Abstract
Patients with early Parkinson’s disease (PD) may not complain of gait difficulties but subtle gait abnormalities may be revealed as part of a “preclinical gait syndrome” when they are challenged by dual tasks. 21 early PD patients (n = 21, mean age 63.5 years, H&Y 1.62, disease duration <5 years, mean UPDRS-III 7.7) who did not have gait complaints were as compared to age- and gender-matched healthy controls (n = 21). Memory function was not different between the two groups. Under normal walking conditions, there were no significant differences in gait parameters between the patients and the control group. In both groups, normalized gait velocity decreased in response to dual tasking in a parallel fashion (p < 0.001). Similarly, gait variability increased in both groups with dual tasking although not statistically significant. In PD patients, the performance of an additional task resulted in an increased number of cadences (p = 0.04), a reduction in swing time (p = 0.02) and cycle time (p = 0.04) compared with the control group but there was no significant reduction in normalized velocity. Stride width also increased in the PD patients. The addition of a cognitive task may affect certain aspects of gait and is able to elicit subclinical deficits in early PD patients. In an attempt to maintain velocity, early PD patients develop compensatory mechanisms by increasing cadence and decreasing swing time and cycle time. Increased step width helps support balance, and prevents going beyond the base-of-support which may predispose to unsteadiness and falls. We propose that these findings occur as part of a spectrum of a “preclinical gait syndrome” and longitudinal studies are needed to assess the predictive values of these early markers of gait deficits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The United Kingdom Parkinson’s Disease Society Brain Bank (UKPDSBB) clinical diagnostic criteria identify postural instability as one of the main features in the diagnosis of a parkinsonian syndrome (Gibb and Lees 1988). However, most patients with newly diagnosed or early Parkinson’s disease (PD) seldom complain of any gait difficulties and most physicians interpret early postural instability or falls as a suggestive feature of atypical parkinsonian syndromes (Gilman et al. 2008; Litvan et al. 1996). The current emphasis of gait dysfunction still focuses on patients with at least moderate severity as reflected by Hoehn and Yahr (H&Y) in their original and modified motor staging of PD with some degree of postural instability defining H&Y stage 2.5–3 and gait disturbances for H&Y stage 4 (Goetz et al. 2004; Hoehn and Yahr 1967). However, recent evidence suggests that gait and postural abnormalities which may be subtle can develop in PD patients even at an earlier stage (Baltadjieva et al. 2006; Carpinella et al. 2007; Chastan et al. 2008; Lewek et al. 2010; Yang et al. 2008).
Gait disturbances, although subtle in the early stages, will eventually affect all patients as the disease progresses. Among all symptoms of PD, gait and postural symptoms evolve more rapidly than other motor features of PD and appear to be the best index of disease progression (Evans et al. 2011). Not surprisingly, gait disturbances can lead to falls, fear, loss of mobilization, independence, and have a significant impact on quality of life (Evans et al. 2011). Although the best predictor of falling is two or more falls in the previous year (Pickering et al. 2007), an attempt to intervene should be made even at the stages before PD patients begin to fall. Therefore, early detection of gait dysfunction at the earliest stage to identify possible “preclinical gait syndrome” in PD may provide the basis for early interventions and thereby prevent or delay the risk of falling, and maintain independent mobility for as long as possible.
Not much information is available on gait dysfunction in early PD. In a study involving de novo patients with H&Y between 1 and 2.5, patients walked more slowly with reduced swing times and increased left/right swing asymmetry, when compared with age- and sex-matched healthy controls (Baltadjieva et al. 2006). Although a number of studies involving quantitative gait analysis in early PD patients demonstrated altered gait patterns, including arm swing asymmetry, greater postural sway, reduction of walking speed and prolonged period of gait initiation(Carpinella et al. 2007; Chastan et al. 2008; Ferrarin et al. 2006; Fioretti et al. 2004; Geurts et al. 2011; Lewek et al. 2010; Yang et al. 2008), only a few studies have examined the effects of cognitive challenges on gait dynamics in early PD patients (Plotnik et al. 2009; Yogev et al. 2005). Recent evidence indicates that cognitive loading while walking or balancing can lead to marked deterioration in postural performance, and there is some evidence to suggest that such “dual tasking” is particularly difficult for elderly patients with dementia or depression. However, this type of study has not focused on PD patients at the early stages with the intention of identifying indicators for early interventions (Bloem et al. 2006; Yogev-Seligmann et al. 2008). Therefore, our study was designed to test patients in the early stages of PD, who did not have axial involvement clinically or any gait complaints. They were cognitively normal and were tested by performing dual tasks to bring out subtle gait abnormalities which may further provide a basis for early interventions in this group of patients.
Patients and methods
Subjects
Twenty-one patients with idiopathic PD, as defined by the UKPDSBB clinical diagnostic criteria (Gibb and Lees 1988) were recruited from the Outpatient Clinic of the Chulalongkorn Center of Excellence on Parkinson’s Disease and Related Disorders (CUPD) between June 2010 and January 2012. Patients were invited to participate if their disease stage was 1–2 on the modified H&Y scale (Goetz et al. 2004), the disease duration was within 5 years since the first noticeable symptoms which led to the diagnosis of PD, and they did not experience motor complications as determined by “0” scoring on the sections A and B of the fourth part of the Unified Parkinson’s Disease Rating Scale (UPDRS-IV). Moreover, subjects must have no complaints of gait or postural instability as demonstrated by “0” scoring on item 13–15 of the second part of the UPDRS. No lower limbs tremor was observed in the patient group as they all scored “0” on item 20 (lower extremities) of the third part of the UPDRS. Medication usage was not altered. All patients were assessed in the morning, at least 12 h after they took their last antiparkinsonian medications. Subjects were excluded if they had clinically significant musculoskeletal disease, cardiovascular disease, respiratory disease, other neurological disease, depression, or uncorrected visual disturbances which may limit their ability to walk optimally. None of the patients were taking sedatives. Subjects were excluded if they scored less than 25 on the validated Thai version of the Mini-Mental State Examination (Thai MMSE) (Folstein et al. 1975) or if they had major depression as defined by DSM-IV criteria. None of the patients reported falls in the past 6 months.
The PD patients were compared to 21 age- and sex-matched healthy control subjects. Controls were recruited from several sources in the community, i.e. patient’s spouses, volunteers from the Chulalongkorn University Hospital, the Thai Red Cross Society and the National Blood Bank Center. Control subjects fulfilled the same inclusion and exclusion criteria as the patients with PD and neurological examinations did not detect any abnormalities. The sample size was based on the study by Yang et al. (2008) The study was approved by the Human Ethics Committee of the Faculty of Medicine of Chulalongkorn University. All subjects gave their written informed consent before entering the study in accordance with the declaration of Helsinki.
Procedures
Each participant’s gait performance was assessed using an electronic walkway system (GAITRite®) with a single and a dual task requirement. Three trials per condition were found in the power analysis to be the optimum number of trials needed to obtain enough number of strides to be able to compute reliability assessments for the quantitative gait variables of interest (Donner and Eliasziw 1987).
The GAITRite® system includes a portable electronic walkway mat (782 cm in length and 61 cm in width) for the automated measurement of spatiotemporal gait parameters. The mat was located in a well-lit, 12-m long hallway with starting and ending limits marked one meter from the mat to avoid recording acceleration and deceleration phases. As participants walked along the mat, imbedded sensors were activated by the foot pressure and were deactivated when the pressure is released. A computer processed the footsteps, providing data for both spatial and temporal parameters. To ensure that gait parameters were collected during steady-state walking, participants started walking at least 2 m before reaching the electronic walkway and completed their walk at least 2 m beyond it (Kressig and Beauchet 2006). The following 10 gait variables were selected based on their clinical relevance and their reported association with dual tasking in previous studies: gait velocity (cm/s), cadence (steps/min), step length (cm), stride length (cm), step time (s), cycle time (s), swing time (s), double support time (s), and stride width (cm). The velocity was normalized to leg length in all subjects. Gait parameters were recorded using only the footprint of the participants, thereby eliminating the need for external sensors attached to the body or lower limbs which may interfere with the gait performance.
To quantify gait variability under both single and dual task instructions, the coefficient of variation (CoV = SD/mean × 100) of each gait variable was calculated at each time point. The CoV assessed the magnitude of the deviations of the stride time with respect to each subject’s mean value. Stride time variability was considered as a marker for the control of limb-coordinated movements (Hausdorff 2007) while the swing time variability is a measure of dynamic balance that is not influenced by gait speed (Frenkel-Toledo et al. 2005). Stride width variability was also assessed as it may relate to fall risk (Hausdorff 2005).
We also evaluated the Functional Ambulation Performance (FAP) scoring of both groups. The FAP score consists of the linear relationship of step length/leg length ratio to step time when the velocity is “normalized” to leg length in healthy adults and it provides a quantitative means of assessing gait without the subjective qualification that most rating scales require (Nelson 1974). The FAP score is a valid, reliable, and objective method of measuring various gait parameters in both the control group and the PD patients (Nelson et al. 2002).
Assessment of gait and dual tasking
Gait was evaluated during a single task of normal walking and when doing an additional arithmetic task. During the arithmetic task, subjects walked while reciting out loud serial subtractions of seven, starting from a three-digit number (e.g. 200, 193, 186). Subjects were requested to walk at their normal pace under each of the two sets of tasks. No instructions were given for prioritizing one of the tasks, walking or counting, as more important than the other. The order of the tasks was as follows: walking as a single task and walking while subtracting serial 7’s. Evaluation of performance on serial 7 subtractions included the number of mistakes they made during the calculation. Serial 7 subtractions have been widely used as means of providing a distraction and a cognitive challenge and the attention devoted to serial subtractions is not likely to change over time during a given test (Ganguli et al. 1990; Karzmark 2000; Yogev-Seligmann et al. 2008).
Data acquisition of the quantitative gait variables
GAITRite® software version 3.95 (CIR Systems, Inc., New Jersey) was used to process footstep data using the settings for light and short footsteps because individuals with PD may be more likely to slow down or hesitate while doing two tasks simultaneously. If a participant’s first and last footstep did not fall completely within the active area of the walkway, these footsteps were manually removed from the recorded walk. Further, to minimize environmental variability, evaluations were conducted on the same weekday (±1 day) and in the morning with participants instructed to wear the same pair of shoes for both sessions.
Statistical analysis
Baseline characteristics and gait parameters were summarized using either means and standard deviations, or frequencies and percentages as appropriate. For each gait parameter and for both conditions, the mean of the three trials was used in the analysis. The paired t test was used to compare the results from normal walking with those from walking while doing the serial 7 subtraction task. A p < 0.05 (2-tailed) was considered statistically significant. Statistical analysis was performed using SPSS version 14.0 software (SPSS Inc., Chicago IL).
Results
Demographic and medical characteristics are summarized in Table 1. In the patient group, they were nine males and 12 females, with a mean age of 63.52 (SD = 9.41). Among the PD patient group, ten subjects were in H&Y stage 1 and eleven were in disease stage 2. The majority of patients (61.9 %) were a tremor predominant type with a mean disease duration of 2.77 years (SD = 1.67) and a relatively low mean score (7.71; SD = 3.9) on the motor section of the UPDRS (UPDRS-III) representing an early stage of PD. The mean levodopa equivalent dose (LED) was 396 mg (SD = 233). The relatively high LED, despite the fact that they were all in the early stage of the disease was due to the predominant tremor in the majority of patients. They were high functioning in terms of walking independently and did not report any falls in a past 6 months. Both groups were similar with respect to age, gender, height, weight, body mass index (BMI) and MMSE (Table 1).
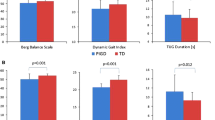
All participants were able to complete normal and dual task walking without falling. There were no significant differences in all gait parameters between the patients and the control groups with normal walking (Fig. 1a, b). Table 2 summarizes the effects of dual tasking on gait in patients with PD and control subjects. In both groups, the normalized velocity decreased significantly during dual tasking, compared to the normal walking in a parallel fashion (p < 0.001). When compared with the control group during dual tasking, PD patients had more cadences (p = 0.04) but less swing time (p = 0.02) and cycle time (p = 0.04) while the normalized velocity between both groups was not statistically significant (Fig. 1c, d). Interestingly, in comparison to normal walking, stride width was increased in PD patients doing two tasks simultaneously and became statistically larger than control subjects during the dual task exercise (p = 0.04) (Fig. 1c). The significant difference was not observed on the parameters of stride length and double support time between PD patients and control subjects. Subjects of both groups exhibited larger swing time, stride time and stride width variability during the dual task condition but there was no significant difference on these parameters between the PD patients and control subjects (Fig. 2). The mean FAP scores were not significantly different between PD subjects and the control group on both normal walking and dual task exercises (Table 3).
Discussion
Our main findings in this study occurred when PD subjects performed dual tasks which resulted in an increased number of cadences and a reduction in swing time and cycle time in comparison to normal subjects with no significant reduction in normalized velocity. However, in both PD patients and control groups, both velocity and normalized velocity significantly decreased in a parallel fashion in response to dual tasking. Significant increases in stride width were also observed in PD patients, compared with control subjects. During normal walking, no differences between PD and control subjects were observed on various gait parameters. Because these PD patients are all in their very early stage (mean H&Y = 1.62) without any axial involvement or any gait complaints, it is likely that they are still able to perform well and their performance is equal to the control subjects when they walk normally. However, in this study, attentional demands such as performing arithmetic tasks while walking, compromise their walking abilities, thus revealing subtle abnormalities in gait dynamics.
Current evidence suggests that gait is affected in all stages of PD, but it does not cause significant functional disturbances at the early stage (Baltadjieva et al. 2006; Carpinella et al. 2007; Ferrarin et al. 2006; Yang et al. 2008). However, when their walking were measured quantitatively, patients with early stage PD exhibited slower walking speed, shorter stride length, increased right/left swing asymmetry and inconsistencies in the timing of gait (Baltadjieva et al. 2006; Yang et al. 2008). Moreover, performing additional tasks often worsen these parameters, particularly on gait speed, variability, and rhythmicity (Rochester et al. 2004; Yogev et al. 2005). Although some of these profiles were also observed in our PD patients, this study provides a number of new findings which include an increased number of cadences, a reduction of swing time and cycle time. In PD patients, who are in the very early H&Y stage with minimal motor deficits, these observed abnormalities may be viewed as a compensatory increase in stepping in an attempt to maintain the velocity. Increased stride width may be an attempt to maintain the center of pressure not to go beyond the base-of-support which may predispose to unsteadiness and falls. When the compensatory mechanism is still considered sufficient and patients are still able to maintain their desired velocity, variability on stride time, swing time, and stride width may not be as yet apparent. Once the compensatory process is diminished, we may start to observe the increase in gait variability, slow speed, reduction in stride length with a later involvement of balance when the equilibrium is jeopardized.
Attentional demands of gait are often tested using dual tasking methodologies and serial 7 subtractions have been determined as a reliable assessment tool that can be used to evaluate executive function (EF) in the clinic and can be utilized for dual tasking when directly assessing the effect on walking in such settings (Yogev-Seligmann et al. 2008). However, serial 7 subtractions as part of the MMSE is not a very sensitive test that can fully evaluate EF or the whole spectrum of cognitive abnormalities in PD patients and additional EF tests, including the Frontal Assessment Battery, the Executive Interview and CLOX (executive clock drawing task), are recommended for completed EF evaluation (Yogev-Seligmann et al. 2008). Therefore, it is still possible that our PD patients may have a mild degree of cognitive impairment and the changes in gait parameters that we observed in our patients may be the results of wrong prioritization in which patients sacrifice their gait performances to optimize their cognitive task (“posture second” strategy) (Bloem et al. 2006). If this is the case, we should also observe the prioritization strategy in the control subjects in which they would maintain their gait and balance at the expenses of their cognitive function (“posture first” strategy). Moreover, the increased numbers of cadence may reflect the actual gait disorders in PD subjects when they are challenged with attention-demanding tasks. While our PD subjects took more steps in order to maintain a desired velocity, taking shorter steps can be interpreted as an early phenomenon leading to festination and eventually freezing of gait in PD (Chee et al. 2009).
Compensatory or reserve mechanisms are evident in PD. It is well established that classic motor signs that are sufficient for the diagnosis of PD occur when striatal dopamine loss reaches 60 % (Fearnley and Lees 1991). Moreover, several mechanisms exist in early PD in an attempt to maintain adequate intrasynaptic dopamine, including upregulation of dopamine synthesis, downregulation of the dopamine transporter, and postsynaptic dopamine receptor supersensitivity (Lee et al. 2000; Nandhagopal et al. 2011). These mechanisms may develop very early during the premotor phase in those who are at-risk of PD (van Nuenen et al. 2012). Although these biochemical and pathological findings support several brain mechanisms to maintain striatal dopamine level, evidence on compensatory gait mechanisms in early PD is much less clear. Early PD patients have infraclinical postural instability, manifested as greater sway area, which is compensated when it is more difficult to maintain good balance (Chastan et al. 2008). One study demonstrated that there was an increase in step frequency that was a compensation for reduced stride length when subjects walked at a slow to medium speed (Morris et al. 1994). Another study indicated that PD patients maintain a narrow stance as a compensation for their inability to sufficiently increase the size of their lateral anticipatory postural adjustments to allow fast step initiation in wide stance (Rocchi et al. 2006). Although both later studies were conducted in patients at moderate to advanced stages, the findings confirmed that compensatory gait mechanisms also occur in PD.
It is well recognized that there is a long prodromal phase in PD in which patients may not experience any symptoms related to their subtle deficits (Lang 2011). It is most likely that there is also a long period of “preclinical gait syndrome” when early PD patients may exhibit several subtle abnormalities in their walking and balance without any clinical complaints, for example reduced arm swing, changes in walking patterns and increased sway. The development of these abnormalities may indeed occur in a prodromal phase with subsequent progression of symptoms and signs to become clinically evident when they reach H&Y stage 2.5–3, manifested with postural instability, slow walking speed, freezing of gait, and falls (Fig. 3). To confirm these ‘preclinical’ manifestations, one may need to follow this particular population over a long period to determine if they clinically develop postural and gait abnormalities. Although this period may take several years before becoming clinically apparent, therapeutic options focusing on early interventions at this stage are limited and are usually for research purposes (Bhidayasiri and Truong 2012). As axial (gait and postural) symptoms tend to evolve more rapidly than other motor features of PD and appear to be a good index of disease progression (Evans et al. 2011), the identification of the manifestations of a preclinical gait syndrome has the potential to better ascertain disease progression and may serve as a tool for early gait and balance interventions once longitudinal studies have confirmed their predictive values of these early markers of gait deficits.
Proposed timeline from prodromal period to early stage of PD. Pathology, Braak staging, clinical correlates, and gait manifestations with an emphasis on the “preclinical gait syndrome” are shown. A 40-year course is assumed although there will be considerable individual variability. Modified from Bhidayasiri and Truong 2012
References
Baltadjieva R, Giladi N, Gruendlinger L, Peretz C, Hausdorff JM (2006) Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson’s disease. Eur J Neurosci 24(6):1815–1820
Bhidayasiri R, Truong DD (2012) Therapeutic strategies for nonmotor symptoms in early Parkinson’s disease: the case for a higher priority and stronger evidence. Parkinsonism Relat Disord 18(Suppl 1):S110–S113
Bloem BR, Grimbergen YA, van Dijk JG, Munneke M (2006) The “posture second” strategy: a review of wrong priorities in Parkinson’s disease. J Neurol Sci 248(1–2):196–204
Carpinella I, Crenna P, Calabrese E, Rabuffetti M, Mazzoleni P, Nemni R et al (2007) Locomotor function in the early stage of Parkinson’s disease. IEEE Trans Neural Syst Rehabil Eng 15(4):543–551
Chastan N, Debono B, Maltete D, Weber J (2008) Discordance between measured postural instability and absence of clinical symptoms in Parkinson’s disease patients in the early stages of the disease. Mov Disord 23(3):366–372
Chee R, Murphy A, Danoudis M, Georgiou-Karistianis N, Iansek R (2009) Gait freezing in Parkinson’s disease and the stride length sequence effect interaction. Brain 132(Pt 8):2151–2160
Donner A, Eliasziw M (1987) Sample size requirements for reliability studies. Stat Med 6(4):441–448
Evans JR, Mason SL, Williams-Gray CH, Foltynie T, Brayne C, Robbins TW et al (2011) The natural history of treated Parkinson’s disease in an incident, community based cohort. J Neurol Neurosurg Psychiatry 82(10):1112–1118
Fearnley JM, Lees AJ (1991) Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114(Pt 5):2283–2301
Ferrarin M, Carpinella I, Rabuffetti M, Calabrese E, Mazzoleni P, Nemni R (2006) Locomotor disorders in patients at early stages of Parkinson’s disease: a quantitative analysis. Conf Proc IEEE Eng Med Biol Soc 1:1224–1227
Fioretti S, Guidi M, Ladislao L, Ghetti G (2004) Analysis and reliability of posturographic parameters in Parkinson patients at an early stage. Conf Proc IEEE Eng Med Biol Soc 1:651–654
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198
Frenkel-Toledo S, Giladi N, Peretz C, Herman T, Gruendlinger L, Hausdorff JM (2005) Effect of gait speed on gait rhythmicity in Parkinson’s disease: variability of stride time and swing time respond differently. J Neuroeng Rehabil 2:23
Ganguli M, Ratcliff G, Huff FJ, Belle S, Kancel MJ, Fischer L et al (1990) Serial sevens versus world backwards: a comparison of the two measures of attention from the MMSE. J Geriatr Psychiatry Neurol 3(4):203–207
Geurts AC, Boonstra TA, Voermans NC, Diender MG, Weerdesteyn V, Bloem BR (2011) Assessment of postural asymmetry in mild to moderate Parkinson’s disease. Gait Posture 33(1):143–145
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51(6):745–752
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ et al (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71(9):670–676
Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C et al (2004) Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 19(9):1020–1028
Hausdorff JM (2005) Gait variability: methods, modeling and meaning. J Neuroeng Rehabil 2:19
Hausdorff JM (2007) Gait dynamics, fractals and falls: finding meaning in the stride-to-stride fluctuations of human walking. Hum Mov Sci 26(4):555–589
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17(5):427–442
Karzmark P (2000) Validity of the serial seven procedure. Int J Geriatr Psychiatry 15(8):677–679
Kressig RW, Beauchet O (2006) Guidelines for clinical applications of spatio-temporal gait analysis in older adults. Aging Clin Exp Res 18(2):174–176
Lang AE (2011) A critical appraisal of the premotor symptoms of Parkinson’s disease: potential usefulness in early diagnosis and design of neuroprotective trials. Mov Disord 26(5):775–783
Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE et al (2000) In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann Neurol 47(4):493–503
Lewek MD, Poole R, Johnson J, Halawa O, Huang X (2010) Arm swing magnitude and asymmetry during gait in the early stages of Parkinson’s disease. Gait Posture 31(2):256–260
Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC et al (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele–Richardson–Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47(1):1–9
Morris ME, Iansek R, Matyas TA, Summers JJ (1994) Ability to modulate walking cadence remains intact in Parkinson’s disease. J Neurol Neurosurg Psychiatry 57(12):1532–1534
Nandhagopal R, Kuramoto L, Schulzer M, Mak E, Cragg J, McKenzie J et al (2011) Longitudinal evolution of compensatory changes in striatal dopamine processing in Parkinson’s disease. Brain 134(Pt 11):3290–3298
Nelson AJ (1974) Functional ambulation profile. Phys Ther 54(10):1059–1065
Nelson AJ, Zwick D, Brody S, Doran C, Pulver L, Rooz G et al (2002) The validity of the GaitRite and the Functional Ambulation Performance scoring system in the analysis of Parkinson gait. NeuroRehabilitation 17(3):255–262
Pickering RM, Grimbergen YA, Rigney U, Ashburn A, Mazibrada G, Wood B et al (2007) A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov Disord 22(13):1892–1900
Plotnik M, Giladi N, Hausdorff JM (2009) Bilateral coordination of gait and Parkinson’s disease: the effects of dual tasking. J Neurol Neurosurg Psychiatry 80(3):347–350
Rocchi L, Chiari L, Mancini M, Carlson-Kuhta P, Gross A, Horak FB (2006) Step initiation in Parkinson’s disease: influence of initial stance conditions. Neurosci Lett 406(1–2):128–132
Rochester L, Hetherington V, Jones D, Nieuwboer A, Willems AM, Kwakkel G et al (2004) Attending to the task: interference effects of functional tasks on walking in Parkinson’s disease and the roles of cognition, depression, fatigue, and balance. Arch Phys Med Rehabil 85(10):1578–1585
van Nuenen BF, Helmich RC, Ferraye M, Thaler A, Hendler T, Orr-Urtreger A et al (2012) Cerebral pathological and compensatory mechanisms in the premotor phase of leucine-rich repeat kinase 2 parkinsonism. Brain 135(Pt 12):3687–3698
Yang YR, Lee YY, Cheng SJ, Lin PY, Wang RY (2008) Relationships between gait and dynamic balance in early Parkinson’s disease. Gait Posture 27(4):611–615
Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM (2005) Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding? Eur J Neurosci 22(5):1248–1256
Yogev-Seligmann G, Hausdorff JM, Giladi N (2008) The role of executive function and attention in gait. Mov Disord 23(3):329–342 (quiz 472)
Acknowledgments
The study was supported by the Rachadaphiseksomphot Endowment Fund Part of the “Strengthen CU’s Researcher’s Project” and research unit (RU) grant number GRU 52-026-30-005 of Chulalongkorn University, Bangkok, Thailand. We would like to thank Wannipat Buated for her assistance in patients recruitment and the data assortment.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panyakaew, P., Bhidayasiri, R. The spectrum of preclinical gait disorders in early Parkinson’s disease: subclinical gait abnormalities and compensatory mechanisms revealed with dual tasking. J Neural Transm 120, 1665–1672 (2013). https://doi.org/10.1007/s00702-013-1051-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-013-1051-8