Abstract
Rasagiline protects neuronal cells from cell death caused by various lines of insults. Its neuroprotective function is due to suppression of mitochondrial apoptosis signaling and induction of neuroprotective genes, including Bcl-2 and neurotrophic factors. Rasagiline inhibits the mitochondrial membrane permeabilization, an initial stage in apoptosis, but the mechanism has been elusive. In this paper, it was investigated how rasagiline regulates mitochondrial death cascade in apoptosis induced in SH-SY5Y cells by PK11195, a ligand of the outer membrane translocator protein of 18 kDa. Rasagiline prevented release of cytochrome c (Cyt-c), and the following caspase 3 activation, ATP depletion and apoptosis, but did not inhibit the mitochondrial membrane potential collapse, in contrast to Bcl-2 overexpression. Rasagiline stabilized the mitochondrial contact site and suppressed Cyt-c release into cytoplasm, which should be the critical point for the regulation of apoptosis. Monoamine oxidase was not associated with anti-apoptotic activity of rasagiline in PK11195-induced apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The neuroprotective function of rasagiline and selegiline [(−)deprenyl], inhibitors of type B monoamine oxidase [monoamine oxidase: oxygen oxidoreductase (deaminating), EC 1.4.3.4, MAO-B], has been proved in animal and cellular models of neurodegenerative disorders induced by neurotoxins, oxidative stress, ischemia, and serum withdrawal (Ebadi et al. 2006; Youdim et al. 2006; Naoi and Maruyama 2010). Their neuroprotective activity is ascribed to the prevention of death cascade activation in mitochondria, and the induction of neuroprotective genes, including anti-apoptotic Bcl-2 family protein and neurotrophic factors, like glial cell line-derived and brain-derived neurotrophic factor (Akao et al. 2002; Tatton et al. 2002; Maruyama et al. 2004).
Rasagiline suppresses the activation of mitochondrial apoptosis pathway initiated with mitochondrial permeability permeabilization (MMP) (Maruyama et al. 2002; De Marchi et al. 2003). The MMP is an increase of permeability of the mitochondrial membrane to ions and molecules with an exclusion size of about 1.5 kDa, such as cytochrome c (Cyt-c) and apoptosis inducing proteins. The MMP is induced by opening of the mitochondrial permeability transition pore (mPTP) or forming the mitochondrial apoptosis induced channel (MAC) at the outer membrane (Kinnally and Antinsson 2007). The mPTP is a voltage-dependent, cyclosporin-sensitive channel, and composed mainly of the voltage-dependent anion channel (VDAC) in the outer membrane, the adenine nucleotide translocator (ANT) in the inner membrane, and cyclophilin D (CypD) in the mitochondrial matrix (Green and Kroemer 2004). In addition, the mPTP contains mitochondrial creatine kinase, hexokinase isoforms, and the outer membrane translocator protein 18 kDa (TSPO, previously called as the peripheral benzodiazepine receptor) (Grimm and Brdiczka 2007). MAC is a channel formed with Bax alone or in combination of other proteins and inserted in the outer membrane. Opening of the channel leads to Cyt-c release, a critical point in apoptosis. Cyt-c binds with apoptosis-activating factor 1 (APAF-1) and pro-caspase 9 and forms a complex called the apoptosomes, where caspase 9 is activated. Caspase 9 cleaves pro-caspase 3 into caspase 3, an executor of apoptosis, which activates a range of enzymes to rearrange the nucleus, cytoskeleton, and plasma membrane to form the typical apoptotic features (Green and Kroemer 2004).
Bcl-2 protein family regulates the mPTP opening by either in a preventing (Bcl-2, Bcl-xL) or a promoting way (Bax, BAD, BID) (Tsujimoto and Shimizu 2000). Bcl-2 is a membrane protein localized in mitochondria, the endoplasmic reticulum and the nuclear envelope. Bcl-2 is expressed at low basal levels in neurons of the adult brains, but induced under pathological conditions. Rasagiline and related neuroprotective MAO inhibitors enhance in situ synthesis of anti-apoptotic Bcl-2 in SH-SY5Y cells (Akao et al. 2002) and type A monoamine oxidase (MAO-A) mediates the Bcl-2 induction by rasagiline (Inaba-Hasegawa et al. 2012). The direct interactions with the apoptosis executors Bax and Bak and the inducer BH3-only protein, are considered as the mechanism for the anti-apoptotic function of Bcl-2. However, it remains unclear what level in the apoptosis process the anti-apoptotic Bcl-2 proteins interfere with.
The molecular mechanism behind anti-apoptotic function of rasagiline has been elucidated in intrinsic apoptosis induced by an endogenous neurotoxin, N-methyl(R)salsolinol (NMRSal) in human SH-SY5Y cells expressing MAO-A. This neurotoxin binds to MAO-A and induces apoptosis, as shown in the cells, where MAO-A expression was down-regulated with short interfering siRNA (Yi et al. 2006). NMRSal induced the MMP: loss of mitochondrial membrane potential (∆Ψm), mitochondrial swelling and release of Cyt-c (Naoi et al. 2000; Maruyama et al. 2001; Akao et al. 2002). Rasagiline and Bcl-2 overexpression completely prevent the MMP and mitochondrial swelling, not only in the cells, but also in isolated mitochondria (Akao et al. 2002). Cyclosporin A (CysA), an inhibitor of CypD, prevents the MMP and apoptosis by NMRSal, indicating the opining of the mPTP by this toxin and its suppression by rasagiline.
In this paper, we investigated the detailed mechanism behind the intervention of rasagiline in apoptosis cascade, using PK11195, a ligand of the TSPO (Gavish et al. 1999; Seleikyte et al. 2011). TSPO and MAO were co-localized in the digitonin-extracted preparation of mitochondrial outer membrane (Anholt et al. 1986), and also in mitochondrial fraction of human U251 glioma cells (Olson et al. 1992). The protection by rasagiline against apoptosis caused by PK11195 was investigated in MAO-A expressing wild, MAO-B transfected (MAO-B SH cells), and Bcl-2 overexpressed SH-SY5Y cells (Bcl-2 SH cells) to clarify roles of MAO-A, MAO-B, and Bcl-2, respectively. The effects of rasagiline on the step-wise activation of apoptosis pathway were investigated to confirm the critical point for rasagiline to halt the death progression.
Materials and methods
Materials
Rasagiline was kindly donated by Teva Pharmaceutical (Netanya, Israel). MitoTracker Orange and Green, Alexa Fluor 488 (Annexin V conjugates) and propidium iodide were purchased from Molecular Probes (Eugene, OR, USA); PK11195, CysA, digitonin, Dextran-40, poly-l-aspartate-Na and kynuramine, from Sigma (St Louis, MO, USA), Dulbecco’s modified Eagle’s medium (DMEM), phosphate-buffered saline (PBS), and other drugs were purchased from Wako (Osaka, Japan). SH-SY5Y cells were cultured in Cosmedium-001 tissue culture medium (CosmoBio, Tokyo, Japan) supplemented by 5 % fetal calf serum (FCS) in an atmosphere of 95 % air–5 % CO2. Bcl-2 overexpressed SH-SY5Y cells (Bcl-2 SH cells) (Akao et al. 2002) and MAO-B transfected SH-SY5Y cells (MAO-B SH cells) (Yi et al. 2006) were established as reported previously.
Assessment of apoptosis by FACS and XTT assay
Apoptotic cells were determined by fluorescence-augmented cell sorter (FACS), which was performed over 30,000 events by three-color flow cytometry with a modified FACSCaliber cytometer and CellQuest software (Becton Dickson, San Jose, CA, USA). Dead cells were detected with propidium iodide staining without Triton X-100, and apoptotic cells were detected by staining in the presence of 0.1 % Triton X-100 to differentiate from necrotic ones. Fluorescence intensity at 530 nm and that above 575 nm were measured with excitation at 488 nm. Cells with low DNA content less than G1 (subG1 peak) were defined to be apoptotic (Eckert et al. 2001). Apoptosis was also determined from the Annexin V fluorescence detected at 530 nm with excitation at 488 nm by FACS.
Cell death was quantitatively measured by use of 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay for metabolic activity/proliferation/cell viability (MD Biosciences, Zürich, Switzerland). Wild, MAO-B and Bcl-2 SH cells (1 × 105 cells/well) were cultured in 96-well culture flasks for 24 h, and treated with PK11195 further 18 h. The absorbance at 450 nm was measured after 2-h incubation, and that at 630 nm was used as the reference. The decrease in the absorbance from that of control cells was assigned as the cell death and presented as the ratio to the absorbance of control.
Quantitative determination of ∆Ψm by FACS
SH-SY5Y cells were cultured in a 6-well poly-l-lysine-coated tissue culture plate for 18 h, washed with DMEM, and incubated with 1–10 μM PK11195 in DMEM for 1–3 h. After trypsinized, washed with the culture medium and gathered, the cells were suspended in DMEM and stained with 10 nM MitoTracker Orange and Green for 30 min in an ice bath. After washed and suspended with PBS, the cells were subjected to FACS. The laser emission at 534–606 and 515 nm were used for detection of Orange and Green fluorescence, respectively, with excitation at 488 nm. The MitoTracker Orange fluorescence was used for the ∆Ψm quantitation in mitochondrial fraction visualized with MitoTracker Green.
Assay for the enzymatic activity of caspase 3
Wild and Bcl-2 SH cells cultured in a 75 cm2 culture flask were gathered, washed, and suspended in PBS. The cells were treated with 50 μM PK11195 in the absence or presence of 10 μM rasagiline for 4 h. Enzymatic activity of caspase 3 was measured in the reaction mixture composed of 20 mM HEPES buffer, pH 7.5, containing 10 % glycerol, 2 mM dithiothreitol, and 10 μM digitonin, using 100 μM of a substrate, acetyl-l-aspartyl-l-asparginyl-l-leucyl-l-aspartic acid a-(4-methyl-coumaryl-7-amide) (Ac-DNLD-MCA, Peptide Research Institute, Minoh, Japan) for 1 h at 37 °C. Fluorescence at 460 nm was measured with excitation at 380 nm. The activity was expressed as pmoles of 7-amino-4-methyl-cumarin (AMC) produced per min per mg protein. Protein concentration was determined according to Bradford (1976).
Detection of released Cyt-c and the effect of rasagiline
To detect Cyt-c released from mitochondria by PK11195, wild and Bcl-2 SH cells suspended in DMEM were incubated with 1–10 μM PK11195 for 1–6 h after pre-treatment with 1–0.01 μM rasagiline. Gathered cells were lyzed in the extraction buffer [50 mM PIPES–KOH buffer, pH 7.4, containing 220 mM mannitol, 68 mM sucrose, 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM dithiothreitol and complete protease inhibitor cocktail (Roche, Mannheim, Germany)] (Akao et al. 2002). The lysates (6 μg protein) were subjected to SDS-PAGE and then electroblotted onto a PVDF membrane (Du Pont, Boston, MA, USA). The membrane was blocked with 5 % non-fat milk containing 0.1 % Tween 20, and incubated overnight at 4 °C with monoclonal anti-Cyt-c antibody (1,000× dilution, R&D Systems, Minneapolis, MN, USA). The membrane was incubated with alkaline phosphate-conjugated goat anti-mouse antibody (3,000× dilution, Promega, Madison, WI, USA) at room temperature. Immunoblots were visualized by use of an enhanced chemiluminescence detection kit (New England Biolabs, Beverly, MA, USA) and β-actin was used as control.
Assay for ATP content in cells and the enzymatic activity of mitochondrial complex I
Wild SH cells were gathered, suspended in PBS containing 2 mM pyruvic acid, and treated with 100–0.1 μM PK11195 for 15–120 min at 37 °C, without or after pre-treatment with 10–0.01 μM rasagiline for 20 min. ATP levels were measured in the cells treated with 100–0.1 μM PK11195 for 60 min, or at appropriate time for 15–120 min in those incubated with 50 μM PK11195. ATP contents were quantitatively determined with ATP Bioluminescence Assay Kit HS II (Roche) and expressed as nmoles of ATP per mg protein.
The effects of PK11195 on the activity of mitochondrial complex I (NADH-ubiquinol reductase) were examined in mitochondria prepared from wild SH cells. The activity was measured as oxidation of NADH using 125 μM CoQ10 as electron acceptor (Lambert and Brand 2004). The decrease of NADH fluorescence at 450 nm with excitation at 365 nm was measured in 30 mM HEPES buffer, pH 7.2, containing 1.2 M KCl and 10 mM EGTA, using 25 μM NADH. Mitochondria were treated with 250, 100, and 50 μM PK11195 for 60 min, and the activity was determined as nmoles of NADH decrease per min per mg protein.
Assay for reactive oxygen and nitrogen species (ROS–RNS)
Wild SH cells cultured in a 150-cm2 tissue culture flask were gathered, washed, and suspended in PBS. 2′,7′-Dichlorodihydrofluorescein diacetate (2′,7′-H2DCFDA) (Molecular Probes) was used to detect ROS–RNS (hydrogen peroxide, nitric oxide, peroxynitrite). The cells were treated with 100 μM PK11195 with 10 μM 2′,7′-H2DCFDA for 60 min at 37 °C and the fluorescence at 520 nm was measured at every 10 min with excitation at 504 nm. 2′,7′-Dichlorofluorescein (DCF) (Sigma) was used as standard. ROS–RNS production was calculated from the increased fluorescence intensity and expressed as pmoles of DCF produced per min per mg protein.
Assay for MAO activity
MAO activity in mitochondria was measured fluorometrically by use of kynuramine as a substrate (Kraml 1965). Mitochondria prepared from wild SH cells were used as a MAO-A sample, and those from MAO-B SH cells were pre-treated with 1 μM clorgyline at 37 °C for 20 min and used as a MAO-B sample. The enzymatic activity was measured with seven graded concentrations of kynuramine, and presented as nmoles of 4-hydroxyquinoline produced from kynuramine per min per mg protein. The maximal velocity (V max) and the Michaelis constant (K m) of MAO activities, and the type of inhibition and the inhibitor constant (K i) of PK11195 were determined by kinetic analyses using the double-reciprocal plot of the reaction velocity against the substrate concentration.
Statistics
Experiments were repeated 4–6 times with quadruplicate measurements, and the results were expressed as the mean and SD. Differences were statistically evaluated by analysis of variance (ANOVA) by Sheffe’s F test. A p value less than 0.05 was considered to be statistically significant.
Results
Induction of apoptosis by PK11195
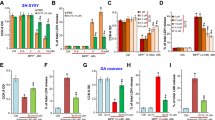
PK11195 induced apoptosis in SH-SY5Y cells after treatment for 18 h, as shown in Fig. 1a–c. After staining with propidium iodine, FACS quantitatively determined apoptosis. PK11195 increased apoptotic cells to 51.4 % of the total from 5.6 % in control cells (Fig. 1a). Annexin V-FITC staining of phosphatidylserine exposed on the outer plasma membrane also confirmed apoptosis and 55.7 % of the total cells were apoptotic, compared to 1.7 % in control (Fig. 1b). PK11195 induced apoptotic death in wild SH cells at concentration higher than 50 μM, and more markedly in MAO-B SH cells, whereas Bcl-2 overexpression almost completely prevented apoptosis (Fig. 1c). Cell death was also measured by XTT assay, and MAO-B transfection increased the sensitivity to PK11195 cytotoxicity at the lower concentrations, 50 and 75 μM (Fig. 1d). Apoptosis was accompanied with the collapse of ∆Ψm, as shown by FACS after staining with MitoTracker Orange and Green (Fig. 1e). ∆Ψm began to decrease after 30-min exposure with 100 μM PK11195 and reached to the lowest level after 120 min treatment.
Induction of apoptosis by PK11195. a Apoptotic cells were quantified in the subG1 peak by FACS after propidium iodine staining. b Annexin V-FFITC staining. c Apoptotic cells were quantified in wild, MAO-B, and Bcl2 SH cells. *significantly different from control, p < 0.01. d The cell viability was quantitatively determined by XTT assay. e ∆Ψm decline was followed in wild SH cells for 180 min by FACS, after stained with MitoTracker Orange in mitochondrial fraction detected with MitoTracker Green
Rasagiline protected cells from apoptosis induced by PK11195
Rasagiline pre-treatment prevented apoptosis by PK11195. Hoechst 33342 staining showed that 50 μM PK11195 induced the typical apoptotic features in wild SH cells with condensation and shrinking of cell body and nuclei, which rasagiline at 10 μM completely prevented (Fig. 2a). XTT assay confirmed also that rasagiline at 10 and 1 μM completely suppressed the cell death caused by 50 μM PK11195 (Fig. 2b). Rasagiline and Bcl-2 overexpression inhibited the activation of caspase 3 in wild SH cells. Rasagiline protected the cells by suppressing the caspase 3 activation in a similar way as Bcl-2 overexpression.
Inhibition of PK11195-induced apoptosis by rasagiline. a Cells were treated with 100 μM PK11195 (PK) without or with 10 μM rasagiline (PK + Ras) and stained with Hoechst 33342. b Quantitative determination of cell death by XTT assay. The number of dead cells was expressed as the relative values to that in control cells treated without PK11195. The column and bar represent the mean and SD of three independent experiments with quadruplicate measurements. ** and æ significantly different from control and from PK111195-treated cells, p < 0.01. c Increased caspase 3 activity by PK11195 in wild SH cells. *significantly different from control, p < 0.05. æsignificantly different from PK11195-treated cells, p < 0.01
Bcl-2 prevented PK11195-induced ∆Ψm decline in contrast to rasagiline
The effects of PK11195 on ∆Ψm were examined in wild, MAO-B and Bcl-2 SH cells. In wild SH cells PK11195 decreased ∆Ψm at 100 μM, but did not at 10 μM, as in the case of apoptosis (Fig. 3a). Bcl-2 overexpression suppressed the ∆Ψm decline induced by PK11195 (Fig. 3b). In wild (Fig. 3c) and MAO-B SH cells (Fig. 3d), rasagiline at 10 μM could not inhibit ∆Ψm decline by PK11195, in contrast to the suppression of apoptosis shown in Fig. 2b. 5-HT (1 mM) did not affect ∆Ψm collapse by PK11195, suggesting that PK11195 did not bind to the active site of MAO-A to decrease ∆Ψm (Fig. 3e). The anti-apoptotic activity of rasagiline was independent from suppressing ∆Ψm decline, in contrast to Bcl-2 overexpression.
Effects of PK11195 on ∆Ψm in wild and Bcl-2 SH cells. The ∆Ψm was measured by FACS after staining with MitoTracker Orange and Green. a and b Wild and Bcl-2 SH cells were treated with 100 and 10 μM PK11195. c and d Wild SH and MAO-B cells were treated with 50 μM PK11195 in the absence (I) or presence (II) of 10 μM rasagiline. e Wild SH cells were treated with 50 μM PK11195 without (I) or with 10 mM 5HT (II), or with 5-HT alone (III)
Release of Cyt-c release into cytoplasm by PK11195 and the suppression by rasagiline
Release of Cyt-c from mitochondria was determined in the cytoplasmic fraction isolated from wild SH cells treated with PK11195 (Fig. 4a). Cyt-c levels were markedly increased after 2-h incubation with 50 μM PK11195 and reached to the maximal at 4 h, then declined. Bcl-2 overexpression completely inhibited Cyt-c release by PK11195, whereas transfection of the vector did not (Fig. 4b). Rasagiline at 10 μM markedly prevented Cyt-c release in the cytoplasm induced by PK11195 (Fig. 4c), which accounted for the inhibition of caspase 3 activation by rasagiline (Fig. 2c).
Release of Cyt-c from mitochondria by PK11195. a The effect of the incubation time on Cyt-c release. Wild SH cells were treated with 100 μM PK11195 for 0, 2, 4, and 6 h at 37 °C, gathered, and Cyt-c was detected as described in “Materials and methods”. b Prevention of Cyt-c release by Bcl-2 overexpression. Cells transfected with Bcl-2 or with vector were incubated with 50 and 10 μM PK11195, and Cyt-c in cytoplasmic fraction was detected. c The effect of rasagiline on Cyt-c release by PK11195
Depletion of mitochondrial ATP and the protection by rasagiline
The MPP causes uncoupling of oxidative phosphorylation in mitochondria and the effects of rasagiline on ATP depletion were investigated. PK11195 decreased ATP levels in wild SH cells in a dose- and time-dependent way (Fig. 5a, b). PK11195 depleted ATP at the concentration higher than 1 μM and after 30 min of treatment. Rasagiline at the concentration higher than 0.1 μM prevented the reduction of ATP by 10 μM PK11195 (Fig. 5c).
Depletion of ATP levels by PK11195 and the effects of rasagiline. Wild SH cells were treated with PK11195 and ATP contents were determined with ATP biofluorescence assay. a The effects of PK11195 concentrations and on the ATP levels. The columns and bar represent the mean and SD of three independent experiments with quadruplicate measurements. ** and * significantly different from control ATP level before the treatment, p < 0.01 and 0.05. b The effects of the incubation time on ATP levels. Cells were treated with PK11195 and at appropriate time the ATP content was quantified. c The effect of rasagiline on the reduction of ATP contents by PK11195. Cells were treated with 10–0.01 μM rasagiline for 20 min, then with 10 μM PK11195 for 60 min at 37 °C. ** and * significantly different from cells treated with PK11195 alone, p < 0.01 and 0.05. d The effects of PK11195 on complex I activity in isolated mitochondria. Mitochondria isolated from wild SH cells were treated with PK11195 for 60 min and the complex I activity was measured
The direct effects of PK11195 on the enzymatic activity of complex I were examined in mitochondria prepared from wild SH cells (Fig. 5d). PK11195 at concentrations up to 250 μM did not inhibit complex I activity, suggesting that PK11195 depleted ATP by mechanism other than the direct inhibition of complex I activity, or as the consequence of the MMP.
Inhibition of the enzymatic activity of MAO-A and MAO-B by PK11195
The effect of PK11195 to the enzymatic activity of MAO-A and MAO-B was examined to clarify the association of MAO with apoptosis by PK11195 using mitochondria prepared from wild and MAO-B SH cells. Kinetic studies were carried out with kynuramine at 7 graded concentrations, and the values of the V max and K m of MAO-A and MAO-B for kynuramine, and the K i of PK11195 and the type of inhibition were determined. Transfection of MAO-B into wild SH increased the enzymatic activity significantly: the V max of MAO-B sample for kynuramine increased to 10.0 ± 2.5 nmol/min/mg protein from 0.11 nmol/min/mg protein in MAO-A sample, whereas the K m value for MAO-A and MAO-B were 37.0 ± 8.9 and 100 ± 15.6 μM, respectively (Inaba-Hasegawa et al. 2012). Figure 6a and b shows that the inhibition of MAO-A and MAO-B activity by PK11195 was non-competitive to the substrate. The K i values of PK11195 were obtained to be 333 ± 22 and 183 ± 13 μM for MAO-A and MAO-B, respectively. MAO-B had higher affinity to PK11195, which might be relevant with the higher vulnerability of MAO-B SH cells to PK11195 at lower than 75 μM (Fig. 1c, d).
Kinetic studies on the inhibition of MAO-A and MAO-B by PK11195. a and b MAO-A and MAO-B activities were measured in mitochondria isolated from wild (a) and MAO-B SH cells (b), using kynuramine in the absence (I) or presence of 100 μM PK11195 (II). The reciprocal of the enzyme activity was plotted against that of the substrate concentration and the values for V max and the K m for kynuramine, and the K i values of PK11105 were determined
PK11175 did not increase, but inhibited ROS–RNS production
PK11195 was reported to open the mPTP via ROS–RNS generation or vise versa to produce ROS as a by-product of the MPP (Veeman et al. 2010). The effects of PK11195 on ROS–RNS generation were investigated in wild SH cells (Fig. 7). In MAO-A-expressing wild SH cells, PK11195 (100 μM) significantly reduced DCF production, which was further decreased by clorgyline and rasagiline, the inhibitor of MAO-A and MAO-B. Inhibition of the enzymatic oxidation of endogenous monoamines by MAO-A by clorgyline, and direct scavenging of ROS by rasagiline might contribute the decrease of ROS–RNS levels. The reduced production of ROS–RNS by PK11195 might be due to the inhibition of mitochondrial respiratory chain at downstream from complex I, which might cause the ATP decrease shown in Fig. 6. Reduced glutathione (GSH) and ascorbic acid decreased ROS–RNS production, but the difference from PK11195 alone-treated cells was not statistically significant. These results suggest that ROS–RNS might not cause the MMP by PK11195 in SH-SY5Y cells.
ROS–RNS production in cells treated with PK11195. Wild SH cells were treated with 100 μM PK11195 alone (II), or in the presence of 1 mM GSH (III), 1 mM ascorbic acid (IV), 10 μM clorgyline (V) or 10 μM rasagiline (VI). I Control. Column and bar represent three independent experiments with quadruplicate measurements. * and æ significantly different from control or cells treated PK11195 alone, respectively, p < 0.05
Dextran-40 and poly-l-aspartate prevented apoptosis by PK11195
Dextran diminishes the inter-membrane space (IMS) of mitochondria, increases the number of contact sites between the inner and outer mitochondrial membrane (OMM), and decreases the outer membrane permeability (Wicker et al. 1993; Doran and Halestrap 2000). Wild SH cells were treated with 100 μM PK11195 in the presence of 1, 2.5, and 5 % of dextran-40 (40 kDa), and the cell viability and ∆Ψm were determined by FACS. Dextran-40 prevented apoptosis significantly (Fig. 8a, c), and suppressed ∆Ψm collapse at 5 % (Fig. 8b). Poly-l-aspartate, which binds to the VDAC at the outer membrane and favors its low conductance state (Mangan and Colombini 1987), inhibited apoptosis induced by 100 μM PK11195 (Fig. 8c), but did not prevent the ∆Ψm by PK11195 (data, not shown).
Protection by dextran-40 and poly-l-aspartate against the cytotoxicity of PK11195. a Wild SH cells were treated with 100 μM PK11195 in the absence or presence dextran-40 and subjected to FACS. b Effects of dextran-40 on ∆Ψm decline by PK11195. Cells treated with 100 μM PK11195 in the absence (I) or presence of 2.5 % (II) or 5 % (III) dextran-40 for 2 h. c Quantitation of apoptosis by FACS after treated with PK11195 in the presence of dextran-40, or poly-l-aspartate (Poly-l-As). *Significantly different from control, p < 0.01. Dextran-40 and poly-l-aspartate at any concentrations significantly suppressed apoptosis, p < 0.01 from PK11195-treated cells
Cyclosporin A (CysA) could not prevent apoptosis induced by PK11195
The effects of CysA, an ANT inhibit, on apoptosis induced by PK11195 were investigated. As shown in Fig. 9. CysA could not reduce cell death by PK11195, but rather increased apoptotic cells. ANT was neither required for the MMP by PK11195 nor the anti-apoptotic function of rasagiline.
The effects of CysA on cell death induced by PK11195. Wild SH cells were treated without or with 50 μM PK11195 in the absence or presence of 10 μM CysA at 37 °C for 4 h, then subjected to FACS. The number in each figure represents the percentage of apoptotic cells in the subG1 peak to the total cell number
Discussion
Rasagiline and related propargylamines show neuroprotective functions in in vivo and in vitro models of Parkinson’s disease prepared with MPTP (Kupsch et al. 2001), 6-hydroxydopamine (Blandini et al. 2004), NMRSal (Maruyama et al. 2000), peroxynitrite (Maruyama et al. 2002), oxidative stress, (Speiser et al. 2007) and withdrawal of serum or neurotrophic factors (Tatton et al. 2002; Weireb et al. 2004). The anti-apoptotic function is ascribed directly to the stabilization of mitochondria, and indirectly to the inhibition of MAO-B, reduced production of ROS–RNS, scavenge of free radicals. The gene induction of anti-apoptotic Bcl-2, anti-oxidant enzymes and pro-survival neurotrophic factors is now proposed as the most important mechanism for the neuroprotection in in vivo in animal models and clinical trials (Maruyama and Naoi 2013; Naoi et al. 2011, 2013). However, only few papers are available in concern to the molecular mechanism behind the regulation of the MPP by MAO inhibitors. Selegiline inhibited the MMP induced by pro-oxidant 2-methyl-1,4-naphthoquinone (menadione) in liver mitochondria (De Marchi et al. 2003). Direct interaction of a protonated amino group of the propargylamine structure with aromatic or anionic amino acid residue of the pore forming structure was proposed as the mechanism, but this hypothesis requires more evidences for the final acceptance. In addition, selegiline did not suppress the MMP caused by dopamine and peroxynitrite in rat brain mitochondria (Lee et al. 2002) and even enhanced dopamine-induced cytotoxicity in SH-SY5Y cells (Lai and Yu 1997).
Rasagiline inhibits the swelling and release of trapped Rhodamine 123 in isolated mitochondria treated with NMRSal, suggesting the interaction with the mPTP components (Akao et al. 2002). However, a direct physical interaction between rasagiline and components of mitochondrial channels has not been confirmed. Figure 10 shows the schematic process of the MMP in apoptosis by NMRSal and PK11195, and the anti-apoptotic function of rasagiline and other compounds. Bcl-2 overexpression completely inhibited the MMP in mitochondria prepared from Bcl-2 transgenic mice and also in Bcl-2 SH cells, indicating the decisive role of Bcl-2. Bcl-2 itself might regulate the MMP through formation of the heterodimers with apoptogenic Bax and inhibits formation of autonomous channels, such as the MAC. In healthy cells, Bcl-2 regulates the function of mitochondrial carrier proteins, like VDAC, ANT, and mitochondrial carrier homolog 2 (Mitch2) (Schwarz et al. 2007). Direct interaction of Bcl-2 protein family with VDAC was reported using proteoliposomes: Bcl-XL promotes the closure of VDAC, whereas Bax forms a mega channel with VDAC and releases Cyt-c (Shimizu et al. 1999). As indirect anti-apoptotic activities, Bcl-2 overexpression increases mitochondrial Ca2+ buffering capacity and protects against Ca2+-induced MPP (Murphy et al. 1996), increases cellular resistance to oxidative stress through its influence over NAD(P)H, glutathione redox state, elevated anti-oxidant enzymes, glutathione and thioredoxin peroxidase (Kowaltowski and Fiskum 2005), and Bcl-2 itself regulates caspase activity (Enari et al. 1995). The systemic administration of rasagiline might increase the in situ synthesis of Bcl-2 and promote cell survival in the brain.
Schematic mechanism of the MMP by NMRSal and PK11195 and of the inhibition of Cyt-c release by rasagiline. a NMRSal binds to MAO-A and opens the MPP composed of VDAC at the outer mitochondrial membrane (OMM) and ANT at the inner mitochondrial membrane (IMM), and causes ∆Ψm decline and Cyt-c release with swelling of the inter-membrane space (IMS). b Rasagiline completely inhibits the MPP opening and suppresses the ∆ΨM decline, Cyt-c release and mitochondrial swelling. Rasagiline increases also Bcl-2 protein via MAO-A and sequesters Bax as the heterodimer with Bcl-2. CysA inhibits cyclophilin D (CypD) prevents the MPP opening. c PK11195 forms a channel composed of Bax homo-tetramer, or Bax bound to VDAC or other protein and releases Cyt-c release, decreases ∆Ψm and mitochondrial swelling. PK11195 may be not associated with TSPO to induce the MMP. d Dextran-40 suppresses the mitochondrial swelling by increasing the contact site of the IMS and poly-l-As interacts with VDAC, and both of them inhibit Cyt-c release and apoptosis. Rasagiline prevents Cyt-c release and following progression of apoptotic cascade independently from ∆Ψm
PK11195 caused apoptosis by opening mitochondrial channel not associated with the MPP, whereas NMRSal opened the MPP. Even though PK11195 is a well-known TSPO ligand, it induced apoptosis independently from the TSPO in the cells, where TSPO expression was knockdown with siRNA (Gonzalez-Polo et al. 2005). Apart from binding to the TSPO, PK11195 induces the MMP and apoptosis by a down-regulation of Bcl-2, translocation of Bax to form a pore, and increased ROS–RNS production (Maaser et al. 2005), but these results could not be confirmed in our experiments.
Dextran-40 and poly-l-aspartate suppressed the MMP and apoptosis by PK11195, but CysA, a specific inhibitor of CypD, did not. Poly-l-aspartate (Mangan and Colombini 1987) bound to VDAC and stabilized the low conductance state of the VDAC, which inhibited the Cyt-c permeation (Shimizu et al. 1999). Dextran-40 prevented apoptosis by PK11195, through maintaining the structure of contact site between the outer and inner membrane and inhibiting Cyt-c release (Wicker et al. 1993; Doran and Halestrap 2000). This paper presents that intervention of Cyt-c release is essential for rasagiline to protect cells from apoptosis, as proposed to be the “point of no return” in the cell death process (Garrido et al. 2006). Rasagiline might change the protein–protein interaction or the conformation of VDAC and the contact site to inhibit Cyt-c release.
Our previous results show that anti-apoptotic activity of propargylamine MAO-B inhibitors depends on the three-dimensional structure: R-enantiomers are protective, but S-enantiomers are ineffective. MAO-A, but not MAO-B, mediates the induction of anti-apoptotic Bcl-2 by rasagiline, whereas selegiline increases Bcl-2 independently from MAO-A (Inaba-Hasegawa et al. 2012). Rasagiline also increases MAO-A itself through the reduction of R1, a MAO-specific repressor, and the activation of Sp1 (Inaba-Hasegawa et al. 2013). These results suggest that MAO-A might play a role in signal transduction in mitochondria, but the mechanism remains elusive how MAO transmits signal from mitochondrial outer membrane to nuclei, and induces the transcription of Bcl-2 and MAO-A.
Rasagiline and related MAO-B inhibitors now proposed as disease-modifying agents in PD and other age-dependent neurodegenerative disorders (Olanow et al. 2009; Riederer and Laux 2011). However, the role of MAO itself in the neuroprotective function of these inhibitors has not been well confirmed. Chronic treatment with TVP-1012, the racemic form of rasagiline, and selegiline reduced MAO-B activity in the rat striatum by 90 %, but MAO-A was also inhibited by TVP-1012 (15 %) and selegiline (40 %), suggesting their binding to MAO-A (Lamensdorf et al. 1996). This result might be comparable to our results on the involvement of MAO-A in the protective functions of these MAO-B inhibitors.
However, we should be cautious to apply our results using SH-SY5Y cells to the effects on dopamine neurons in the brain. SH-SY5Y cells have been commonly used as a cellular model of dopaminergic neurons, which express the enzymes related to catecholamine synthesis and metabolism, such as tyrosine hydroxylase and dopamine-β-hydroxylase, the rate-limiting enzyme of dopamine,and noradrenalin synthesis, MAO-A, and noradrenalin transporter (Hong-Rong et al. 2010). However, this cell line expresses undetectable protein level of tyrosine hydroxylase through the feedback inhibition with dopamine (Mazzulli et al. 2006), and the distinct agents differentiate SH-SY5Y cells to dopaminergic, noradrenergic, or cholinergic cells (Hong-Rong et al. 2010). Previously, we studied the induction of apoptosis in SH-SY5Y cells differentiated with retinoic acid in comparison with those without differentiation and the increased vulnerability was observed, but the treatment did not affect the cellular mechanism of apoptosis induced by NMRSal (Maruyama et al. 1997).
The binding of rasagiline-related propargylamines to MAO-A and MAO-B was clarified in details using recombinant MAO (Hubalek et al. 2004; Binda et al. 2005), but the possible binding of rasagiline and selegiline to MAO at not the substrate-binding site, and protein other than MAO cannot be excluded. Further studies on the binding sites of rasagiline in neuronal cells and the molecular mechanism to intervene the MPP will give us a way to find the novel anti-apoptotic agents as the disease-modifying therapy in neurodegenerative disorders.
Abbreviations
- ANT:
-
Adenine nucleotide translocator
- CysA:
-
Cyclosporin A
- Cyt-c:
-
Cytochrome c
- ∆Ψm:
-
Mitochondrial membrane potential
- FACS:
-
Fluorescence-augmented flow cytometry
- MAC:
-
Mitochondrial apoptosis induced channel
- MAO-A and MAO-B:
-
Type A and B monoamine oxidase
- MMP:
-
Mitochondrial permeability permeabilization
- mPTP:
-
Mitochondrial permeability transition pore
- TSPO:
-
Outer membrane translocator protein of 18 kDa
- VDAC:
-
Voltage-dependent anion channel
References
Akao Y, Maruyama W, Shimizu S et al (2002) Mitochondrial permeability transition mediates apoptosis induced by N-methyl(R)salsolinol, an endogenous neurotoxin, and is inhibited by Bcl-2 and rasagiline, N-propargyl-1(R)-aminoindan. J Neurochem 82:913–923
Anholt RRH, Pedersen L, De Souza EB, Snyder SH (1986) The peripheral-type benzodiazepine receptor. J Biol Chem 261:576–583
Binda C, Hubalek F, Li M, Herzig Y, Sterling J, Edmondson DE, Mattevi A (2005) Binding of rasagiline-related inhibitors to human monoamine oxidases; a kinetic and crystallographic analysis. J Med Chem 48:8148–8154
Blandini F, Armentero MT, Fancellu R, Blaugrund E, Nappi G (2004) Neuroprotective effect of rasagiline in a rodent model of Parkinson’ disease. Exp Neurol 187:455–459
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye binding. Anal Biochem 72:248–254
De Marchi U, Pietrangeli P, Marcocci L, Mondovi B, Toninello A (2003) l-Deprenyl as an inhibitor of menadione-induced permeability transition in liver mitochondria. Biochem Pharmacol 66:1749–1754
Doran E, Halestrap AP (2000) Cytochrome c release from isolated rat liver mitochondria can occur independently of outer-membrane rupture: possible role of contact sites. Biochem J 348:343–350
Ebadi M, Brown-Borg H, Ren J, Sharma S, Shavali S, ReFacy HE, Carlson EC (2006) Therapeutic efficacy of selegiline in neurodegenerative disorders and neurological diseases. Curr Drug Targets 7:1513–1529
Eckert A, Steiner B, Marques C, Leutz S, Roming H, Hass C, Muller WE (2001) Elevated vulnerability to oxidative stress-induced cell death and activation of caspase-3 by the Swedish amyloid precursor protein mutation. N Neurosci Res 64:183–192
Enari M, Hase A, Nagata S (1995) Apoptosis by a cytosolic extract from Fas-activated cells. EMBO J 14:5201–5208
Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G (2006) Mechanism of cytochrome c release from mitochondria. Cell Death Differ 13:1423–1433
Gavish M, Bachman I, Shoukrun R, Katz Y, Veenman L, Weisingwe G, Weizman A (1999) Enigma of the peripheral benzodiazepine receptor. Pharmacol Rev 51:629–650
Gonzalez-Polo R, Carvalho G, Braun T et al (2005) PK11195 potently sensitizes to apoptosis induction independently from the peripheral benzodiazepine receptor. Oncogene 24:7503–7513
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305:626–629
Grimm S, Brdiczka D (2007) The permeability transition pore in cell death. Apoptosis 12:841–855
Hong-Rong XIE, Lin-Sen HU, Guo-Yi LI (2010) SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin Med 123:1086–1092
Hubalek F, Binda C, Ki M, Herzig Y, Sterling J, Youdim MBH, Mattevi A, Edmondson DE (2004) Inactivation of purified human recombinant monoamine oxidase A and B by rasagiline and its analogues. J Med Chem 47:1760–1768
Inaba-Hasegawa K, Akao Y, Maruyama W, Naoi M (2012) Type A monoamine oxidase is associated with induction of neuroprotective Bcl-2 by rasagiline, an inhibitor of type B monoamine oxidase. J Neural Transm 119:405–414
Inaba-Hasegawa K, Akao Y, Maruyama W, Naoi M (2013) Rasagiline and selegiline, inhibitors of type B monoamine oxidase, induce type A monoamine oxidase in human SH-SY5Y cells. J Neural Transm 120:435–444
Kinnally KW, Antinsson B (2007) A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis 12:857–868
Kowaltowski AJ, Fiskum G (2005) Redox Mechanisms of cytoprotection by Bcl-2. Antioxid Redox Signal 7:508–514
Kraml M (1965) A rapid microfluorometric determination of monoamine oxidase. Biochem Pharmacol 14:1684–1686
Kupsch A, Sautter J, Götz ME et al (2001) Monoamine oxidase-inhibition and MPTP-induced neurotoxicity in the non-human primate: comparison of rasagiline (TVP1012) with selegiline. J Neural Transm 108:985–1009
Lai CT, Yu PH (1997) R(−)-deprenyl potentiates dopamine-induced cytotoxicity towards catecholaminergic neuroblastoma SH-SY5Y cells. Appl Pharmacol 142:186–191
Lambert AJ, Brand MD (2004) Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH: ubiquinone oxidoreductase (complex I). J Biol Chem 279:39414–39420
Lamensdorf I, Youdim MBH, Finberg JPM (1996) Effect of long-term treatment with selective monoamine oxidase A and B inhibitors on dopamine release from rat striatum. J Neurochem 67:1532–1539
Lee CS, Lee CS, Ko HH, Song JH, Han ES (2002) Effect of R-(−)-deprenyl and harmaline on dopamine- and peroxynitrite-induced membrane permeability transition in brain mitochondria. Neurochem Res 27:215–224
Maaser K, Sutter AP, Scherübl H (2005) Mechanisms of mitochondrial apoptosis induced by benzodiazepine receptor ligands in human colorectal cancer cells. Biochem Biophys Res Commun 332:646–652
Mangan PS, Colombini M (1987) Ultrasteep voltage dependence in a membrane channel. Proc Natl Acad Sci USA 84:4896–4900
Maruyama W, Naoi M (2013) “70th Birthday Professor Riederer” Induction of glial cell-line-derived and brain-derived neurotrophic factors by rasagiline and (−)deprenyl: a way to a disease-modifying therapy? J Neural Transm 120:83–89
Maruyama W, Strolin Benedett M, Takahasi T, Naoi M (1997) A neurotoxin, N-methyl(R)salsolinol induces apoptosis in human differentiated dopaminergic neuroblastoma SH-SY5Y cells. Neurosci Lett 237:147–150
Maruyama W, Akao Y, Youdim M, Naoi M (2000) Neurotoxin induced apoptosis in dopamine neurons: protection by N-propargylamine-1(R)- and (S)-aminoindan, Rasagiline and TV1022. J Neural Transm 60(Suppl):171–186
Maruyama W, Akao Y, Youdim MBH, Davis BA, Naoi M (2001) Transfection-enforced Bcl-2 overexpression and an anti-Parkinson drug, rasagiline, prevent nuclear accumulation of glyceraldehydes-3-phosphate dehydrogenase induced by an endogenous dopaminergic neurotoxin, N-methyl(R)salsolinol. J Neurochem 78:727–735
Maruyama W, Takahashi T, Youdim M, Naoi M (2002) The anti-parkinson drug, rasagiline, prevents apoptotic DNA damage induced by peroxynitrite in human dopaminergic neuroblastoma SH-SY5Y cells. J Neural Transm 109:467–481
Maruyama W, Nitta A, Shamoto-Nagai M et al (2004) N-propargyl-1(R)-aminoindan, rasagiline, increases glial cell line-derived neurotrophic factor (GDNF) in neuroblastoma SH-SY5Y cells through activation of NF-κB transcription factor. Neurochem Int 44:393–400
Mazzulli JR, Mishizen AJ, Giasson BI et al (2006) Cytosolic catechols inhibit a-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J Neurosci 26:1068–10078
Murphy AN, Bredesen DE, Cortopassi G, Wang E, Fiskum G (1996) Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc Natl Acad Sci USA 93:9893–9898
Naoi M, Maruyama W (2010) Monoamine oxidase inhibitors as neuroprotective agents in age-dependent neurodegenerative disorders. Curr Pharm Design 16:2799–2817
Naoi M, Maruyama W, Akao Y, Zhang J, Parvez H (2000) Apoptosis induced by an endogenous neurotoxin, N-methyl(R)salsolinol, in dopamine neurons. Toxicology 153:123–141
Naoi M, Maruyama W, Inaba-Hasegawa K, Akao Y (2011) Type A monoamine oxidase regulates life and death of neurons in neurodegeneration and neuroprotection. Int Rev Neurobiol 100:85–106
Naoi M, Maruyama W, Inaba-Hasegawa K (2013) Revelation in neuroprotective functions of rasagiline and selegiline: the induction of distinct genes by different mechanisms. Expert Rev Neurother (in press)
Olanow CW, Rascol O, Hauser R et al (2009) A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Eng J Med 361:1268–1278
Olson JM, McNeed W, Young AB, Mancini WR (1992) Localization of peripheral-type benzodiazepine binding site to mitochondria of human glioma cells. J Neurosci 13:35–42
Riederer P, Laux G (2011) MAO-inhibitors in Parkinson’s disease. Exp Neurobiol 20:1–17
Schwarz M, Andrade-Navarro M, Gross A (2007) Mitochondrial carriers and pores: key regulators of the mitochondrial apoptotic program? Apoptosis 12:869–876
Seleikyte J, Petronilli V, Zulian A et al (2011) Regulation of the inner membrane mitochondrial permeability transition by the outer membrane translocator protein (peripheral benzodiazepine receptor). J Biol Chem 28:1046–1053
Shimizu S, Narita M, Tsujimoto Y (1999) Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399:483–487
Speiser Z, Mayk A, Litinetsky L, Fine T, Nyska A, Blaugrund E, Cohen S (2007) Rasagiline is neuroprotective in an experimental model of brain ischemia in the rat. J Neural Transm 114:595–605
Tatton WG, Chalmers-Redman RME, Ju WJH et al (2002) Propargylamines induce antiapoptotic new protein synthesis in serum- and nerve growth factor (NGF)-withdrawn, NGF-differentiated PC-12 cells. J Pharmacol Exp Ther 301:753–764
Tsujimoto Y, Shimizu S (2000) VDAC regulation by the Bcl-2 family of proteins. Cell Death Differ 7:1174–1181
Veeman L, Alten J, Linnemannstöns K et al (2010) Potential involvement of FF1-ATP(syth)ase and reactive oxygen species in apoptosis induction in the antineoplastic agent erucylphosphohomocholine in glioblastoma cell lines. Apoptosis 15:753–768
Weireb O, Bar-Am O, Amit T, Chillag-Talmor O, Youdim MBH (2004) Neuroprotection via pro-survival protein kinase C isoforms associated with Bcl-2 family members. FASEB J 18:1471–1473
Wicker U, Bucheler K, Gellerich FN, Wagner M, Kapischke M, Brdiczka D (1993) Effect of macromolecules on the structure of the mitochondrial intermembrane space and the regulation of hexokinase. Biochim Biophys Acta 1142:228–239
Yi H, Akao Y, Maruyama W, Chen K, Shih J, Naoi M (2006) Type A monoamine oxidase is the target of an endogenous dopaminergic neurotoxin, N-methyl(R)salsolinol, leading to apoptosis in SH-SY5Y cells. J Neurochem 96:541–549
Youdim MBH, Edmondson D, Tipton KF (2006) The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 7:295–309
Acknowledgments
This work was supported by the Research Grant for Longevity Sciences (21A-13) from the Ministry of Health, Labour and Welfare, Japan (W. M and M. N), and the Promotion of Fundamental Studies in Health Sciences of National Institute of Biomedical Innovation, Japan (W. M).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naoi, M., Maruyama, W. & Yi, H. Rasagiline prevents apoptosis induced by PK11195, a ligand of the outer membrane translocator protein (18 kDa), in SH-SY5Y cells through suppression of cytochrome c release from mitochondria. J Neural Transm 120, 1539–1551 (2013). https://doi.org/10.1007/s00702-013-1033-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-013-1033-x