraindividual data pre- versus postsurgery. Individual deterioration was analysed as change/DBS year for each cognitive domain and for global cognition score and was correlated with age at surgery, disease duration, DBS-years, and preoperative baseline levels of each cognitive domain.
Affective state was assessed using multidimensional mood state questionnaire (MDMQ) (Steyer et al. 1997), Beck Depression and Anxiety Inventory (BDI, BAI) (Beck 1987; Beck et al. 1988), Snaith–Hamilton-Pleasure-Scale (SHAPS-D) (Snaith et al. 1995), Bech-Rafaelsen-Mania Scale (BRMS) (Bech et al. 1979), Montgomery and Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg 1979), Brief Psychiatric Rating Scale (BPRS) (Flemenbaum and Zimmermann 1973) and quality of life (QoL) by Parkinson’s Disease Questionnaire (PDQ 39) (Peto et al. 1995) and the 36-item Short-Form Health Survey (SF 36) (Bullinger 1996).
Statistical analysis
Longterm outcomes were compared to baseline using the Wilcoxon signed-rank test for matched pairs. Statistical tests were two-tailed and were adjusted for multiple testing according to Bonferroni, and the level of significance was calculated as p ≤ 0.001(Kim 2014). Spearman’s ρ coefficient for ordinal and metric data was analysed, for nominal data exact Fishers test was used. Next, variables that showed a potential association in the correlation analysis (p < 0.2) were used in the logistic regression analysis, with conversion to dementia as the dependent variable.
Logistic regression was used to calculate odds ratio (OR) with 95% confidence interval (CI) for PD-D with and without adjustment for age at surgery, sex, and education. Statistical analyses were performed with the SPSS® Statistics, version 22.
Results
Preoperative neuropsychological results were available in 79/104 of the patients. Thirty-seven of these patients at age of 67.6 ± 6.9 years (range 53–81 years) with a disease duration of 17.1 ± 5.1 years (range 6–28 years) were additionally assessed after 6.3 ± 2.2 years (range 3.6–10.5 years) postsurgery (Table 1) via neuropsychological and motor test batteries. In the other 42 patients follow-up assessments were not available due to death (n = 21), loss of patient contact (n = 9; mean age 74.2 ± 7.9 years, mean disease duration 21.6 ± 5.2 years) or patients’ refusal of retesting (n = 12; mean age 76.1 ± 14.2 years, mean disease duration 17.9 ± 6.1 years) (Fig. 1).
At a follow-up of 6.3 ± 2.2 years in 37/79 patients motor function measured by UPDRSm worsened by trend post- versus presurgery (p < 0.01) by a difference of 7.1 ± 14.1 points (i.e., 36.2%) and Levodopa equivalent dose (LED) showed a trend of sustained reduction of 39.1% compared to presurgical levels (p < 0.01). Importantly, all patients had at least one replacement of their implantable pulse generator during an observation period (October 2018), suggesting continued benefit of motor symptoms via STN-DBS.
Presurgical state
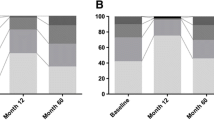
Presurgically, 24.4% of patients (n = 9/37) showed normal cognitive functioning (PD-NC), while 75.7% showed mild cognitive impairment (PD-MCI; n = 28/37) according to level 1 criteria (Litvan et al. 2012). None of the patients displayed dementia (PD-D) presurgically (Fig. 2a).
a Cognitive classification of all patients as absent (PD-NC) or mild cognitive impairment (PD-MCI) or dementia (PD-D) according to level-1 criteria of movement disorders society (Emre et al. 2007; Litvan et al. 2012) in pre- and postsurgical states. b Significant correlation of Mattis Dementia-Ratings Scale-Change/DBS-year and disease duration (r = 0.42; p = 0.017, Spearman’s-ρ correlation coefficient), rhombs represent each individual patient score. Note that the rapid decline in one patients most likely reflects long disease duration (19 years) since no clinical signs for atypical PD were present in this patient. c Non-significant correlation of Mattis Dementia-Ratings Scale-Change/DBS-year and age (r = − 0.09; p = 0.62, Spearman’s-ρ correlation coefficient), points represent each individual patient score
Age, disease duration, LED, motor function (med-off, med-on), levodopa-response, educational level did not differ in PD-MCI versus PD-NC patients, respectively. PD-MCI and PD-NC also did not differ with respect to affective states and QoL.
Presurgery, PD-MCI versus PD-NC patients differed in premorbide verbal intelligence measured by MWT-B (p = 0.008) by trend, a German equivalent of National Adult Reading Test. Preoperative digit span forward (p = 0.008) and backward (p = 0.008), and memory subscore of mMDRS (p = 0.04) were lower by trend in PD-MCI patients compared to PD-NC patients.
Postsurgical state
Postsurgically, 18.9% (n = 7/37) showed no cognitive impairment, 40.5% (n = 15/37) displayed MCI and 40.5% (n = 15/37) developed dementia (Fig. 2a) according to level 1-criteria (Emre et al. 2007). Thirteen of 28 (46.4%) presurgically identified PD-MCI patients converted to PD-D following 6.3 DBS years. In contrast, the conversion rate to dementia of PD-NC patients was 2/9 (22.2%).
Notably, a trend to association between preoperative mild cognitive impairment and conversion to dementia during the postoperative observation period was observed with respect to age at baseline, sex and education (adjusted OR, 10.8, 95% CI 1.0–119.0, p = 0.052).
In comparison to the non-demented PD patients (PD-NC/MCI) PD-D patients showed longer disease durations (20.2 ± 4.8 years vs. 15.0 ± 4.1 years, PD-NC/MCI versus PD-D; p < 0.01) and more severe motor impairment (UPDRSm 36.1 ± 12.9 versus 23.7 ± 7.8, PD-NC/MCI versus PD-D; p < 0.01) by trend. Additionally, PD-D patients showed more pronounced deterioration of UPDRS motor subscores (bradykinesia, p = 0.012; rigidity, p = 0.018; axial symptoms—summary score of speech, posture, gait and postural stability, p = 0.032) by trend. LED, presurgical levodopa-response as well as age, age of disease onset and DBS-years did not significantly differ between non-demented and demented patients.
In general, there was a deterioration of various cognitive domains (global cognition, memory, language) in all STN-DBS treated patients 6.3 years after surgery (cf. Table 2), while partial working memory was preserved and slightly improved by trend (digit span forward, p < 0.01).
Specifically, PD-D patients worsened significantly compared to PD-MC/NC patients with respect to global cognition (mMDRS, p < 0.001), comprising the domains (1) memory (RAVLT, immediate and delayed recall), (2) executive function (TMT B), (3) language (semantic and phonematic fluency), (4) attention (TMT A), while also working memory did not differ between the two groups (digit span, data not shown).
Overall, patients showed a global cognitive change/DBS years measured by mMDRS of − 1.6 ± 3.8/140 points/DBS year (range − 2.2 to 19.8 points/DBS year), which inversely correlated with disease duration (r = − 0.4, p = 0.02), but not with age at surgery (Fig. 2b/c).
Various cognitive changes/DBS years of single testing domains correlated with disease duration: (1) executive function: TMT B (r = 0.6, p = 0.03), TMT B–A (r = 0.5, p = 0.05); (2) language: semantic fluency “supermarket” (r = − 0.5, p < 0.01), phonemic fluency “F” (r = − 0.5, p = 0.03); (3) longterm memory: verbal memory, RAVLT total score (r = − 0.4, p = 0.03), RAVLT free recall (r = − 0.6, p < 0.01), RAVLT delayed recall (r = − 0.6, p = 0.01); (4) attention TMT A (r = 0.5, p = 0.02); (5) for working memory digit span backward (r = − 0.4, p = 0.05).
Finally, the conversion to dementia (PD-D) according to level-1 criteria of movement disorders society is also positively correlated (Spearman’s ρ r = 0.4, p = 0.01) with disease duration. Furthermore, logistic regression analysis showed significant relation beetween development of dementia and disease duration with respect to age at postsurgical investigation (adjusted odds ratio 1.2, 95% CI 1.0–1.4, p = 0.02). Development of dementia did not correlate with hallucinosis and levodopa-response.
Investigation of affective states in all PD patients showed a trend of postsurgical deterioration of mood measured by patient-related score BDI (p = 0.02), in contrast to the patient-related score MDMQ (p = 0.2) and physician-related score MADRS (p = 0.7). Anxiety (BAI), hedonia (SHAPS-D) and positive psychiatric symptoms (BRMAS, BPRS) did not change postsurgery compared to presurgery (Table 2).
Mood evaluated via BDI positively correlated with MADRS (r = 0.78, p < 0.01) and PDQ 39 (r = 0.69, p < 0.01). Particularly, there was no association between mood and disease duration, LED, motor function and global cognition (data not shown).
Postsurgery, QoL worsened by trend assessed via PDQ 39 (p < 0.01), although not reflected by SF 36 (SF 36 physical summery subscore, p = 0.1, SF 36 mental summery subscore, p = 0.7, Table 2).
QoL investigated by PDQ 39 negatively correlated with SF 36 mental summary subscore (r = − 0.8, p < 0.01), the global cognition score mMDRS (r = − 0.5, p = 0.02), and positively associated with the mood score MADRS (r = 0.7, p < 0.01), motor function scales UPDRSm (r = 0.5, p = 0.03), including the UPDRS motor subscores bradykinesia (r = 0.5, p = 0.03), the summary subscore for axial symptoms (r = 0.7, p < 0.01) and isolated gait (r = 0.67, p < 0.01) as well as Hoehn and Yahr Stage (r = 0.6, p < 0.01).
PD-D patients did not differ from PD-MCI/NC-patients with respect to affective state and QoL (data not shown).
Mortality rate
Approximately 27% (21/79) of all patients died at mean age of 71.5 ± 5.7 years following a mean disease duration of 16.3 ± 5.3 years and mean chronic STN-DBS of 5.4 ± 2.4 years. Causes of death were not available. 9/79 (11.3%) of patients were not available.
Discussion
This observational “real life” study of 37 initially non-demented Parkinson patients provides longterm data on cognition, affective state and QoL of Parkinson’s patients treated with STN-DBS up to 10.5 years.
Study limitations of the present study comprise a missing control group (i.e., a comparable drug-treated group), precluding differential analyses of effects of STN-DBS and natural disease course. Furthermore the high drop out rate of 53% might have biased the present results since PD dementia may be associated with reduced study participation (n = 12/79) and death (n = 21/79). However, this bias is difficult to avoid in real-life studies despite home visits as performed in the present study, but may certainly alter the real dementia prevalence and the correlation between dementia and age. Thus, in 2 other studies on STN-DBS and development of PD dementia dropout distinctly higher rates of 76% or even 87% have been reported (Kim et al. 2014); Fig. 1, approximately 25/103 patients, i.e., 76% drop out rate at 5–7 years post-DBS (Merola et al. 2014) (Fig. 1; 25/189 patients with completed evaluation, i.e., 87% drop out rate 5-year post-DBS).
Following approximately 6 years of STN-DBS and a disease duration of 17 years, 41% of all patients developed either dementia or MCI, whereas 18% continued to not be affected by cognitive deficits. These data comply with the natural disease course of PD in medically treated patients with a dementia rate of 48% following 15 years, which rises to 83% after 20 years of PD duration (Aarsland et al. 2001; Hely et al. 2008).
The presurgical prevalence of MCI of 75.7% after 11.3 years disease course seems to be relatively high in our study population contrasting for instance 25–30% after 6.8 years PD duration and 43% after 6 years in non-surgically treated PD patients, respectively (Aarsland et al. 2010; Pigott et al. 2015).
The presurgical prevalence in other DBS studies is heterogeneous and varies between 23% and 14.2 years (Merola et al. 2014) and 63.1% and 10.6 years (Kim et al. 2014) PD duration, which likely reflects differential selection criteria of the different DBS studies.
In the present study population presurgically identified PD-MCI patients converted to dementia in 46.6% of the cases after an observation period of 6 years. Presence of MCI non-significantly correlated (trend; p = 0.052) with development of dementia. In contrast, only 22.2% of PD-NC patients developed dementia in the investigated study population following a disease duration of 17.1 years. Thus, presurgically existing MCI may predict a conversion to dementia of PD patients, complying with other studies assessing PD patients with (Kim et al. 2014; Merola et al. 2014) and without neurostimulation (Anang et al. 2014, 2017).
Interestingly, the annual deterioration of global cognition measured via mMDRS of 1.6/140 points/DBS-years and subsequently the conversion to dementia correlated with disease duration, but not with age at surgery.
Natural history studies of PD patients identified several predictors of conversion to dementia such as age, disease duration, male sex, baseline RBD, hallucinations orthostatic hypotension, axial symptoms like particular postural and gait disturbances, color discrimination ability, and MCI (Anang et al. 2014, 2017; Aarsland and Kurz 2010; Marder et al. 1995; Hely et al. 2008; Domellof et al. 2015; Cereda et al. 2016; Aarsland et al. 2001, 2003). Additionally, recent studies of PD patients with STN-DBS (Aybek et al. 2007; Smeding et al. 2011; Kim et al. 2014) partially provided similar predictors for global cognitive decline such as age, hallucinosis, impaired executive function, or lower levodopa-response after 1- or 3-year post-STN-DBS. Except for poor presurgical cognitive performance, these factors including age were not identified in the present analyses. However, we can not exclude a bias for instance with respect to age due to differential selection criteria for STN-DBS and/or older deceased patients, who could not be reassessed.
Overall, a deterioration of various cognitive domains was observed in all patients (PD-NC/MCI) postsurgery. Demented versus nondemented patients performed worse in multiple cognitive domains: executive function, language, memory and attention, less pronounced working memory. The progression of each of the multiple domain deficits correlated with disease duration and not with age of PD patients.
Mood and QoL slightly declined by trend postsurgery, whereas declined QoL positively correlated with diminished global cognitive function, mood and motor function (especially axial symptoms), that underlies the impact of nonmotor and motor symptoms on QoL (Martinez-Martin et al. 2011; Perez-Lloret et al. 2017; Schrag et al. 2000; Rahman et al. 2008).
The mortality of 27% (maximal 38% including lots of follow-up) in our study population (n = 79) is lower compared with previously reported epidemiological studies, amounting to 38%and 66% following 10 and 15 years PD disease duration (Hely et al. 2008), possibly reflecting selection criteria of patients evaluated for surgery.
Motor function, represented by UPDRSm showed by trend a progressive worsening over the observational period of 3.6–10.5 years, and complying with previous reports (Merola et al. 2011, 2014; Rizzone et al. 2014; Castrioto et al. 2011). LED was persistently diminished by trend postsurgery, likely due to beneficial DBS-effects, levodopa-resistent symptoms and/or adverse effects of increased levodopa dosage, that often induces hallucinosis or orthostatic dysregulation in severely affected PD patients. Thus, despite the slightly pronounced motor impairment of PD-D-patients versus PD-NC/MCI patients, the LED did not differ in the subgroups, possibly due to potential side effects of levodopa or levodopa-resistent symptoms.
Demented patients suffered by trend from a longer disease duration and were more affected by trend by axial symptoms, rigidity and bradykinesia compared to non-demented patients, which likely reflects the natural history of PD (Hely et al. 2008; Coelho and Ferreira 2012; Anang et al. 2014).
In summary, lack of a matched control group precludes final conclusions regarding possible influence of STN-DBS on disease course with respect to conversion to dementia. However, the present data complies with previous studies on cognitive decline in medically treated PD patients. The gradual deterioration of global cognitive impairment measured by MDRS and the conversion to dementia was associated with disease duration, but not with age of patients.
Abbreviations
- BAI:
-
Beck Anxiety Inventory
- BDI:
-
Beck Depression Inventory
- BPRS:
-
Brief Psychiatric Rating Scale
- BRMS:
-
Bech-Rafaelsen-Mania Scale
- DBS:
-
Deep brain stimulation
- LED:
-
Levodopa equivalent dosage
- MADRS:
-
Montgomery and Asberg Depression Rating Scale
- mMDRS:
-
Modified Mattis Dementia Rating Scale
- MDMQ:
-
Multidimensional Mood State Questionnaire
- OR:
-
Odds ratio
- PD:
-
Parkinson’s disease
- PD-D:
-
Parkinson’s disease with dementia
- PD-MCI:
-
Parkinson’s disease with mild cognitive impairment
- PD-NC:
-
Parkinson’s disease with normal cognition
- PDQ 39:
-
Parkinson’s Disease Questionnaire
- QoL:
-
Quality of life
- RBD:
-
REM sleep behaviour disorder
- RAVLT:
-
Rey Auditory Verbal Learning Test
- SF 36:
-
36-item Short-Form Health Survey
- SHAPS-D:
-
Snaith–Hamilton-Pleasure-Scale
- STN:
-
Nucleus subthlamicus
- TMT:
-
Trail Making Test
- UPDRSm:
-
Unified Parkinson’s Disease Rating Scale, motor subscore
References
Aarsland D, Kurz MW (2010) The epidemiology of dementia associated with Parkinson disease. J Neurol Sci 289(1–2):18–22. https://doi.org/10.1016/j.jns.2009.08.034
Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sorensen P (2001) Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology 56(6):730–736
Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P (2003) Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 60(3):387–392
Aarsland D, Zaccai J, Brayne C (2005) A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov Disord 20(10):1255–1263. https://doi.org/10.1002/mds.20527
Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, Burn D, Barone P, Pagonabarraga J, Allcock L, Santangelo G, Foltynie T, Janvin C, Larsen JP, Barker RA, Emre M (2010) Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology 75(12):1062–1069. https://doi.org/10.1212/WNL.0b013e3181f39d0e
Anang JB, Gagnon JF, Bertrand JA, Romenets SR, Latreille V, Panisset M, Montplaisir J, Postuma RB (2014) Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology 83(14):1253–1260. https://doi.org/10.1212/WNL.0000000000000842
Anang JB, Nomura T, Romenets SR, Nakashima K, Gagnon JF, Postuma RB (2017) Dementia predictors in parkinson disease: a validation study. J Parkinsons Dis 7(1):159–162. https://doi.org/10.3233/JPD-160925
Aybek S, Gronchi-Perrin A, Berney A, Chiuve SC, Villemure JG, Burkhard PR, Vingerhoets FJ (2007) Long-term cognitive profile and incidence of dementia after STN-DBS in Parkinson’s disease. Mov Disord 22(7):974–981. https://doi.org/10.1002/mds.21478
Balzer-Geldsetzer M, Costa AS, Kronenburger M, Schulz JB, Roske S, Spottke A, Wullner U, Klockgether T, Storch A, Schneider C, Riedel O, Wittchen HU, Seifried C, Hilker R, Schmidt N, Witt K, Deuschl G, Mollenhauer B, Trenkwalder C, Liepelt-Scarfone I, Graber-Sultan S, Berg D, Gasser T, Kalbe E, Bodden M, Oertel WH, Dodel R (2011) Parkinson’s disease and dementia: a longitudinal study (DEMPARK). Neuroepidemiology 37(3–4):168–176. https://doi.org/10.1159/000331490
Bech P, Bolwig TG, Kramp P, Rafaelsen OJ (1979) The Bech–Rafaelsen Mania Scale and the Hamilton Depression Scale. Acta Psychiatr Scand 59(4):420–430
Beck AT (1987) Beck Depression Inventory: manual. Psychological Corporation, San Antonia
Beck AT, Epstein N, Brown G, Steer RA (1988) An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 56(6):893–897
Bullinger M (1996) Assessment of health related quality of life with the SF-36 health survey. Rehabilitation (Stuttg) 35(3):XVII–XXVII (quiz XXVII–XXIX)
Castrioto A, Lozano AM, Poon YY, Lang AE, Fallis M, Moro E (2011) Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol 68(12):1550–1556. https://doi.org/10.1001/archneurol.2011.182
Cereda E, Cilia R, Klersy C, Siri C, Pozzi B, Reali E, Colombo A, Zecchinelli AL, Mariani CB, Tesei S, Canesi M, Sacilotto G, Meucci N, Zini M, Isaias IU, Barichella M, Cassani E, Goldwurm S, Pezzoli G (2016) Dementia in Parkinson’s disease: is male gender a risk factor? Parkinsonism Relat Disord 26:67–72. https://doi.org/10.1016/j.parkreldis.2016.02.024
Coelho M, Ferreira JJ (2012) Late-stage Parkinson disease. Nat Rev Neurol 8(8):435–442. https://doi.org/10.1038/nrneurol.2012.126
Combs HL, Folley BS, Berry DT, Segerstrom SC, Han DY, Anderson-Mooney AJ, Walls BD, van Horne C (2015) Cognition and depression following deep brain stimulation of the subthalamic nucleus and globus pallidus pars internus in Parkinson’s disease: a meta-analysis. Neuropsychol Rev 25(4):439–454. https://doi.org/10.1007/s11065-015-9302-0
Daniels C, Krack P, Volkmann J, Pinsker MO, Krause M, Tronnier V, Kloss M, Schnitzler A, Wojtecki L, Botzel K, Danek A, Hilker R, Sturm V, Kupsch A, Karner E, Deuschl G, Witt K (2010) Risk factors for executive dysfunction after subthalamic nucleus stimulation in Parkinson’s disease. Mov Disord 25(11):1583–1589. https://doi.org/10.1002/mds.23078
Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, Daniels C, Deutschlander A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider GH, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J (2006) A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 355(9):896–908
Domellof ME, Ekman U, Forsgren L, Elgh E (2015) Cognitive function in the early phase of Parkinson’s disease, a five-year follow-up. Acta Neurol Scand 132(2):79–88. https://doi.org/10.1111/ane.12375
Eggert K, Oertel W, Reichmann H (2012) Parkinsonsyndrome-Diagnostik und Therapie. Leitlinien für Diagnostik und Therapie in der Neurologie. Thieme Verlag, Stuttgart
Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22(12):1689–1707. https://doi.org/10.1002/mds.21507 (quiz 1837)
Fahn S, Elton R, Members of the UPDRS Development Committee (1987) The unified parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M (eds) Recent developments in parkinson’s disease, vol 2. McMellam Health Care Information, Florham Park, pp 153–163
Flemenbaum A, Zimmermann RL (1973) Inter- and intra-rater reliability of the Brief Psychiatric Rating Scale. Psychol Rep 32(3):783–792
Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG (2008) The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 23(6):837–844. https://doi.org/10.1002/mds.21956
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17(5):427–442
Kim HY (2014) Statistical notes for clinical researchers: nonparametric statistical methods: 2. Nonparametric methods for comparing three or more groups and repeated measures. Restor Dent Endod 39(4):329–332. https://doi.org/10.5395/rde.2014.39.4.329
Kim HJ, Jeon BS, Paek SH, Lee KM, Kim JY, Lee JY, Kim HJ, Yun JY, Kim YE, Yang HJ, Ehm G (2014) Long-term cognitive outcome of bilateral subthalamic deep brain stimulation in Parkinson’s disease. J Neurol. https://doi.org/10.1007/s00415-014-7321-z
Lezak M (1995) Neuropsychological assessment. Oxford University Press, New York
Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH, Aarsland D, Kulisevsky J, Rodriguez-Oroz MC, Burn DJ, Barker RA, Emre M (2012) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement disorder society task force guidelines. Mov Disord 27(3):349–356. https://doi.org/10.1002/mds.24893
Marder K, Tang MX, Cote L, Stern Y, Mayeux R (1995) The frequency and associated risk factors for dementia in patients with Parkinson’s disease. Arch Neurol 52(7):695–701
Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR, Group NV (2011) The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord 26(3):399–406. https://doi.org/10.1002/mds.23462
Mattis S (1988) Dementia Rating Scale: professional manual. Psychological Assessment Resources, Odessa
Merola A, Zibetti M, Angrisano S, Rizzi L, Ricchi V, Artusi CA, Lanotte M, Rizzone MG, Lopiano L (2011) Parkinson’s disease progression at 30 years: a study of subthalamic deep brain-stimulated patients. Brain 134(Pt 7):2074–2084. https://doi.org/10.1093/brain/awr121
Merola A, Rizzi L, Artusi CA, Zibetti M, Rizzone MG, Romagnolo A, Bernardini A, Lanotte M, Lopiano L (2014) Subthalamic deep brain stimulation: clinical and neuropsychological outcomes in mild cognitive impaired parkinsonian patients. J Neurol 261(9):1745–1751. https://doi.org/10.1007/s00415-014-7414-8
Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389
Odekerken VJ, Boel JA, Schmand BA, de Haan RJ, Figee M, van den Munckhof P, Schuurman PR, de Bie RM, Group Ns (2016) GPi vs STN deep brain stimulation for Parkinson disease: three-year follow-up. Neurology 86(8):755–761. https://doi.org/10.1212/WNL.0000000000002401
Pedersen KF, Larsen JP, Tysnes OB, Alves G (2017) Natural course of mild cognitive impairment in Parkinson disease: a 5-year population-based study. Neurology 88(8):767–774. https://doi.org/10.1212/WNL.0000000000003634
Perez-Lloret S, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destee A, Meissner WG, Tison F, Rascol O, of the CSG (2017) L-DOPA-induced dyskinesias, motor fluctuations and health-related quality of life: the COPARK survey. Eur J Neurol. https://doi.org/10.1111/ene.13466
Peto V, Jenkinson C, Fitzpatrick R, Greenhall R (1995) The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res 4(3):241–248
Pigott K, Rick J, Xie SX, Hurtig H, Chen-Plotkin A, Duda JE, Morley JF, Chahine LM, Dahodwala N, Akhtar RS, Siderowf A, Trojanowski JQ, Weintraub D (2015) Longitudinal study of normal cognition in Parkinson disease. Neurology 85(15):1276–1282. https://doi.org/10.1212/WNL.0000000000002001
Rahman S, Griffin HJ, Quinn NP, Jahanshahi M (2008) Quality of life in Parkinson’s disease: the relative importance of the symptoms. Mov Disord 23(10):1428–1434. https://doi.org/10.1002/mds.21667
Rizzone MG, Fasano A, Daniele A, Zibetti M, Merola A, Rizzi L, Piano C, Piccininni C, Romito LM, Lopiano L, Albanese A (2014) Long-term outcome of subthalamic nucleus DBS in Parkinson’s disease: from the advanced phase towards the late stage of the disease?. Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2014.01.012
Schrag A, Jahanshahi M, Quinn N (2000) What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry 69(3):308–312
Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, Halbig TD, Hesekamp H, Navarro SM, Meier N, Falk D, Mehdorn M, Paschen S, Maarouf M, Barbe MT, Fink GR, Kupsch A, Gruber D, Schneider GH, Seigneuret E, Kistner A, Chaynes P, Ory-Magne F, Brefel Courbon C, Vesper J, Schnitzler A, Wojtecki L, Houeto JL, Bataille B, Maltete D, Damier P, Raoul S, Sixel-Doering F, Hellwig D, Gharabaghi A, Kruger R, Pinsker MO, Amtage F, Regis JM, Witjas T, Thobois S, Mertens P, Kloss M, Hartmann A, Oertel WH, Post B, Speelman H, Agid Y, Schade-Brittinger C, Deuschl G (2013) Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med 368(7):610–622. https://doi.org/10.1056/NEJMoa1205158
Smeding HM, Speelman JD, Koning-Haanstra M, Schuurman PR, Nijssen P, van Laar T, Schmand B (2006) Neuropsychological effects of bilateral STN stimulation in Parkinson disease: a controlled study. Neurology 66(12):1830–1836. https://doi.org/10.1212/01.wnl.0000234881.77830.66
Smeding HM, Speelman JD, Huizenga HM, Schuurman PR, Schmand B (2011) Predictors of cognitive and psychosocial outcome after STN DBS in Parkinson’s disease. J Neurol Neurosurg Psychiatry 82(7):754–760. https://doi.org/10.1136/jnnp.2007.140012
Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P (1995) A scale for the assessment of hedonic tone the Snaith–Hamilton Pleasure Scale. Br J Psychiatry 167(1):99–103
Steyer R, Schwenkmezger P, Notz P, Eid M (1997) Der Mehrdimensionale Befindlichkeitsfragebogen (MDBF). Hogrefe, Göttingen
Volkmann J, Daniels C, Witt K (2010) Neuropsychiatric effects of subthalamic neurostimulation in Parkinson disease. Nat Rev Neurol 6(9):487–498. https://doi.org/10.1038/nrneurol.2010.111
Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ Jr, Rothlind J, Sagher O, Reda D, Moy CS, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein J, Stoner G, Heemskerk J, Huang GD, Group CSPS (2009) Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 301(1):63–73. https://doi.org/10.1001/jama.2008.929
Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, Scott R, Ives N, Rick C, Daniels J, Patel S, Wheatley K, Group PSC (2010) Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol 9(6):581–591. https://doi.org/10.1016/S1474-4422(10)70093-4
Witt K, Daniels C, Reiff J, Krack P, Volkmann J, Pinsker MO, Krause M, Tronnier V, Kloss M, Schnitzler A, Wojtecki L, Botzel K, Danek A, Hilker R, Sturm V, Kupsch A, Karner E, Deuschl G (2008) Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurol 7(7):605–614. https://doi.org/10.1016/S1474-4422(08)70114-5
York MK, Dulay M, Macias A, Levin HS, Grossman R, Simpson R, Jankovic J (2008) Cognitive declines following bilateral subthalamic nucleus deep brain stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry 79(7):789–795. https://doi.org/10.1136/jnnp.2007.118786
Zangaglia R, Pacchetti C, Pasotti C, Mancini F, Servello D, Sinforiani E, Cristina S, Sassi M, Nappi G (2009) Deep brain stimulation and cognitive functions in Parkinson’s disease: a three-year controlled study. Mov Disord 24(11):1621–1628. https://doi.org/10.1002/mds.22603
Zangaglia R, Pasotti C, Mancini F, Servello D, Sinforiani E, Pacchetti C (2012) Deep brain stimulation and cognition in Parkinson’s disease: an eight-year follow-up study. Mov Disord 27(9):1192–1194. https://doi.org/10.1002/mds.25047
Acknowledgements
We thank our patients for their continued support of our work.
Funding
University Medicine Berlin, Charité.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DG has received honoraria for speaking from Medtronic, outside the submitted work. AAK is a consultant to Boston Scientific and has received honoraria for speaking from Medtronic, Boston Scientific and Abbott, also grants from Medtronic, all outside the submitted work. GHS has received honoraria for speaking from Medtronic, and Boston Scientific, outside the submitted work. AK has received honoraria for speaking from Medtronic and Abbott, outside the submitted work. LC and UK declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gruber, D., Calmbach, L., Kühn, A.A. et al. Longterm outcome of cognition, affective state, and quality of life following subthalamic deep brain stimulation in Parkinson’s disease. J Neural Transm 126, 309–318 (2019). https://doi.org/10.1007/s00702-019-01972-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-01972-7