Abstract
While cortical thinning has been associated with HIV infection, it is unclear whether this reflects a direct effect of the virus, whether it is related to disruption of subcortical function or whether it is better explained by epiphenomena, such as drug abuse or comorbid medical conditions. The present study investigated the relationship between cortical thickness and subcortical function in HIV+ patients. Specifically, we examined the relationship between prefrontal cortical thickness and striatal function. Twenty-three largely treatment naïve, non-substance abusing HIV+ participants and 19 healthy controls matched for age, gender, and educational status were included. Cortical morphometry was performed using FreeSurfer software analysis. Striatal function was measured during an fMRI stop-signal anticipation task known to engage the striatum. Any cortical regions showing significant thinning were entered as dependent variables into a single linear regression model which included subcortical function, age, CD4 count, and a measure of global cognitive performance as independent predictors. The only cortical region that was significantly reduced after correction for multiple comparisons was the right superior frontal gyrus. Striatal activity was found to independently predict superior frontal gyral cortical thickness. While cortical thinning in HIV infection is likely multifactorial, viral induced subcortical dysfunction appears to play a role.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
HIV infection produces a typical fronto–subcortical pattern of neuropsychiatric impairment, (Navia et al. 1986), characterized by specific motor (Hardy and Hinhn 2002), cognitive (Antinori et al. 2007), and behavioral symptoms (Castellon et al. 1998). Despite the widespread availability of combination antiretroviral therapy (cART), HIV infection continues to have a negative impact on cognitive function in up to 52 % of infected individuals (Heaton et al. 2010). HIV is thought to enter the brain via infected monocytes in the early stage of illness (An et al. 1999) and to concentrate primarily in subcortical regions, such as the striatum (Wiley et al. 1998). Here, it is thought to cause synapto-dendritic damage (Moore et al. 2006)/apopotosis either by the induction of neuroinflammation (Desplats et al. 2013) or due to direct toxic effects of viral proteins, such as tat (Bruce-Keller et al. 2003) and GP120 proteins (Agrawal et al. 2010).
Neuroimaging studies consistently report generalized cortical volume loss in patients with HIV infection (Elovaara et al. 1990; Chang et al. 2011; Pfefferbaum et al. 2012). Additionally, in keeping with the clinical features of subcortical dementia, imaging studies have reported loss of gray matter in the basal ganglia (Elovaara et al. 1990; Aylward et al. 1993; Ances et al. 2012), and subcortical volume reductions have been found even in patients receiving cART treatment (Ances et al. 2012). These volume reductions have been related to viral load (Dewey et al. 2010) as well as the AIDS dementia complex stage (Aylward et al. 1993). It is surprising, therefore, that other studies have not found striatal atrophy in HIV+ patients (Melrose et al. 2008; Heaps et al. 2010). This is all the more unexpected given that frontal striatal dysfunction was consistently found in HIV patients in a recent meta-analysis of working memory studies (Ernst et al. 2002) as well as in studies of episodic memory(Maki et al. 2009) and delay discounting paradigms (Meade et al. 2011). Striatal dysfunction has also been shown to be specifically affected in a simple motor paradigm (Ances et al. 2011), reward processing (du Plessis et al. 2015a) as well as with an inhibition task (du Plessis et al. 2015b). Furthermore, a fMRI study in HIV patients has demonstrated reduced functional connectivity between subcortical and cortical regions, in the presence of normal striatal volumes (Melrose et al. 2008). Thus, the exact relationship between structural and functional changes observed in HIV remains to be determined. Taken together, these findings suggest that it may be that structural and functional imaging techniques are sensitive to different aspects of HIV-related neuropathology.
There are several possible explanations for the cortical thinning reported in HIV infection. First, it could be a direct consequence of viral infection in cortical structures. Second, indirect viral mediated immune processes may be involved (Thompson et al. 2005). Third, epiphenomena such as substance abuse (Jernigan et al. 2005) and age (Ances et al. 2012) should be considered. Finally, cortical thickness reductions could be secondary to the observed subcortical dysfunction in these patients. To understand how subcortical pathology could influence cortical morphology (Draganski et al. 2008), the functional relationship of these two systems needs to be considered. Current thinking is that the cortex and basal ganglia are connected in parallel, as functionally segregated circuits (Alexander and Crutcher 1990). This is based on anatomical (Middleton and Strick 2002), functional connectivity (Zandbelt and Vink 2010) and probabilistic tracking studies (Draganski et al. 2008). Being functionally connected in this manner, it is reasonable to propose that subcortical dysfunction caused by direct viral infection could result in reductions in cortical thickness in areas that are functionally connected.
In the present study, we investigated the relationship between cortical thickness and subcortical function in HIV+ patients compared to healthy controls. Specifically, we examined the relationship between prefrontal cortical thickness and striatal function, since HIV has been shown to affect the frontostriatal system specifically (du Plessis et al. 2013). We predicted that the striatal hypofunction we have shown in a group of HIV+ participants in a previously published analysis would be related to frontal atrophy in the same group. For the fMRI, we selected an inhibition task that we previously used to demonstrate that the striatum, particularly the putamen, is hypoactive in HIV+ participants (i.e., during successful versus unsuccessful STOPs), whereas frontal functioning was not affected during proactive inhibition (du Plessis et al. 2015b). The morphometric measures for the new analysis in the same cohort, were obtained using the standard Desikan–Killiany Atlas in FreeSurfer to generate regions of interest (Desikan et al. 2006).
These include a number of bilateral prefrontal regions. In particular, activity in the right inferior and superior frontal gyrus is normally functionally coupled with activity in the left putamen (Zandbelt and Vink 2010) during the correct versus incorrect STOPs BOLD contrast.
Methods and materials
Participants
The study was approved by the Health Research Ethics Committee (HREC) of Stellenbosch University and the Human Research Ethics Committee of the University of Cape Town, Cape Town, South Africa. Prior to enrolment, all participants provided written consent after receiving a full description of this study. Our sample was recruited from a medically stable clinic-attending population during routine HIV care and testing. A total of 23 HIV+ participants were included in the study together with 19 gender, education, and ethnicity matched healthy controls. The controls were HIV negative, as confirmed by the enzyme-linked immunosorbent assay (ELISA).
Participants were screened using the Mini International Neuropsychiatric Interview (MINI) 6.0.0/MINI-PLUS 6.0.0. (Sheehan et al. 1998). All HIV+ participants received a full physical examination and were excluded if there was: a current comorbid psychiatric/neurological or general medical condition that could confound the diagnosis of HIV-associated neurocognitive disorders (HAND); any history of substance use/abuse as assessed by the Substance Abuse and Mental Illness Symptoms Screener (SAMISS) screening questionnaire (Pence et al. 2005); a score for smoking greater than 1 according to the Kreek–McHugh–Schluger–Kellogg (KMSK) scale (Kellogg et al. 2003); current active treatment for tuberculosis. All participants were right-handed as confirmed by the Edinburgh Handedness Inventory (Oldfield 1971).
HIV+ participants underwent detailed neuropsychological assessment within two weeks of neuroimaging and controls within one year, for characterization purposes only. The test battery assessed the following cognitive domains: abstraction/executive function, memory, learning, speed of information processing, verbal fluency, motor and sensory/perception (Grant 2008). From these scores, a composite global deficit score (GDS) was derived (Carey et al. 2004) using normative data from a larger parent study (Joska et al. 2010).
The following laboratory measures were performed in the HIV+ participants, within two weeks of neuroimaging: CD4 count, HIV viral load, rapid plasma reagin for syphilis (RPR), and thyroid stimulating hormone (TSH) level. Urinary drug screen was performed on all participants prior to undergoing the MRI assessments. While hepatits C co-infection has been associated with increased risk and severity of cognitive impairment in HAND, participants were not screened as it is not endemic to the region (Amin et al. 2004). An experienced radiologist reviewed all of the scans for intracranial pathology that could potentially confound functional imaging measurement results.
Image acquisition
All scans were acquired on a 3T Siemens Allegra at the Combined Universities Brain Imaging Centre (CUBIC). For the study of brain morphology, a T1 ME-MPRAGE-weighted structural scan was acquired (TR = 2530 ms; TE1 = 1.53 ms TE2 = 3.21, ms, TE3 = 4.89 ms, TE4 = 6.57 ms, flip-angle: 7°, FOV: 256 mm, 128 slices, 1 mm isotropic voxel size) (van der Kouwe et al. 2008).
Structural imaging analysis
Preprocessing of structural scans
Cortical reconstruction and volumetric segmentation were performed with the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/). As FreeSurfer is computationally intensive, reconstructions were performed utilizing custom batching scripts, on the Centre for High Performance Computing (CHPC) Rosebank, Cape Town, Sun Intel Nehalem cluster (http://www.chpc.ac.za/).
Scans were processed and analyzed using FreeSurfer stable release version 5.1. The technical details of these procedures are described in prior publications (Dale et al. 1999). Briefly, slices were resampled to a three-dimensional image with 1-mm isotropic voxels. Non-uniform intensity normalization was then performed, and images were registered to the Montreal Neurological Institute (MNI) space. A second normalization step was performed with a different algorithm in which control points were automatically identified and normalized to a standard intensity value. This was followed by an automated skull strip procedure. Gross brain anatomy was then delineated into cortical and subcortical labels. All scans were visually checked for errors in Talaraich transformation, skull stripping as well as segmentation and corrected if possible.
Structural regions of interest analysis
Initially we compared whole brain cortical volumes as well as subcortical volumes, corrected for total intracranial volume (Buckner et al. 2004) between groups to ascertain the general impact of HIV on brain volume in our sample. Then, we subdivided the frontal lobes into regions using standard FreeSurfer regions of interest (Desikan et al. 2006) for their cortical thickness measures. We specifically selected the frontal lobe regions as they are functionally connected to both right and left putamen activation during successful inhibition (Zandbelt and Vink 2010). Apart from playing an important role in executive functions, such as response inhibition (Zandbelt and Vink 2010) and working memory [i.e., rostral middle frontal gyrus (Kikinis et al. 2010)], these regions also play an important role in functions associated with motivational control, such as reward processing [i.e., rostral anterior cingulate, lateral orbitofrontal gyrus, medial orbitofrontal gyrus (Hoogendam et al. 2013)].The following frontal regions were included: bilateral rostral anterior cingulate thickness, medial orbitofrontal thickness, pars opercularis thickness, superior frontal thickness, lateral orbitofrontal thickness, and rostral middle frontal thickness. To limit the number of comparisons, we restricted our a priori analysis to these regions. The regions were compared between groups using a two sample t test. All statistical tests were corrected for multiple comparisons using the Bonferroni method.
Stop-signal anticipation fMRI task
During the fMRI experiment, participants performed the STOP-signal anticipation task (Zandbelt and Vink 2010). Not all participants recruited in the present study could be included in a combined functional and structural analysis due to poor structural and/or poor fMRI data quality (see “Demographics” section). The present set of patients, therefore, represent a new subsample of data previously reported on (du Plessis et al. 2015b).
Functional imaging analysis
Image preprocessing
Image data were modeled using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Preprocessing and first-level statistical analyses were performed as previously described (Zandbelt and Vink 2010). In brief, preprocessing involved correction for slice timing differences, realignment to correct for head motion, spatial normalization to the Montreal Neurological Institute template brain, and spatial smoothing to accommodate inter-individual differences in functional and gyral anatomy during inter-subject averaging. Head motion parameters were analyzed to ensure that the maximum motion did not exceed a predefined threshold (scan-to-scan >3 mm) (Van Dijk et al. 2012).
Individual fMRI analyses
The fMRI data were modeled voxel-wise, using a general linear model, in which successful STOP-signal trials and failed STOP-signal trials events were included as regressors. The fMRI data were high-pass filtered (cutoff 128 s) to remove low-frequency drifts. For each participant, we computed a contrast image of activation during successful STOP-signal trials versus failed STOP-signal trials.
Regions of interest analyses
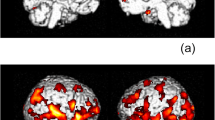
As previously described (du Plessis et al. 2015b), group activation differences in the left and right putamen were determined by a region-of-interest analysis in predefined a priori regions, defined by a previous experiment (Zandbelt and Vink 2010), in which an independent sample of healthy volunteers performed the same task. These ROIs were defined using a cluster-level threshold (cluster-defining threshold p < 0.001, cluster probability of p < 0.05, family-wise error corrected for multiple comparisons). For each participant, the mean activation level (expressed as percent signal change) during the contrasts of interest specific to successful STOP-signal trials versus failed STOP-signal trials was calculated over all the voxels of each ROI.
Regression analysis
Brain regions showing significant cortical thinning were entered into a linear regression model as the dependant variable. Contralateral left putamen activation during successful versus unsuccessful stops was entered as a predictor as it has been previously shown to be primarily associated with right superior frontal lobe activity during inhibition (Zandbelt and Vink 2010). Age, CD4 count, and GDS scores were also included in the model, as they are known to affect cortical thickness (Thompson et al. 2005; Schnack et al. 2015). We did not include subcortical volume measures, as there we no differences between patients and controls. Viral load was also not included, as there were two missing data points. Finally, we did not compare cortical activation with cortical thickness as we found no differences in cortical function between HIV+ participants and controls in our previously reported fMRI results in this cohort (du Plessis et al. 2015b).
Post hoc exploratory correlation analysis
Lastly, we wished to clarify whether the relationship between striatal activation and frontal cortical thinning was specific to these regions or whether it represents part of a more general association between frontal cortical structure and subcortical function. To this end, we performed an uncorrected correlation analyses between all of the FreeSurfer regions known to be related to response inhibition.
Results
Demographics
Twenty-five HIV+ participants and 19 matching controls were initially included in the study. Two HIV+ participants and one control were excluded from the structural analysis due to poor scan quality. Thus, 23 HIV+ participants and 18 healthy controls were included in the final structural analysis (see Table 1). A further six HIV+ participants were excluded from the regression analysis for the following reasons: Tested positive for benzodiazepines (n = 1); uncertainty regarding general medical condition (n = 1); technical problems with the response box (n = 2); poor scan quality on their functional scans (n = 1); and missing data (n = 1).
The groups did not differ with regards to age, gender, educational level or final GDS score (see Table 1). All subjects were from a similar socioeconomic background. All of the HIV+ participants were ambulant and healthy enough to participate in the fMRI task. No intracranial pathology was found on inspection of the MRI scans.
Imaging results
Structural regions of interest analysis
Compared to healthy volunteers, the HIV+ participants showed a smaller total cortical volumes [t(39) = 3.14, p = 0.003], suggestive of general cortical atrophy. However, no differences were found in subcortical volumes between the groups [t(39) = 1.14, p = 0.26]. Specifically, we found no differences in right [t(39) = 0.453, p = 0.653] or left [t(39) = 0.381, p = 0.705] putamen volumes.
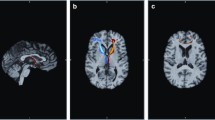
For the cortical thickness measures, the only region of interest to be significantly reduced compared with healthy controls after Bonferrroni correction was the right superior frontal gyrus [t(39) = 3.15, p = 0.003]. At an uncorrected threshold of p = 0.05, we found reduced cortical thickness in patients in the right medial orbitofrontal gyrus [t(39) = 2.56, p = 0.01], left pars opercularis [t(39) = 2.07, p = 0.05], left [t(39) = 1.98, p = 0.05] superior frontal gyrus as well as left [t(39) = 2.62, p = 0.01] rostral and right [t(39) = 2.05, p = 0.05] middle frontal gyrus. This suggests a trend to overall frontal cortical thickness reductions in HIV (see Table 2).
Functional regions of interest analysis
Successful inhibition
We found hypo-activation in the left [t(32) = 2.279, p = 0.029] as well as the right [t(32) = 2.233, p = 0.033] putamen in this particular subsample confirming our results from our previous analysis in the larger cohort (du Plessis et al. 2015b).
Regression analysis
Reduced right superior frontal gyral thickness in the HIV+ group was predicted by a decrease in left putamen BOLD activity (β = 0.381, p = 0.033), as well as by lower GDS score (β = −0.531, p = 0.003), more advanced age (β = −0.381, p = 0.030), and lower CD4 count (β = 0.543, p = 0.004). Together, these predictors accounted for 76.5 % of the total variance [R 2 = 0.765, R 2adjusted = 0.687, F(4,12) = 9.763, p = 0.001] (Fig. 1).
To determine whether striatal activation was related to cortical thickness in the other frontal regions that did not show significant thinning, we also sought correlations between putamen activation and these other regions of interest. We did not correct for multiple comparisons in this additional exploratory analysis. Significant positive correlations were found between task-related putamen BOLD activity during inhibition and cortical thickness in 9 out of our 12 frontal lobe regions of interest for the left and 3 out of 12 for the right putamen (see Table 3).
Discussion
In this study, we investigated the relationship between prefrontal cortical thickness and striatal function in 23 HIV+ participants and 18 matched healthy controls. To the best of our knowledge, this is the first study to explore the relationship between subcortical function and cortical thickness in HIV infection. Our main finding was that decreased striatal activity was associated with reduced prefrontal cortical thickness in a region that is involved in inhibition, i.e., the superior frontal gyrus. Rather than being an isolated association with the superior frontal gyrus, our uncorrected post hoc analyses suggest that this was part of a general trend toward reduced cortical thickness being related to decreased striatal function. While we cannot infer causality from our findings, one possible explanation is that the frontal cortical thinning is a consequence of viral induced striatal hypofunction. This would be consistent with neuropathological evidence suggesting that HIV concentrates primarily in subcortical regions, such as the striatum (Wiley et al. 1998), and would also explain the typical pre-treatment clinical presentation of a subcortical dementia (Navia et al. 1986). However, an alternative explanation that should be kept in mind is that cortical thinning is a consequence of a more global insult of HIV on the brain, and that striatal hypofunction is secondary to this process. Our study confirms previous findings that a decreased CD4 count is associated with reductions in cortical thickness (Stout et al. 1998; Thompson et al. 2005). Also, as with previous functional imaging (Ances et al. 2010), we were able to show that our findings were independent of age-related changes. However, as our sample is relatively young, future work in older populations should further investigate the effects of age on brain function and morphology in HIV infection. Taken together, our results suggest that cortical thinning in HIV infection is likely multifactorial, and that in addition to factors such as immune status, age, and substance abuse, striatal dysfunction may be an important factor to consider.
Our finding of generalized cortical volume reduction in HIV-infected individuals confirms previous studies (Elovaara et al. 1990; Aylward et al. 1993; Thompson et al. 2005; Ances et al. 2010; Dewey et al. 2010). On the other hand, our failure to find evidence of subcortical volume reduction is not consistent with earlier studies reporting subcortical atrophy in HIV (Elovaara et al. 1990; Aylward et al. 1993; Dewey et al. 2010; Ances et al. 2012). However, our results are similar to more recent studies reporting no striatal volume loss (Melrose et al. 2008; Heaps et al. 2012). At face value, these results seem to contradict the long held classification of HIV as primarily a fronto–subcortical neurodegenerative process (Navia et al. 1986). One possible explanation is that the more recent studies included patients identified at an earlier stage of illness. Also, volume reductions due to neuronal damage could possibly be masked by active glial proliferation in these regions, as the striatum is thought to be a site of active viral replication (Wiley et al. 1998).
Our study is limited by a small sample size, which limits our ability to detect more subtle relationships between HIV-inducted striatal function and brain morphology. Also, we only used one functional paradigm, therefore limiting our ability to determine whether functional loss in other cortico–striatal circuits is also related to changes in brain morphology. As our sample included participants who were relatively cognitively intact, we could not effectively investigate the potential interaction between cognitive impairment, subcortical dysfunction, and cortical atrophy. We did, however, find that, when controlling for cognitive performance, the significant relationship between subcortical dysfunction and frontal cortical thickness persisted. Our sample was carefully selected to control for recognized confounding variables. Although representative of the local clinic-going population, we excluded factors such as severe comorbid depression, the elderly, and a history of substance use/abuse. Although this resulted in a relatively homogenous sample, it also limits the generalizability of our findings. Further studies should investigate the role of such comorbidities.
In conclusion, we have shown that striatal function is related to frontal cortical thickness in HIV infection, even in the presence of normal striatal volumes. This effect was found to be independent of factors such as age, cognitive impairment, and CD4 count.
References
Agrawal L, Louboutin J-P, Marusich E et al (2010) Dopaminergic neurotoxicity of HIV-1 gp120: reactive oxygen species as signaling intermediates. Brain Res 1306:116–130. doi:10.1016/j.brainres.2009.09.113
Alexander GE, Crutcher MD (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13:266–271
Amin J, Kaye M, Skidmore S et al (2004) HIV and hepatitis C coinfection within the CAESAR study. HIV Med 5:174–179. doi:10.1111/j.1468-1293.2004.00207.x
An SF, Groves M, Gray F, Scaravilli F (1999) Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J Neuropathol Exp Neurol 58:1156–1162
Ances BM, Vaida F, Yeh MJ et al (2010) HIV infection and aging independently affect brain function as measured by functional magnetic resonance imaging. J Infect Dis 201:336–340. doi:10.1086/649899
Ances BM, Vaida F, Cherner M et al (2011) HIV and chronic methamphetamine dependence affect cerebral blood flow. J Neuroimmune Pharmacol 6:409–419. doi:10.1007/s11481-011-9270-y
Ances BM, Ortega M, Vaida F et al (2012) Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr 59:469–477. doi:10.1097/QAI.0b013e318249db17
Antinori A, Arendt G, Becker JT et al (2007) Updated research nosology for HIV associated neurocognitive disorders. Neurology 69:1789–1799
Aylward EH, Henderer JD, Mcarthur JC et al (1993) Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology 43:2099–2104
Bruce-Keller AJ, Chauhan A, Dimayuga FO et al (2003) Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci 23:8417–8422
Buckner RL, Head D, Parker J et al (2004) A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage 23:724–738. doi:10.1016/j.neuroimage.2004.06.018
Carey CL, Woods SP, Gonzalez R et al (2004) Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 26:307–319. doi:10.1080/13803390490510031
Castellon SA, Hinkin CH, Wood S, Yarema KT (1998) Apathy, depression, and cognitive performance in HIV-1 infection. J Neuropsychiatry Clin Neurosci 10:320–329
Chang L, Andres M, Sadino J et al (2011) Impact of apolipoprotein E ε4 and HIV on cognition and brain atrophy: antagonistic pleiotropy and premature brain aging. Neuroimage 58:1017–1027. doi:10.1016/j.neuroimage.2011.07.010
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–194. doi:10.1006/nimg.1998.0395
Desikan RS, Ségonne F, Fischl B et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. doi:10.1016/j.neuroimage.2006.01.021
Desplats P, Dumaop W, Smith D et al (2013) Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology 80:1415–1423. doi:10.1212/WNL.0b013e31828c2e9e
Dewey J, Hana G, Russell T et al (2010) Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. Neuroimage 51:1334–1344. doi:10.1016/j.neuroimage.2010.03.033
Draganski B, Kherif F, Klöppel S et al (2008) Evidence for Segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci 28:7143–7152. doi:10.1523/JNEUROSCI.1486-08.2008
du Plessis S, Vink M, Joska JA et al (2013) HIV infection and the fronto-striatal system: a systematic review and meta-analysis of fMRI studies. AIDS 28:803–811. doi:10.1097/QAD.0000000000000151
du Plessis S, Vink M, Joska JA et al (2015a) HIV infection results in ventral-striatal reward system hypo-activation during cue processing. AIDS 29:1335–1343. doi:10.1097/QAD.0000000000000680
du Plessis S, Vink M, Joska JA et al (2015b) HIV infection is associated with impaired striatal function during inhibition with normal cortical functioning on functional MRI. J Int Neuropsychol Soc 21:722–731. doi:10.1017/S1355617715000971
Elovaara I, Poutiainen E, Raininko R et al (1990) Mild brain atrophy in early HIV infection: the lack of association with cognitive deficits and HIV-specific intrathecal immune response. J Neurol Sci 99:121–136
Ernst T, Chang L, Jovicich J et al (2002) Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology 59:1343–1349
Grant I (2008) Neurocognitive disturbances in HIV. Int Rev Psychiatry 20:33–47. doi:10.1080/09540260701877894
Hardy DJ, Hinhn CH (2002) Reaction time performance in adults with HIV/AIDS. J Clin Exp Neuropsychol 24:912–929
Heaps J, Niehoff J, Lane E et al (2010) Brain imaging in behavioral medicine and clinical neuroscience. Springer, New York
Heaps JM, Joska J, Hoare J et al (2012) Neuroimaging markers of human immunodeficiency virus infection in South Africa. J Neurovirol 18:151–156. doi:10.1007/s13365-012-0090-5
Heaton RK, Clifford DB, Franklin DR Jr et al (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy. Neurology 75:2087–2095
Hoogendam JM, Kahn RS, Hillegers MHJ et al (2013) Different developmental trajectories for anticipation and receipt of reward during adolescence. Dev Cogn Neurosci 6:113–124. doi:10.1016/j.dcn.2013.08.004
Jernigan TL, Gamst AC, Archibald SL et al (2005) Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry 162:1461–1472. doi:10.1176/appi.ajp.162.8.1461
Joska JA, Westgarth-Taylor J, Myer L et al (2010) Characterization of HIV-associated neurocognitive disorders among individuals starting antiretroviral therapy in south Africa. AIDS Behav 15:1197–1203. doi:10.1007/s10461-010-9744-6
Kellogg SH, McHugh PF, Bell K et al (2003) The Kreek–McHugh–Schluger–Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend 69:137–150
Kikinis Z, Fallon JH, Niznikiewicz M et al (2010) Gray matter volume reduction in rostral middle frontal gyrus in patients with chronic schizophrenia. Schizophr Res 123:153–159. doi:10.1016/j.schres.2010.07.027
Maki PM, Cohen MH, Weber K et al (2009) Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology 72:1661–1668. doi:10.1212/WNL.0b013e3181a55f65
Meade CS, Lowen SB, MacLean RR et al (2011) fMRI brain activation during a delay discounting task in HIV-positive adults with and without cocaine dependence. Psychiatry Res Neuroimaging 192:167–175. doi:10.1016/j.pscychresns.2010.12.011
Melrose RJ, Tinaz S, Castelo JMB et al (2008) Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behav Brain Res 188:337–347. doi:10.1016/j.bbr.2007.11.021
Middleton FA, Strick PL (2002) Basal-ganglia “projections” to the prefrontal cortex of the primate. Cereb Cortex 12:926–935
Moore DJ, Masliah E, Rippeth JD et al (2006) Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS 20:879–887. doi:10.1097/01.aids.0000218552.69834.00
Navia BA, Jordan BD, Price RW (1986) The AIDS dementia complex: I. Clinical features. Ann Neurol 19:517–524. doi:10.1002/ana.410190602
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Pence BW, Gaynes BN, Whetten K et al (2005) Validation of a brief screening instrument for substance abuse and mental illness in HIV-positive patients. J Acquir Immune Defic Syndr 40:434–444
Pfefferbaum A, Rosenbloom MJ, Sassoon SA et al (2012) Regional brain structural dysmorphology in human immunodeficiency virus infection: effects of acquired immune deficiency syndrome, alcoholism, and age. Biol Psychiatry 72:361–370. doi:10.1016/j.biopsych.2012.02.018
Schnack HG, van Haren NEM, Brouwer RM et al (2015) Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cereb Cortex 25:1608–1617. doi:10.1093/cercor/bht357
Sheehan DV, Lecrubier Y, Sheehan KH et al (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl 20:22–33 (quiz 34–57)
Stout JC, Ellis RJ, Jernigan TL et al (1998) Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. HIV Neurobehavioral Research Center Group. Arch Neurol 55:161–168
Thompson PM, Dutton RA, Hayashi KM et al (2005) Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci USA 102:15647–15652. doi:10.1073/pnas.0502548102
van der Kouwe AJW, Benner T, Salat DH, Fischl B (2008) Brain morphometry with multiecho MPRAGE. Neuroimage 40:559–569. doi:10.1016/j.neuroimage.2007.12.025
Van Dijk KRA, Sabuncu MR, Buckner RL (2012) The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59:431–438. doi:10.1016/j.neuroimage.2011.07.044
Wiley CA, Soontornniyomkij V, Radhakrishnan L et al (1998) Distribution of brain HIV load in AIDS. Brain Pathol 8:277–284
Zandbelt BB, Vink M (2010) On the role of the striatum in response inhibition. PLoS One 5:e13848. doi:10.1371/journal.pone.0013848
Acknowledgments
The authors would like to thank Dr Hetta Gouse from the University of Cape Town, as well as Prof Rob Paul from the University of Missouri-St. Louis, for their assistance in determining the neuropsychological assessment battery as well as giving input into the neuropsychological assessments. The authors would also like to thank Mr Teboho Linda from the University of Cape Town for assisting in the recruiting of participants. S.D.P has received support from a National Research Fund International Research Training Grant (IRTG 1522), as well as support from the Medical Research Council of South Africa, Biological Psychiatry Special Interest Group of the South African Society of Psychiatrists as well as the HIV research Trust (HIVRT14-049). M.V and A.B. have reported no biomedical financial interests or potential conflicts of interest. J.J has received support from the Medical Research Council of South Africa. E.K. is supported by an EDCTP Strategic Primary Grant (SP. 2011.41304.065.) as well as a “Verein für Parkinson-Forschung” grant. D.S. has received research grants and/or consultancy honoraria from Abbott, Astrazeneca, Biocodex, Eli-Lilly, GlaxoSmithKline, Jazz Pharmaceuticals, Johnson & Johnson, Lundbeck, Novartis, Orion, Pfizer, Pharmacia, Roche, Servier, Solvay, Sumitomo, Sun, Takeda, Tikvah, and Wyeth. He is supported by the MRC of South Africa. R.E. has participated in speakers/advisory boards and received honoraria from AstraZeneca, Bristol-Myers Squibb, Janssen, Lilly, Lundbeck, Servier and Otsuka. He has also received research funding from Janssen, Lundbeck, and AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
du Plessis, S., Vink, M., Joska, J.A. et al. Prefrontal cortical thinning in HIV infection is associated with impaired striatal functioning. J Neural Transm 123, 643–651 (2016). https://doi.org/10.1007/s00702-016-1571-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-016-1571-0