Abstract
Repetitive transcranial magnetic stimulation (rTMS) enables the local and non-invasive modulation of cortical activity and has proved to achieve antidepressant effects. To a lesser extent, rTMS is investigated as a treatment option for anxiety disorders. As the prefrontal cortex and the amygdala represent key components of human emotion regulation, we investigated how prefrontally applied rTMS affects the responsiveness of the subcortical amygdala during a fear-relevant study paradigm to examine potential cortico-limbic effects. Sham-controlled, randomised inhibitory rTMS (continuous theta burst stimulation, TBS) was applied to 102 healthy subjects (female = 54) over the right dorsolateral prefrontal cortex. Subsequently, the emotion-potentiated (unpleasant, neutral, and pleasant International Affective Picture System pictures) acoustic startle response was investigated. Subjective anxiety ratings (anxiety sensitivity, trait and state anxiety) were considered. Picture category affected the startle magnitude as expected for both TBS intervention groups (highest startle response for unpleasant, lowest for pleasant pictures). However, no modulatory effects of TBS on startle potentiation were discerned. No significant interaction effects of TBS intervention, subjective anxiety ratings, and gender were identified. Interestingly, startle habituation was influenced by TBS intervention on a trend-level, with verum TBS leading to an accelerated habituation. We found no evidence for the hypothesis that prefrontal inhibitory TBS affects the responsiveness of the amygdala during the presentation of emotionally relevant stimuli in healthy subjects. Instead, we found accelerated habituation under verum TBS on a statistical trend-level. Hence, some preliminary hints for modulatory effects of inhibitory TBS on basic learning mechanisms could be found.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A complex neuronal network enables the modulation of human emotions. Important structures—among others—are the amygdala and the prefrontal cortex (PFC; Davidson 2002). The activation of the amygdala plays a key role in the development of fear and anxiety (Davidson 2002; Charney 2003). Anxiety disorders are discussed to be associated with a stronger activation of the amygdala after the presentation of fear-relevant stimuli, compared with healthy controls (Etkin and Wager 2007).
The valence hypothesis states that the processing of negative emotions is located in the right brain hemisphere, whereas positive emotional responses are located in the left hemisphere (Davidson 1998, 2002; Wiedemann et al. 1999). Another approach argues for an inhibitory top-down control of the PFC on the amygdala (Hariri et al. 2000; Hariri et al. 2003; Kim and Whalen 2009). According to this theory, a hyperactivated amygdala, insufficiently inhibited by the prefrontal cortex, is associated with anxiety disorders (Etkin and Wager 2007; Hariri et al. 2003).
The responsiveness of the amygdala can be indexed through an established paradigm—the acoustic startle response (ASR; Dawson et al. 2008). A potentiation of the startle response during fear-inducing conditions could be consistently demonstrated (Davis et al. 1993; Grillon and Davis 1995; Grillon 2008). In addition, altered ASR in baseline amplitude or during emotion-potentiated startle paradigms was found in patients with anxiety disorders (for review, see Grillon 2008; McTeague and Lang 2012).
Via electromagnetic induction, repetitive transcranial magnetic stimulation (rTMS) enables a non-invasive and local modulation of cortical activity (Wassermann and Zimmermann 2012). RTMS can enhance or inhibit local cortical excitability, depending on the frequency of stimulation and the protocol applied (Wassermann and Zimmermann 2012; Huang et al. 2005). In this context, theta burst stimulation (TBS) as an innovative form of rTMS could demonstrate extended after-effects in addition to a more comfortable application compared with conventional rTMS protocols (Huang et al. 2005). RTMS has been able to achieve antidepressant effects in controlled trials (George et al. 2013; Berlim et al. 2013), although also inconsistent results were found (Couturier 2005; Martin et al. 2002). In accordance with the valence hypothesis, activating rTMS over the left PFC has demonstrated moderate antidepressant effects (Garcia-Toro et al. 2006), as has inhibitory rTMS over the right PFC, a finding that, in turn, supports the theory of functional brain asymmetry (Garcia-Toro et al. 2006). Less research has been conducted so far with respect to rTMS effects on anxiety or trauma- and stressor-related disorders, although promising results exist, e.g., for posttraumatic stress disorder or panic disorder (Machado et al. 2012; Zwanzger et al. 2009).
Considering interindividual differences in the ASR, a significantly stronger fear-potentiated startle response was found in subjects reporting high trait fear (Vaidyanathan et al. 2009).
With respect to gender differences, it could be demonstrated that women exhibit significantly greater baseline and emotion-potentiated startle responses than men (Bianchin and Angrilli 2012); however, non-confirming results exist (Hubbard et al. 2011).
In addition, the effects of rTMS seem to be influenced by the degree of anxiety, as demonstrated by Vanderhasselt et al. (2011). It was found that the effects of rTMS on a negative attentional bias were influenced by the baseline state anxiety in 28 healthy female subjects.
To the best of our knowledge, this is the first study that examines whether inhibitory, prefrontally applied TBS influences the emotion-potentiated acoustic startle response in healthy subjects. The effects of TBS were investigated in a sham-controlled, randomised study with a between-subjects design to clarify the link between cortical and limbic structures. The following hypotheses were tested: (1) in accordance with the valence hypothesis, inhibitory rTMS over the right PFC should reduce the emotion-potentiated startle amplitude, while sham stimulation does not affect this phenomenon. (2) The degree of alteration depends on the degree of state and trait anxiety, and gender.
Materials and methods
Participants
A sample of 102 healthy subjects was recruited at the Department of Psychiatry, University of Muenster, between 2011 and 2013. Current or previous diagnosis of DSM-IV axis-I disorders were ruled out using the Mini-International Neuropsychiatric Interview (M.I.N.I, Sheehan et al. 1998). The severity of potential subclinical depressive symptoms was assessed with the Beck Depression Inventory (BDI, Hautzinger et al. 1995; Beck et al. 2013).
To exclude neurological or other somatic disorders, all subjects were interviewed and underwent a neurological and physical examination by a physician prior to the experiment. Additional exclusion criteria were high caffeine consumption (with more than three cups of coffee/tea per day; Andrews et al. 1998), alcohol consumption of more than 140 g per week, daily smoking of more than 10 cigarettes (Duncan et al. 2001), illegal drug consumption, daily intake of any medication (except for hormonal contraception or thyroid hormonal substitution therapy), pregnancy, breastfeeding, history of seizures, tinnitus, age under 18 or over 50 years, metal or magnetic pieces in the head, history of major head trauma or of migraine, hearing loss, and a history of heart or brain diseases. Participants were asked to abstain from caffeine and nicotine on the day of the experiment (Andrews et al. 1998; Duncan et al. 2001). All subjects were naïve to rTMS.
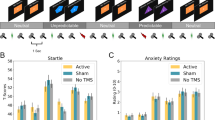
Anxiety sensitivity (AS), meaning the tendency to fear anxiety symptoms, was quantified with the German version of the Anxiety Sensitivity Index-3 (ASI-3, Kemper et al. 2009; Taylor et al. 2007). The extent of state and trait anxiety before the intervention was measured with the State-Trait Anxiety Inventory (STAI; Laux et al. 1981; Spielberger 1989). The study design is shown in Fig. 1.
Study design. ASI-3 Anxiety Sensitivity Index-3, BDI Beck Depression Inventory, cTBS continuous theta burst stimulation, dB decibel, F4 electrode position, International 10–20 system EEG/right dorsolateral prefrontal cortex, IAPS International Affective Picture System, ITI intertrial interval, min minute(s), ms millisecond(s), SAM Self-Assessment-Manikin, STAI State Trait Anxiety Inventory, RMT resting motor threshold, and rTMS repetitive transcranial magnetic stimulation
The procedure was approved by the ethics committee of the medical faculty of the University of Muenster, Germany. All procedures performed in the study were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments. Written informed consent was obtained from all individual participants included in the study.
Theta burst stimulation
The study took place in a double-blind, sham-controlled and between-subjects design. After a screening interview prior to the investigation day, the experiment was carried out from 13:45 to 17:30, or 14:45 to 18:30 h. After physical and neurological examinations ruling out exclusion criteria, self-report anxiety and depression questionnaires were completed. To localise the rTMS stimulation site, electrode position F4 [right dorsolateral prefrontal cortex, (DLPFC)] was identified according to the international 10–20 system for electrode positioning (Jasper 1958; Herwig et al. 2003). Following a baseline recording of the startle magnitude, TBS was applied with a figure-eight coil (MCF-B65, 75 mm outer diameter) with a MagVenture MagPro X100 with Option (MagVenture, Farum, Denmark). Continuous TBS (cTBS) consisted of 200 theta-bursts (5 Hz) and was continuously repeated every 200 ms (600 pulses per session; Huang et al. 2005). It was applied twice within 15 min over the right DLPFC to achieve sufficient aftereffects (Nyffeler et al. 2006, 2009). Active cTBS was applied at 80 % of the resting motor threshold (RMT). The RMT was determined via a visual motor threshold determination of the fingers of the left hand. For this purpose, single TMS pulses over the right primary motor cortex were delivered (Varnava et al. 2011; Pridmore et al. 1998). The RMT was determined this way to allow for an easy, but also reliable application (Varnava et al. 2011; Jung et al. 2010; Rossi et al. 2009). During active cTBS, the coil was held tangentially to the scalp with a 45° angle to the medial sagittal line of the skull, the handle pointing backwards. Sham cTBS was applied identically, but by tilting the coil 90° off the scalp to rule out active stimulation effects (Lisanby and Belmaker 2000).
Safety and tolerance
In general, TBS was well tolerated. No neurological complications occurred. However, one participant (active TBS) discontinued the experiment while receiving rTMS due to pain on the stimulation site and strong muscle contractions of the upper face muscles. The discomforts were transient and vanished quickly after the TBS stop. One participant (sham TBS) discontinued the investigation due to discomfort while watching anxiety-relevant pictures. Again, the discomfort vanished after stopping the experiment.
Outcome parameters: emotion-potentiated startle paradigm and STAI
The startle paradigm here used, including skin preparation, electromyogram (EMG) recording procedures, and affective images, is also described elsewhere (Domschke et al. 2012a, b, 2015; Klauke et al. 2012). Twenty-four threatening unpleasant images were taken from the International Affective Picture System (IAPS, Lang et al. 2005). These pictures were selected as the anxiety-relevant emotional cues. In addition, 24 neutral and 24 pleasant IAPS pictures were chosen. Prior to the TBS intervention, baseline acoustic startle eyeblink magnitude was recorded by presenting 10 auditory stimuli (50-ms, 95-dB white noise with an instantaneous rise-time presented with the help of Bose around-ear headphones) within 6 min (Miller and Gronfier 2006). After the TBS intervention, the emotion-potentiated startle paradigm was started. To reduce outlier startle responses, subjects were first exposed to eight startle stimuli at random intervals of 1–12 s prior to the critical trials. The total procedure consisted of three blocks of 24 unpleasant anxiety-relevant, neutral, and pleasant IAPS pictures. Between each block, 3-min breaks were realised. One experimental block included eight pictures of each valence in a randomised order, with the constraint that no two of the same type (unpleasant, neutral, or pleasant) were presented in succession. Images were shown for 8 s with an inter-trial interval (ITI) of 21 s (mean value; range 16.5–25.5 s). Startle probes were presented 2.5, 4.0, or 5.5 s after picture onset, and also 10 or 12 s after picture offset (during the ITI). In 12.5 % of all trials no startle probes were administered. 75 % of all trials included startle probes during the picture presentation (equally distributed across all valences). In 12.5 % of all trials, startle probes were solely administered during the ITI. Two electrodes were placed under the left eye to quantify the EMG of the musculus orbicularis oculi (Blumenthal et al. 2005). The reference electrode was placed on the forehead, and the ground electrode was placed on the processus mastoideus behind the left ear. The EMG was recorded with a V-Amp 16 (Brain Products GmbH, Gilching, Germany), a 16-channel DC amplifier system using the BrainVision Recorder Software (V-Amp Edition 1.10; Brain Products GmbH). Sampling frequency was set to 1000 Hz, and a notch filter of 50 Hz was used. Pictures and instructions were presented via the software Presentation (Version 13.0; Neurobehavioral Systems, Albany, CA, USA).
To assess subjective anxiety, participants were repeatedly asked to rate their anxiety level by completing the state questionnaire of the STAI (immediately after TBS prior to the emotion-potentiated startle paradigm, directly after completing the emotion-potentiated startle paradigm, cf. Fig. 1).
After the experiment, all electrodes were removed and subjects were asked to rate all pictures by valence (1 = highly pleasant, 9 = highly unpleasant) and arousal (1 = excited, 9 = calm) on the Self-Assessment-Manikin (SAM) scales (Lang 1980). After indicating which TBS intervention they believed they had experienced, all subjects were informed about the real TBS condition and were paid € 50.
Data reduction
To analyse EMG data offline, BrainVision Analyzer 2 (Brain Products GmbH) was used. The signals were rectified, filtered (low cut-off, 28 Hz; high cut off, 499 Hz; notch, 50 Hz), and smoothed (time constant of 50 ms). Startle magnitude was defined as the difference between the highest peak 21–200 ms after and the average during 50 ms before startle probe presentation. For each subject, startle signals were checked for artefacts and zero responses. Startle reactions with no detectable responses (<5 µV) were scored as zero. Artefacts were defined as spontaneous eye blinks during baseline or within 20 ms after startle probe onset, and were scored as missing values. When too many zero responses (more than 2.5 SDs above mean number of zero responses) or less than three sufficient startle responses in one picture category were detected, subjects were excluded from data analysis, as is customary in studies of the human acoustic startle reflex (Blumenthal et al. 2005; Mühlberger et al. 2008; Domschke et al. 2012a; Vaidyanathan et al. 2009). Startle magnitudes were T-transformed within subjects to consider interindividual differences and to achieve comparable data (cf. Blumenthal et al. 2005; Pauli et al. 2010; Berg and Balaban 2008).
Statistical analyses
All analyses were conducted with IBM SPSS Statistics 21. Sample characteristics were analysed by χ 2 tests for gender with TBS intervention (verum vs. sham) as between-subjects factor, and by one-way ANOVAs for age, ASI-3, STAI trait, STAI state prior to TBS intervention and mean startle baseline with TBS intervention, and gender as between-subjects factors. The effects of blinding regarding TBS intervention were analysed using binomial tests (test proportion: 0.5) for the subjective TBS condition in each TBS intervention group, separately.
Picture viewing times (time interval between picture onset and successive ratings) and picture ratings (for valence and arousal) were evaluated with ANOVAs for repeated measures (RM-ANOVAs) with TBS intervention as between-subjects factor and picture category (unpleasant, neutral, and pleasant) as within-subjects factor.
Startle habituation assessed during ITIs was analysed by an RM-ANOVA with measurement time point (12 ITI startle reactions divided into four measurement times, each being the mean of at least two startle responses per measurement time) as within-subjects factor and TBS intervention as between-subjects factor.
Based on the a priori hypotheses, emotion-potentiated startle response was analysed by RM-ANOVA with TBS intervention as between-subjects factor and picture category (unpleasant, neutral, and pleasant) as within-subjects factor.
Further analyses were conducted using gender as an additional between-subjects factor for the above mentioned RM-ANOVA for the emotion-potentiated startle response and startle habituation. In an additional step, ASI-3, STAI state prior to TBS intervention, and STAI trait were considered separately as additional between-subjects factors (median split). State anxiety (STAI) over the course of the experiment and gender were analysed by RM-ANOVA with TBS intervention as between-subjects factors and measurement time point (before TBS intervention; immediately after TBS intervention; directly after emotion-potentiated startle paradigm, cf. Fig. 1) as within-subjects factor.
Post-hoc pairwise comparisons of picture ratings, measurement time, and picture valence (startle modulation) were analysed with paired t tests.
Alpha level was set to p < 0.05, and Greenhouse–Geisser correction was used if appropriate.
Results
Sample characteristics
The participation of two subjects of initially 102 recruited subjects was discontinued (see “Material and methods”). Considering the emotion-potentiated startle response, four subjects showed too many zero startle responses (>41; mean zero responses per subject in the whole group: 7.44, SD 13.34). Therefore, these six subjects were excluded from further analyses. The remaining group of 96 subjects (female = 52) was equally distributed with respect to gender across intervention groups (verum vs. sham; \(\chi_{1}^{ 2}\) = 0.01, p = 0.97). One-way ANOVAs of mean age revealed no differences between intervention groups, or gender (all F 1,95 < 0.60, p > 0.41). One-way ANOVAs of mean baseline anxiety ratings (ASI-3, STAI trait, STAI state I, cf. Fig. 1) displayed no differences between intervention groups, or gender (all F 1,95 < 1.53, p > 0.21; Table 1). Mean ASI-3 was 12.05 (SD 6.76, range 0–33, median 12, N = 96), which was lower than assumed for a healthy sample (mean value of about 19; Kemper et al. 2009).
For the assessment of startle habituation (ITI subsample), 19 additional subjects had to be excluded from analyses due to too many missing responses during at least one measurement time point. The analyses of the sociodemographic characteristics were rerun for this subsample (n = 77), and no significant differences occurred for the variables considered above (cf. Table 1).
Blinding examination: TBS
Thirty-seven participants of the sham group thought that they had been sham stimulated, while 13 subjects assumed that it had been the active intervention. Thirty-three participants in the verum group thought that they had obtained the active protocol, while 13 said they received sham stimulation. For each TBS intervention group, these guesses differed significantly from chance (binomial test; verum group: p = 0.005, verum group: p = 0.001).
Picture ratings and viewing times
The valence of picture ratings conformed to a priori categories (F 2,127 = 2835.93, p < 0.001; linear trend, F 1,94 = 3426.91, p < 0.001; unpleasant < neutral < pleasant; all t 95 > |34.20|, p < 0.001). No interaction or direct effects of the TBS intervention on picture valence were found.
For the arousal ratings, a significant main effect of picture valence was found (F 2,188 = 494.64, p < 0.001; linear trend, F 1,94 = 100.92, p < 0.001). Unpleasant pictures received significantly higher ratings than pleasant pictures. The lowest ratings were given to neutral pictures (all t 95 > |10.1|, p < 0.001). No interaction or direct effects of TBS intervention on the arousal ratings were found.
Picture viewing times were not affected by picture valence or TBS intervention.
Baseline startle response and habituation
Mean baseline startle response prior to TBS intervention did not differ significantly between TBS intervention groups, or gender.
Startle response during the ITIs was significantly influenced by the measurement time (F 3,225 = 31.24, p < 0.001; linear trend, F 1,75 = 70.80, p < 0.001). Moreover, a trendwise interaction of measurement time and TBS intervention was found (F 3,225 = 2.46, p = 0.064). Under verum TBS, mean ITI startle magnitude declined across the first three measurement times (for both, t 35 > 2.40, p ≤ 0.02) with no difference between the third and fourth measurements. In contrast, under sham TBS, mean ITI startle magnitude declined from the first to second measurement time and from the third to fourth measurement time (for both, t 40 > 2.44, p ≤ 0.019) with no significant difference between the second and third measurement times. No main effect of TBS intervention occurred.
Startle modulation: influence of picture valence
A significant main effect of picture category on startle response was found (F 2,162 = 37.13, p < 0.001) with decreasing startle magnitudes from unpleasant to neutral to pleasant pictures (linear trend, F 1,95 = 62.01, p < 0.001; all t 95 > 3.88, p < 0.001, see Fig. 2).
Effects of TBS (verum vs. sham) on startle modulation
No significant interaction effect of TBS intervention and picture category was identified. In addition, no significant main effect of TBS intervention on startle magnitude was found.
Gender effects and anxiety measures
Effects of gender on startle modulation
When using gender as an additional factor, a significant interaction of picture category and gender on the emotion-potentiated startle amplitude was found (F 2,159 = 3.28, p = 0.047). Therefore, post-hoc analyses for both gender groups were conducted separately. For men, no significant difference between unpleasant and neutral pictures occurred, but neutral pictures led to a significantly higher startle amplitude than pleasant pictures (t 43 = 3.26, p = 0.002). In addition, unpleasant pictures caused a significantly higher startle magnitude than pleasant pictures (t 43 = 4.14, p < 0.001). In contrast, for women, mean startle amplitude significantly decreased from unpleasant > neutral > pleasant pictures (for all, t 51 ≥ 2.21, p ≤ 0.03). Furthermore, unpleasant pictures induced a significantly higher startle reaction than pleasant pictures (t 51 = 7.10, p < 0.001).
However, no significant interaction of picture category, gender, and TBS was identified. For the mean ITI startle magnitude, no significant interaction of measurement time and gender or measurement time, gender, and TBS were found.
Additional effects of anxiety ratings and gender on startle modulation
With ASI-3 (median split of ASI-3) as additional between-subjects factor in the analysis of emotion-potentiated startle amplitude, RM-ANOVA showed no significant interaction effects of picture category × ASI-3, or picture category × gender × ASI-3, or picture category × TBS × gender × ASI-3. The same results were found, if STAI state (prior to TBS intervention, median split) or STAI trait (median split) were considered instead of ASI-3.
In addition, no significant interactions of picture category × gender × ASI-3 or picture category × TBS × gender × ASI-3 were found for the mean ITI startle magnitude. This was also the case when STAI state (prior to TBS intervention, median split) or STAI trait (median split) was considered instead of ASI-3.
A significant main effect of measurement time on STAI state ratings was found (F 2,159 = 6.22, p < 0.005) with a significant decrease in ratings from time point 1 (before TBS intervention) to time point 2 (directly after TBS intervention; t 95 = 2.92, p < 0.01) and a significant increase in STAI state ratings from time point 2 to time point 3 (directly after emotion-potentiated startle paradigm; t 95 = −3.89, p < 0.001). No significant effects of TBS intervention or interaction effects with gender on STAI-state ratings were identified.
Discussion
The aim of this sham-controlled, randomised study was to further clarify the role of cortical and limbic structures during emotional processing in healthy subjects. To the best of our knowledge, for the first time, it was examined whether inhibitory TBS, applied to the right DLPFC, could modify the emotion-potentiated acoustic startle response in healthy participants. It was hypothesised that inhibitory TBS over the right DLPFC would reduce the emotion-potentiated startle response. Furthermore, we argued that TBS induced modifications should depend on the degree of state and trait anxiety and gender.
We found a significant emotion-potentiated startle response with a linear increase in startle amplitude during the unpleasant picture category compared with the amplitude during neutral or pleasant pictures. This is in accordance with former results (for a review see Grillon and Baas 2003) and allowed us to investigate our study hypotheses as the emotion-potentiated startle paradigm was effective.
However, in contrast to our first hypothesis, no significant modifications, such as significantly reduced startle amplitudes during unpleasant picture viewing through inhibitory TBS, could be found.
With respect to our second hypothesis, women displayed a significant decrease in startle amplitude from unpleasant to neutral and from neutral to pleasant pictures, whereas for men, no significant differences occurred between unpleasant and neutral images. This is in accordance with results of Bianchin and Angrilli (2012) or Domschke et al. (2012a). In contrast, startle habituation was not affected by gender. Furthermore, no significant interaction effects of TBS intervention and subjective anxiety ratings (trait anxiety, state anxiety, or anxiety sensitivity) on emotion-potentiated startle response or startle habituation were identified. In addition, additional interaction effects of gender were not found.
Beside the two hypotheses considered, in our study, startle habituation (startle responses during the ITIs) seemed to be affected by TBS intervention. Verum TBS was associated with an accelerated decrease in startle amplitude compared with the results of the sham group. As these results were solely found on a statistical trend-level, they have to be considered with reasonable caution. However, it could be worthwhile to consider these results as they might help to deepen the understanding of cortico-limbic associations and brain hemispheric distributions. Startle habituation, defined as a reduction of ASR magnitude during the repeated presentation of a startle stimulus, is not considered to be caused by fatigue or a blunting of sensory receptor responsiveness (Bradley et al. 2008), and is seen as a form of non-associative learning. For instance, the organism has learned that an uncomfortable tone is not followed by a negative consequence. From a broader perspective and with regard to psychotherapy, habituation is discussed as one possible mechanism of action during successful exposure therapy, where pathological anxiety symptoms decline over time (Mineka and Cannon 1999). In line with the valence hypothesis, threat perception and modulation are commonly related to the right hemisphere, including the PFC, the anterior cingulate, or the amygdala (for a review, see Nitschke and Heller 2005). One might speculate that the acoustic ITI-startles of white noise were already threat-relevant, leading to an increased right hemispheric activation. Active inhibitory TBS could probably reduce this enhanced activation and thus led to a faster habituation course, with a faster learning that startle probes were not followed by a negative consequence. Interestingly, De Raedt et al. (2010) demonstrated that facilitating rTMS over the right DLPFC led to an impaired disengagement from angry faces and was associated with an enhanced activation of the right amygdala. Here, one might argue that right hemispheric activity was artificially augmented by rTMS and led to a stronger processing of threat-relevant stimuli. The modified habituation in our study and the results of De Raedt et al. are contradictory to theories which focus on cortical top-down mechanisms for emotion regulation. However, in line with cortical top-down mechanisms, Baeken et al. (2010) demonstrated that right prefrontal HF-rTMS attenuated right amygdala processing of negatively valenced emotional stimuli in healthy females.
Furthermore, it remains unclear why the emotion-potentiated startle response was not affected by inhibitory TBS, in contrast to startle habituation, modulated on a trend-level. One might speculate that distinct mechanisms were involved. Although the DLPFC (rTMS stimulation site in our study) could be found to be activated during explicit emotion regulation tasks (cf. Mitchell 2011), it comprises comparatively few direct connections to the amygdala (Ghashghaei et al. 2007). More recently, other structures like the ventrolateral prefrontal cortex are discussed to be key structures in emotion regulation (for a review, see Mitchell 2011) and might also represent alternative stimulation sites in the treatment of depression (Downar and Daskalakis 2013).
Worthy of note, modulatory effects of gender on the emotion-potentiated startle response were not found for startle habituation. Perhaps, these diverging reaction patterns between men and women overlayed the statistical power with respect to the TBS intervention. In sum, we found hints for an accelerated startle habituation in the verum group, but the result was not significant and has to be considered with caution.
Despite strengths of our study (sample balanced for gender, pilot study examining prefrontal rTMS effects on emotion-potentiated startle response, TBS as an innovative rTMS protocol), limitations have to be mentioned. The majority of participants correctly guessed the applied TBS condition. Hence, we cannot rule out possible placebo effects. However, as a subcortical variable, the startle response seems to be less influenced by intentional processes (Grillon 2008; Grillon and Baas 2003), which is why our results can still be considered as meaningful. We chose a between-subjects study design to prevent our results from potentially disruptive effects caused by a marked habituation on startle amplitude, which may be the case during repeated presentations of the emotion-potentiated startle paradigm. However, this may have reduced the statistical power to detect TBS effects as within-subjects comparisons (verum vs. sham) were not possible. Another important fact could comprise the relatively low mean anxiety sensitivity of our participants, which was lower than reported for German samples (Kemper et al. 2009). This circumstance probably affected the anxiety ratings and might have had a bearing on the non-existent TBS effects on self-rated anxiety outcomes. Furthermore, one could argue that two single inhibitory TBS sessions within 15 min do not reveal sufficiently powerful or long-lasting effects on the emotion-potentiated startle reflex. However, we based our design on studies, demonstrating inhibitory TBS effects of several hours (Nyffeler et al. 2006, 2009). We additionally examined the temporal progress in our data, but still did not find TBS effects on the emotion-potentiated startle response, e.g. for the first or second half of the experiment. Under these circumstances, one might question whether inhibitory TBS of the right PFC is generally capable to modify cortico-limbic connections and emotional processing. Indeed, in a functional near infrared study of healthy subjects, Tupak et al. (2013) demonstrated that inhibitory TBS over the right DLPFC, in contrast to inhibitory TBS over the left DLPFC, did not influence prefrontal oxygenation. On the other hand, inhibitory TBS of the right DLPFC led to significant effects in terms of a changed impulsivity level of healthy subjects (Cho et al. 2010). One aspect that could account for these inconsistent effects might be the initial state of the stimulated brain region. Indeed, state-dependent rTMS effects were described for the perceptional system of vision or motion (for a review, see Silvanto and Pascual-Leone 2008). In the reported studies, the initial state was manipulated by either adaptation or priming of the neuronal network (Silvanto and Pascual-Leone 2008). Furthermore, Perini et al. (2012) could demonstrate state-dependent effects of TMS in six healthy subjects, where visual contrast sensitivity was decreased via TMS in the absence of physiological adaptation but increased if adaptation existed. With respect to the prefrontal cortex, Weigand et al. (2013) investigated whether priming via transcranial direct current stimulation (tDCS) followed by low-frequency rTMS over the right DLPFC affected the emotional working memory. Whereas no general state-dependent effects of rTMS on working memory were discerned, for anger-related stimuli, differential effects of rTMS on task accuracy dependent on the tDCS condition were found, however, on a statistical trend-level.
In conclusion, similar future studies could focus on the left DLPFC (cf. Tupak et al. 2013), alternative stimulation sites like the ventrolateral prefrontal cortex, but also a standardized priming of the neural network via tDCS (cf. Weigand et al. 2013) to enable stronger and more consistent TBS effects on the emotion-potentiated ASR. Alternatively, instead of inhibitory cTBS investigated in our study, facilitatory intermittent TBS (iTBS, Huang et al. 2005) could be applied over the right DLPFC. Considering the results of Baeken et al. (2010), iTBS might enhance the top-down control of emotion regulation and thus could lead to reduced startle amplitudes during unpleasant picture viewing. Finally, a considerable number of studies investigating the effects of prefrontal TBS have been published since 2005. Often, altered TBS-protocols were applied (e.g. Gamboa et al. 2010; Nyffeler et al. 2006). To the best of our knowledge, so far, only systematic reviews for TBS effects on motor cortex areas were published (e.g. Suppa et al. 2016, in press). To deepen the understanding of optimal effects of prefrontal TBS, it might be helpful to address these questions in a systematic review or meta-analysis.
Taken together, in this pilot study, no modulatory effects of inhibitory TBS on the emotion-potentiated startle response were found. In addition, subjective anxiety ratings did not modulate these results. Consistent with previous findings, the emotion-potentiated startle response was influenced by gender. Furthermore, we found some preliminary hints for modulatory TBS effects on startle habituation with an accelerated habituation under verum TBS.
References
Andrews SE, Blumenthal TD, Flaten MA (1998) Effects of caffeine and caffeine-associated stimuli on the human startle eyeblink reflex. Pharmacol Biochem Behav 59:39–44
Baeken C, De Raedt R, Van Schuerbeek P et al (2010) Right prefrontal HF-rTMS attenuates right amygdala processing of negatively valenced emotional stimuli in healthy females. Behav Brain Res 214:450–455. doi:10.1016/j.bbr.2010.06.029
Beck AT, Brown GK, Steer RA (2013) Beck-Depressions-Inventar-FS (BDI-FS). Manual. Deutsche Bearbeitung von Sören Kliem & Elmar Brähler. Pearson, Frankfurt am Main
Berg K, Balaban MT (2008) Startle elicitation: stimulus parameters, recording techniques, and quantification. In: Dawson ME, Schell AM, Bohmelt AH (eds) Startle modification implications for neuroscience, cognitive science, and clinical science, 2nd edn. Cambridge University Press, Cambridge, pp 21–50
Berlim MT, Van den Eynde F, Daskalakis ZJ (2013) High-frequency repetitive transcranial magnetic stimulation accelerates and enhances the clinical response to antidepressants in major depression: a meta-analysis of randomized, double-blind, and sham-controlled trials. J Clin Psychiatry 74:e122–e129. doi:10.4088/JCP.12r07996
Bianchin M, Angrilli A (2012) Gender differences in emotional responses: a psychophysiological study. Physiol Behav 105:925–932. doi:10.1016/j.physbeh.2011.10.031
Blumenthal TD, Cuthbert BN, Filion DL et al (2005) Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology 42:1–15. doi:10.1111/j.1469-8986.2005.00271.x
Bradley MM, Cuthbert BN, Lang PJ (2008) Affect and the startle reflex. In: Dawson ME, Schell AM, Bohmelt AH (eds) Startle modification implications for neuroscience, cognitive science, and clinical science, 2nd edn. Cambridge University Press, Cambridge, pp 157–186
Charney DS (2003) Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand Suppl 108:38–50
Cho SS, Ko JH, Pellecchia G et al (2010) Continuous theta burst stimulation of right dorsolateral prefrontal cortex induces changes in impulsivity level. Brain Stimul 3:170–176. doi:10.1016/j.brs.2009.10.002
Couturier JL (2005) Efficacy of rapid-rate repetitive transcranial magnetic stimulation in the treatment of depression: a systematic review and meta-analysis. J Psychiatry Neurosci 30:83–90
Davidson RJ (1998) Affective style and affective disorders: perspectives from affective neuroscience. Cogn Emot 12:307–331
Davidson RJ (2002) Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry 51:68–80
Davis M, Falls W, Campeau S, Kim M (1993) Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res 58:175–198
Dawson ME, Schell AM, Bohmelt AH (2008) Startle modification implications for neuroscience, cognitive science, and clinical science, 2nd edn. Cambridge University Press, Cambridge
De Raedt R, Leyman L, Baeken C et al (2010) Neurocognitive effects of HF-rTMS over the dorsolateral prefrontal cortex on the attentional processing of emotional information in healthy women: an event-related fMRI study. Biol Psychol 85:487–495. doi:10.1016/j.biopsycho.2010.09.015
Domschke K, Gajewska A, Winter B et al (2012a) ADORA2A Gene variation, caffeine, and emotional processing: a multi-level interaction on startle reflex. Neuropsychopharmacology 37:759–769. doi:10.1038/npp.2011.253
Domschke K, Klauke B, Winter B et al (2012b) Modification of caffeine effects on the affect-modulated startle by neuropeptide S receptor gene variation. Psychopharmacology 222:533–541
Domschke K, Winter B, Gajewska A et al (2015) Multilevel impact of the dopamine system on the emotion-potentiated startle reflex. Psychopharmacology 232:1983–1993
Downar J, Daskalakis ZJ (2013) New targets for rTMS in depression: a review of convergent evidence. Brain Stimul 6:231–240. doi:10.1016/j.brs.2012.08.006
Duncan E, Madonick S, Chakravorty S et al (2001) Effects of smoking on acoustic startle and prepulse inhibition in humans. Psychopharmacology 156:266–272
Etkin A, Wager T (2007) Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, Social anxiety disorder, and specific phobia. Am J Psychiatry 164:1476–1488
Gamboa OL, Antal A, Moliadze V, Paulus W (2010) Simply longer is not better: reversal of theta burst after-effect with prolonged stimulation. Exp Brain Res 204:181–187. doi:10.1007/s00221-010-2293-4
Garcia-Toro M, Salva J, Daumal J et al (2006) High (20-Hz) and low (1-Hz) frequency transcranial magnetic stimulation as adjuvant treatment in medication-resistant depression. Psychiatry Res 146:53–57. doi:10.1016/j.pscychresns.2004.08.005
George MS, Taylor JJ, Short EB (2013) The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry 26:13–18. doi:10.1097/YCO.0b013e32835ab46d
Ghashghaei HT, Hilgetag CC, Barbas H (2007) Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage 34:905–923. doi:10.1016/j.neuroimage.2006.09.046
Grillon C (2008) Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology 199:421–437. doi:10.1007/s00213-007-1019-1
Grillon C, Baas J (2003) A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol 114:1557–1579. doi:10.1016/S1388-2457(03)00202-5
Grillon C, Davis M (1995) Acoustic startle and anticipatory anxiety in humans: effects of monaural right and left ear stimulation. Psychophysiology 32:155–161
Hariri AR, Bookheimer SY, Mazziotta JC (2000) Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport 11:43–48
Hariri AR, Mattay VS, Tessitore A et al (2003) Neocortical Modulation of the amygdala response to fearful stimuli. Biol Psychiatry 53:494–501. doi:10.1016/S0002-3223(03)01786-9
Hautzinger M, Bailer M, Worall H, Keller F (1995) Beck-Depressions-Inventar (BDI). Testhandbuch, 2nd edn. Huber, Bern
Herwig U, Satrapi P, Schönfeldt-Lecuona C (2003) Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr 16:95–99
Huang Y-Z, Edwards MJ, Rounis E et al (2005) Theta burst stimulation of the human motor cortex. Neuron 45:201–206. doi:10.1016/j.neuron.2004.12.033
Hubbard CS, Ornitz E, Gaspar JX et al (2011) Modulation of nociceptive and acoustic startle responses to an unpredictable threat in men and women. Pain 152:1632–1640. doi:10.1016/j.pain.2011.03.001
Jasper H (1958) The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol 10:371–375
Jung NH, Delvendahl I, Kuhnke NG et al (2010) Navigated transcranial magnetic stimulation does not decrease the variability of motor-evoked potentials. Brain Stimul 3:87–94. doi:10.1016/j.brs.2009.10.003
Kemper CJ, Ziegler M, Taylor S (2009) Überprüfung der psychometrischen Qualität der deutschen Version des Angstsensitivitätsindex-3. Diagnostica 55:223–233. doi:10.1026/0012-1924.55.4.223
Kim MJ, Whalen PJ (2009) The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci 29:11614–11618. doi:10.1523/JNEUROSCI.2335-09.2009
Klauke B, Winter B, Gajewska A et al (2012) Affect-modulated startle: interactive influence of catechol-O-methyltransferase Val158Met genotype and childhood trauma. PLoS One 7:e39709
Lang PJ (1980) Behavioral treatment and bio-behavioral assessment: computer applications. In: Sidowski JB, Johnson JH, Williams TA (eds) Technology in Mental Health Care Delivery Systems Ablex, Norwood, pp 119–137
Lang PJ, Bradley MM, Cuthbert BN (2005) International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical Report A-6. University of Florida, Gainesville
Laux L, Glanzmann P, Schaffner P, Spielberger CD (1981) STAI: State-Trait-Angstinventar. Beltz Test GmbH, Göttingen
Lisanby SH, Belmaker RH (2000) Animal models of the mechanisms of action of repetitive transcranial magnetic stimulation (RTMS): comparisons with electroconvulsive shock (ECS). Depress Anxiety 12:178–187. doi:10.1002/1520-6394(2000)12:3<178:AID-DA10>3.0.CO;2-N
Machado S, Paes F, Velasques B et al (2012) Is rTMS an effective therapeutic strategy that can be used to treat anxiety disorders? Neuropharmacology 62:125–134. doi:10.1016/j.neuropharm.2011.07.024
Martin JL, Barbanoj MJ, Schlaepfer TE et al (2002) Transcranial magnetic stimulation for treating depression. Cochrane database Syst Rev. doi:10.1002/14651858.CD003493
McTeague LM, Lang PJ (2012) The anxiety spectrum and the reflex physiology of defense: from circumscribed fear to broad distress. Depress Anxiety 29:264–281. doi:10.1002/da.21891
Miller MW, Gronfier C (2006) Diurnal variation of the startle reflex in relation to HPA-axis activity in humans. Psychophysiology 43:297–301. doi:10.1111/j.1469-8986.2006.00400.x
Mineka S, Cannon T (1999) Mechanisms of change in exposure therapy for anxiety disorders. In: Dalgleish T, Power MJ (eds) Handbook of cognition and emotion. Wiley, New York, pp 747–764
Mitchell DGV (2011) The nexus between decision making and emotion regulation: a review of convergent neurocognitive substrates. Behav Brain Res 217:215–231. doi:10.1016/j.bbr.2010.10.030
Mühlberger A, Wieser MJ, Pauli P (2008) Darkness-enhanced startle responses in ecologically valid environments: a virtual tunnel driving experiment. Biol Psychol 77:47–52
Nitschke JB, Heller W (2005) Distinguishing neural substrates of heterogeneity among anxiety disorders. Int Rev Neurobiol 67:1–42. doi:10.1016/S0074-7742(05)67001-8
Nyffeler T, Wurtz P, Lüscher H-R et al (2006) Extending lifetime of plastic changes in the human brain. Eur J Neurosci 24:2961–2966. doi:10.1111/j.1460-9568.2006.05154.x
Nyffeler T, Cazzoli D, Hess CW, Müri RM (2009) One session of repeated parietal theta burst stimulation trains induces long-lasting improvement of visual neglect. Stroke 40:2791–2796. doi:10.1161/STROKEAHA.109.552323
Pauli P, Conzelmann A, Mucha RF et al (2010) Affect-modulated startle reflex and dopamine D4 receptor gene variation. Psychophysiology 47:25–33. doi:10.1111/j.1469-8986.2009.00923.x
Perini F, Cattaneo L, Carrasco M, Schwarzbach JV (2012) Occipital transcranial magnetic stimulation has an activity-dependent suppressive effect. J Neurosci 32:12361–12365. doi:10.1523/JNEUROSCI.5864-11.2012
Pridmore S, Fernandes Filho JA, Nahas Z et al (1998) Motor threshold in transcranial magnetic stimulation: a comparison of a neurophysiological method and a visualization of movement method. J ECT 14:25–27
Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2009) Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008–2039. doi:10.1016/j.clinph.2009.08.016
Sheehan DV, Lecrubier Y, Sheehan KH et al (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 2):22–33 (quiz 34–57)
Silvanto J, Pascual-Leone A (2008) State-dependency of transcranial magnetic stimulation. Brain Topogr 21:1–10. doi:10.1007/s10548-008-0067-0
Spielberger CD (1989) State-trait anxiety inventory: bibliography, 2nd edn. Consulting Psychologists Press, Palo Alto
Suppa A, Huang YZ, Funke K, et al (2016) Ten years of theta burst stimulation in humans: established knowledge, unknowns and prospects. Brain Stimul. doi:10.1016/j.brs.2016.01.006 (in press)
Taylor S, Zvolensky MJ, Cox BJ et al (2007) Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychol Assess 19:176–188. doi:10.1037/1040-3590.19.2.176
Tupak SV, Dresler T, Badewien M et al (2013) Inhibitory transcranial magnetic theta burst stimulation attenuates prefrontal cortex oxygenation. Hum Brain Mapp 34:150–157. doi:10.1002/hbm.21421
Vaidyanathan U, Patrick CJ, Bernat EM (2009) Startle reflex potentiation during aversive picture viewing as an indicator of trait fear. Psychophysiology 46:75–85. doi:10.1111/j.1469-8986.2008.00751.x
Vanderhasselt M-A, Baeken C, Hendricks M, De Raedt R (2011) The effects of high frequency rTMS on negative attentional bias are influenced by baseline state anxiety. Neuropsychologia 49:1824–1830. doi:10.1016/j.neuropsychologia.2011.03.006
Varnava A, Stokes MG, Chambers CD (2011) Reliability of the “observation of movement” method for determining motor threshold using transcranial magnetic stimulation. J Neurosci Methods 201:327–332. doi:10.1016/j.jneumeth.2011.08.016
Wassermann EM, Zimmermann T (2012) Transcranial magnetic brain stimulation: therapeutic promises and scientific gaps. Pharmacol Ther 133:98–107. doi:10.1016/j.pharmthera.2011.09.003
Weigand A, Richtermeier A, Feeser M et al (2013) State-dependent effects of prefrontal repetitive transcranial magnetic stimulation on emotional working memory. Brain Stimul 6:905–912. doi:10.1016/j.brs.2013.06.004
Wiedemann G, Pauli P, Dengler W et al (1999) Frontal brain asymmetry as a biological substrate of emotions in patients with panic disorders. Arch Gen Psychiatry 56:78–84
Zwanzger P, Fallgatter AJ, Zavorotnyy M, Padberg F (2009) Anxiolytic effects of transcranial magnetic stimulation–an alternative treatment option in anxiety disorders? J Neural Transm 116:767–775. doi:10.1007/s00702-008-0162-0
Acknowledgments
This work was supported by the fund “Innovative Medical Research” of the University of Muenster Medical School (Project ZW 211105), and the SFB-TRR58, project C02 (Deutsche Forschungsgemeinschaft, DFG). We acknowledge the support by Ann-Christine Robertz and Fred Rist.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by the fund “Innovative Medical Research” of the University of Muenster Medical School (Project ZW 211105), and the SFB-TRR58, project C02 (Deutsche Forschungsgemeinschaft, DFG).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Vennewald, N., Winter, B., Limburg, K. et al. Emotional processing and rTMS: does inhibitory theta burst stimulation affect the human startle reflex?. J Neural Transm 123, 1121–1131 (2016). https://doi.org/10.1007/s00702-016-1568-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-016-1568-8