Abstract
Diamine oxidase (DAO) oxidatively deaminates histamine and other diamines. Due to the lack of antibodies for human DAO, many findings on this enzyme had not been confirmed in man. Therefore, we produced a series of monoclonal antibodies by immunizing mice with human DAO protein fragments expressed in vitro. Five different monoclonal antibodies specific for human DAO were obtained that do not recognize any other human protein and can detect DAO with 100-fold greater sensitivity than the most sensitive enzymatic assays currently available. Using these antibodies allowed confirming the expression and cellular localization of DAO in various human tissues such as kidney, intestine and placenta where the presence of the enzyme had previously been deduced from activity measurement and DAO mRNA analysis. Due to the high sensitivity of the novel monoclonal antibodies, DAO was also detected at sites that previously evaded unequivocal proof of DAO enzymatic activity such as the urine. On the other hand, with these antibodies it was possible to show that DAO is normally not present in human liver and blood serum. The new monoclonal antibodies not only allow a comprehensive quantitative evaluation of the expression of DAO at the cellular level in man but will also facilitate sensitive analyses of disease-associated alterations of this enzyme.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diamine oxidase (DAO) is a member of the class of copper-containing amine oxidases (AOC) and oxidatively deaminates histamine and other diamines (Houen 1999; Schwelberger 2004). DAO is a homodimeric glycoprotein of approximately 200 kDa that appears to be secreted to convert its substrates extracellularly (Schwelberger 2004). In man, DAO is encoded by a single gene on chromosome 7q35 that has been designated ABP1 for amiloride binding protein because the cDNA encoding human DAO was cloned originally in a hunt for the protein binding the diuretic drug amiloride (Barbry et al. 1990; Novotny et al. 1994; Chassande et al. 1994). A high-resolution crystal structure of recombinant human DAO expressed in insect cells has been reported confirming that DAO is structurally a typical AOC protein possessing in addition to the Cu2+ ion, which is bound by three conserved histidine side chains, the active-site cofactor 2,4,5-trihydroxyphenylalanine quinone (McGrath et al. 2009).
As determined by activity measurements and mRNA analysis experiments, major sites of DAO expression in man are kidney, intestine and placenta (Schwelberger 2004). However, mRNA analyses give only an estimate of active gene expression while activity measurements have a limited cellular resolution and may be affected by the presence of related enzymes or by inhibitors. Overall, very little information is available on the regulation of the tissue-specific expression of human DAO, its cellular and subcellular localization, the presence of enzyme variants and enzyme complexes as well as disease-associated enzyme alterations, which is mainly due to the lack of antibodies specific for human DAO.
Previously we were able to produce polyclonal antibodies for porcine DAO that were extremely useful to assess the cellular and subcellular localization of the enzyme in pig tissues (Schwelberger and Bodner 1997; Schwelberger et al. 1998a, 1998b). Although these antibodies bind human DAO, their low sensitivity for human DAO and their cross-reactivity with other human proteins precluded their use for DAO expression and localization studies in man. The need of suitable antibodies for human DAO prompted us to produce a panel of highly sensitive and specific monoclonal antibodies for this enzyme.
Materials and methods
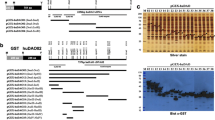
Using the full-length human DAO cDNA (Barbry et al. 1990) four fragments encoding amino acids 23–190 (fragment A), 190–435 (fragment B), 436–751 (fragment C), and 190–751 (fragment D), respectively, were created by site-specific cleavage with restriction enzymes and cloned in frame into the bacterial expression vectors pGEX-5X-1/2/3 (GE Healthcare, Vienna, Austria). Each recombinant plasmid was transformed into the protease deficient strain E. coli BL21 to produce glutathione S-transferase (GST) fusion proteins according to manufacturer’s instructions (GE Healthcare, Vienna, Austria). Briefly, recombinant bacteria were grown at 37 °C with slight agitation (100 rpm) in 500 ml YTA (16 g/l tryptone, 10 g/l yeast extract, 5 g/l NaCl, 100 mg/l ampicillin, pH 7.0) to an OD600nm of 0.5 and fusion protein expression was induced for 4 h by addition of 0.1 mM isopropyl-β-D-thiogalactopyranoside (IPTG, Roche, Vienna, Austria). Bacteria were harvested 5 min at 4,000×g 4 °C, washed with cold deionized water, and lysed in lysis buffer (20 mM bis.Tris.HCl, pH 7.0, 5 mM dithiothreitol) containing Complete Protease Inhibitor Cocktail (Roche, Vienna, Germany) using a French Press at 600 psi. All four constructs were expressed at high levels and produced largely insoluble fusion proteins that were recovered by centrifugation for 5 min at 5,000×g 4 °C, washed three times with cold deionized water, dissolved in 3× SDS sample buffer (60 mM Tris.HCl, pH 6.8, 6 % sodium dodecyl sulfate, 6 mM ethylenediamine tetraacetate, 10 % glycerol, 0.05 % bromphenol blue, 5 % 2-mercaptoethanol) and purified by preparative SDS polyacrylamide gel electrophoresis on 1.5 mm thick 10 % gels (Laemmli 1970) and electroelution using a Mini-Protean Electrophoresis Cell and a Model 422 Electro-Eluter, respectively (Biorad,Vienna, Austria). The purified GST fusion proteins were dialyzed against PBS buffer (10 mM NaH2PO4, pH 7.2, 150 mM NaCl) and used for immunizations.
Balb/c mice (n = 2–4) were immunized i.p. 4–5 times with recombinant antigens (GST-A/-B/-C/-D, Fig. 1) mixed with alhydrogel (9.8 mg/ml). Each mouse received 20 μg antigen + 1 mg Al(OH)3 per injection. After the second and the following injections serum samples were collected and tested by ELISA for the presence of antibodies. When the titer had increased to a level above 1:10,000, selected mice received an i.p. injection of 20 μg antigen in physiological saline (150 mM NaCl). After 4 days, the mice were killed and the spleens removed. Homogenization and disintegration of the spleens, fusion and cloning followed standard procedures essentially as described earlier (Köhler and Milstein 1975), except that the use of fibroblast feeder cells was substituted with HybER™ medium (SSI, Copenhagen, Denmark).
Recombinant DAO antigens used for immunizations. Panel a illustrates the sizes and positions (relative to the complete polypeptide on top) of the four recombinant human DAO fragments A–D cloned into the bacterial expression vector pGEX-5X. Panel b shows a 10 % Coomassie-stained polyacrylamide gel of aliquots of the purified GST fusion proteins A–D used for the immunization of mice. The migration positions of GST-A (44 kDa), GST-B (53 kDa), GST-C (61 kDa), and GST-D (88 kDa) are indicated by arrows and the sizes of molecular weight markers (M) are given on the left in kDa
For testing antibody titers by ELISA, antigens (0.5 μg/ml) were coated in Maxisorp plates (Nunc, Denmark) using 100 μl 50 mM sodium carbonate buffer, pH 9.6 per well. All subsequent blocking (5 min), washing (3 × 5 min) and incubation (60 min) steps were done in TTN buffer (25 mM Tris, pH 7.5, 0.5 % Tween 20, 150 mM NaCl). After blocking, wells were incubated 60 min with sera (starting dilution 1:200 and 2× titrations) or culture supernatants, and after washing wells were incubated 60 min with alkaline phosphatase-conjugated goat IgG against mouse IgG (Sigma, St. Louis, USA). Finally, wells were incubated with para-nitrophenyl phosphate (1 mg/ml) in 1 M diethanolamine, pH 9.8 and the absorbance read at 405 nm with background subtraction at 690 nm. The titer of a serum was defined as the dilution giving half maximal absorbance.
Selected antibody clones (hybridoma culture supernatants) were further tested for binding specificity and sensitivity using filter strips of human tissue homogenates. Tissue homogenates were prepared using the AllPrep DNA/RNA/Protein Mini Kit (Qiagen, Hilden, Germany) and dissolving the total precipitated protein in BUD (20 mM bis.Tris.HCl, pH 7.0, 8 M urea, 50 mM dithiothreitol). The protein was diluted with SDS sample buffer and 100 μg was separated on a 10 % SDS polyacrylamide gel (Laemmli 1970) and blotted onto a polyvinylidene fluoride (PVDF) membrane (Towbin et al. 1979). After washing in TBST (50 mM Tris.HCl, pH 7.5, 150 mM NaCl, 0.1 % Tween 20) and blocking non-specific binding sites by incubation for 60 min at 25 °C in TBSTM (TBST containing 2 % non-fat dry milk), the membrane was cut into vertical filter strips each containing 5 μg of protein. Each filter strip was incubated for 2–16 h at 4 °C with different dilutions of the monoclonal antibodies in TBSTM, washed 4 × 5 min with TBST, incubated 60 min at 25 °C with horseradish peroxidase-conjugated anti-mouse immunoglobulins (Dako, Glostrup, Denmark) diluted 1:1,500 in TBSTM, washed 4 × 5 min with TBST, incubated 5 min with ECL or ECL Plus reagent (GE Healthcare, Vienna, Austria), and exposed to Cronex 5 film (Agfa, Mortsel, Belgium).
For analyses of DAO in human tissues and body fluids we used homogenates of the macroscopically healthy part of surgical resection material not needed for histopathological evaluation as well as blood and urine samples from healthy volunteers (N = 10, 5 male, age 23–49). Tissue samples were immediately frozen and stored at −25 °C until analyzed. Tissue samples (50–100 mg) were homogenized in ten volumes of 20 mM bis.Tris.HCl, pH 7.0 containing 10 mM dithiothreitol and Complete Protease Inhibitor Cocktail (Roche, Vienna, Austria) for 5 min at 30 Hz using a TissueLyser II homogenizer (Qiagen, Hilden, Germany). The homogenate was cleared by centrifugation for 10 min at 20,000xg 4 °C and the supernatant containing the total soluble protein was stored at −25 °C until analyzed. Blood samples drawn from the antecubital vein were allowed to clot for at least 2 h at 4 °C, centrifuged for 10 min at 1,500×g 4 °C and the clear serum was stored at −25 °C until analyzed. Urine samples were cooled on ice, centrifuged for 10 min at 20,000×g 4 °C and the clear supernatant was stored at −25 °C until analyzed. Protein concentration of all samples was determined by the Bradford method (Bradford 1976) using a commercial kit (Biorad, Vienna, Austria). Proteins in different samples were characterized by SDS polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli 1970) and the presence of DAO was analyzed by Western blotting onto PVDF membranes essentially as described for the filter strips above, using the mouse monoclonal antibodies at optimum concentrations. DAO activity was determined using a radiometric assay with [14C]putrescine (GE Healthcare, Vienna, Austria) as the substrate (Schwelberger et al. 1995) and the specific activity was calculated in μU/mg protein where 1 μU converts 1 pmol of putrescine per minute at 37 °C.
For immunohistochemical staining, tissues were fixed for 16–24 h in 4 % paraformaldehyde and embedded in paraffin wax. Sections of 5 μm were cut, mounted on silanized glass slides (Menzel, Braunschweig, Germany), and dried 16 h at 50 °C. Slides were dewaxed 4 × 3 min in xylol, 1 × 3 min each in 100 % ethanol, 96 % ethanol, and 80 % ethanol, rinsed for 5 min with water, and autoclaved for 10 min at 121 °C in 10 mM sodium citrate pH 6.0 for antigen retrieval. Slides were washed 2× with TBS (50 mM Tris.HCl, pH 7.5, 150 mM NaCl) and mounted on Coverplates in Coverplate Racks (Thermo Fisher Scientific, Vienna, Austria) for subsequent incubations. Each incubation step was followed by three washes with TNT (TBS containing 0.05 % Tween 20). Endogenous peroxidase activity was blocked by incubation in 1 % H2O2 for 15 min, endogenous biotin was blocked employing the Biotin Blocking System (Dako, Glostrup, Denmark), and non-specific protein binding sites were blocked by incubation in TNB (TBS containing 0.5 % Blocking Reagent, PerkinElmer, Rodgau, Germany) for 30 min. Slides were incubated for 16 h at 4 °C with the mouse monoclonal antibodies for human DAO or with a rabbit anti-pig DAO polyclonal antibody (α-pkDAO-B7, Schwelberger and Bodner 1997), respectively, diluted 1:500–1:2,000 in TNB and then for 2 h a 25 °C with horseradish peroxidase-conjugated anti-mouse immunoglobulins (Dako, Glostrup, Denmark) or horseradish peroxidase-conjugated anti-rabbit immunoglobulins (Sigma, St. Louis, USA) diluted 1:100 in TNB. The Tyramide Signal Amplification System (PerkinElmer, Rodgau, Germany) was used according to manufacturer’s instructions for signal amplification. For staining of immunocomplexes, slides were incubated for 5 min with DAB substrate (0.05 % 3,3′-diaminobenzidine, 0.01 % H2O2, 50 mM Tris.HCl, pH 7.6) and counterstained with Mayer’s hemalum (Merck, Darmstadt, Germany). Slides were dehydrated by incubation for 3 min each in 70 % ethanol, 80 % ethanol, 96 % ethanol, 2× 100 % ethanol, and 2× xylol and coverslips were mounted with Entellan (Merck, Darmstadt, Germany).
Results
Using the GST fusion proteins of partial DAO protein fragments expressed in bacteria as antigens for immunizations of mice, five clones were selected that produced monoclonal antibodies with high-binding specificity and affinity for human DAO. Four of these clones (HYB313-01/-02/-03/-04) resulted from immunization with fragment B and one clone (HYB311-01) from immunization with fragment D, whereas no suitable clones were obtained from immunizations with fragments A and C, respectively (Fig. 1). The selected antibodies strongly bound to the respective DAO protein fragment used for immunization (results not shown). Initial testing of these five monoclonal antibodies using human kidney homogenates showed that all antibodies produced a single strong band at 102 kDa and could be diluted at least 1:1,000. Further testing of optimum antibody dilutions using filter strips of human kidney homogenates gave suitable titers of 1:1,000–1:3,000 (Fig. 2a).
Test of antibody specificity and sensitivity with human kidney samples. In panel a, filter strips containing 5 μg human kidney homogenate separated on a 10 % polyacrylamide gel were incubated with the indicated dilutions of the five mouse monoclonal antibodies for human DAO (HYB313-01, HYB313-02, HYB313-03, HYB313-04, HYB311-01) in TBSTM and horseradish peroxidase-conjugated anti-mouse immunoglobulins (1:1,500 in TBSTM), followed by ECL Plus substrate, and exposure to film for 1 min. In panels b and d, 10 μg each of eight different kidney homogenates were separated on a 10 % SDS polyacrylamide gel, blotted onto a PVDF membrane, and DAO was detected by incubation with HYB313-03 (1:2,000 in TBSTM) and horseradish peroxidase-conjugated anti-mouse immunoglobulins (1:1,500 in TBSTM), followed by ECL Plus substrate, and exposure to film for 10 s (b) or 15 min (d). Sizes of molecular weight markers (M) are given in kDa on the right and the migration position of monomeric DAO at 102 kDa is indicated by arrows on the left. In panel c, integrated intensity determined for the bands on the blot in panel b using ImageJ were plotted against the DAO activity in the respective sample. A Pearson’s correlation coefficient of 0.894 with p = 0.003 was obtained for this correlation using SPSS Statistics 19 (SPSS Inc., Chicago, USA)
When kidney samples from different individuals were analyzed, the same sharply focused band at 102 kDa was obtained in each case (Fig. 2b) and the intensity of the band was proportional to the DAO enzymatic activity in the respective sample (Fig. 2c) confirming that the antibodies indeed bind to DAO. Even on very long exposures, no other significant bands were visible on the blot showing the absolute specificity of the antibodies (Fig. 2d). With the most sensitive enzymatic assay currently available (Schwelberger et al. 1995), it is possible to detect approximately 200 pg DAO. Using the blotting technique with the monoclonal antibodies described here, we were able to reliably detect 1.5 pg DAO protein in a dilution series of human kidney homogenates (results not shown), which translates into a more than 100-fold higher sensitivity compared with the activity determination. This excellent sensitivity allows excluding the presence of significant amounts of DAO in samples where DAO is not detectable by immunoblotting.
When analyzing the presence of DAO protein in different human samples, the same strong band at 102 kDa observed in kidney samples and corresponding to monomeric DAO was obtained in samples of seminal plasma and small intestine, whereas a relatively diffuse band at ca. 110 kDa was obtained for placenta samples (Fig. 3a). The slower and more diffuse migration of human placenta DAO was observed already earlier and was found to be due to heterogenous glycosylation of the same polypeptide (Wilflingseder et al. 2002). Using the highest possible sensitivity of this immunoblotting technique, DAO was not detectable in any of eight liver samples (Fig. 3b) or any of ten serum samples (Fig. 3c) analyzed. To prove that this was not due to a detection problem, purified seminal plasma DAO was added to pooled serum samples and was detected with the same sensitivity as without serum (Fig. 3c, lanes a, b, c). The lack of detection of DAO protein in human liver and blood serum confirmed earlier activity measurements (Schwelberger 2004). Interestingly, low amounts of DAO could be detected in each of ten urine samples analyzed (Fig. 3d), whereas DAO activity in human urine was below the limits of detection of the enzymatic assay.
Detection of DAO in various human samples. For analysis of the presence of the DAO protein, 5 μg each of 2 seminal plasma samples, 2 placenta homogenates, and 2 small intestine homogenates (panel a), 10 μg each of 8 different liver homogenates (panel b), 50 μg each of 10 different serum samples (panel c), and 5 μg each of 10 different urine samples (panel d) was used. Samples were separated on 10 % SDS polyacrylamide gels, blotted onto PVDF membranes, and DAO was detected by incubation with HYB313-03 (1:2,000 in TBSTM) and horseradish peroxidase-conjugated anti-mouse immunoglobulins (1:1,500 in TBSTM), followed by ECL Plus substrate, and exposure to film for 10 s (a) or 15 min (b, c, d). Sizes of molecular weight markers (M) are given in kDa and the migration position of monomeric DAO is indicated by arrows. For detection control, 1 ng (a), 2 ng (b), and 3 ng (c) of purified human seminal plasma DAO was added to pooled human serum samples (c) or pooled human urine samples (d)
We next tested whether the monoclonal antibodies could detect DAO in tissue sections. When performing immunohistochemical staining on human kidney sections, identical staining patterns were obtained with all five antibodies at dilutions of 1:250–1:500 as shown exemplary for HYB313-01 in Fig. 4. A strong and specific staining of the basolateral plasma membrane of proximal tubular epithelial cells was observed (Fig. 4a, b) that was indistinguishable from the staining obtained with a polyclonal antibody specific for porcine DAO on pig kidney sections (Fig. 4c, d; Schwelberger et al. 1998a). Although DAO is expressed at approximately tenfold higher levels in pig kidney compared with human kidney, the immunohistochemical staining was more intense on human kidney sections and essentially no non-specific staining was observed indicating the excellent sensitivity and specificity of the monoclonal antibodies. Immunohistochemical staining of sections from human small intestine and from human placenta yielded selective staining at the plasma membrane of intestinal epithelial cells and of decidual cells, respectively, whereas no staining was obtained on human liver sections (results not shown). Noteworthy, immunohistochemical staining worked well also on sections from tissues processed for routine histopathological evaluation by fixation in 10 % formaldehyde and embedding in paraffin wax.
Immunohistochemical staining of kidney sections. Thin sections of human kidney (a, b) and pig kidney (c, d) were incubated with HYB313-01 (a, b, diluted 1:500 in TNB) and α-pkDAO-B7 (c, d, diluted 1:2,000 in TNB), respectively. Following incubation with horseradish peroxidase-conjugated secondary antibodies and tyramide signal amplification, slides were developed with 3,3′-diaminobenzidine (brown staining of immunocomplexes) and counterstained with Mayer’s hemalum (blue staining of nuclei and cell membranes). Slides were photographed at 10× (a, c) and 40× (b, d) enlargement. Specific staining of proximal tubular epithelial cells at the basolateral plasma membrane is indicated by arrowheads. Control incubations omitting the primary antibody produced no staining
In order to test the utility of the monoclonal antibodies for routine investigations of DAO in human samples, we analyzed the short- and long-term variations of DAO in urine samples from one individual (Fig. 5). In the series of samples collected continuously over 4 days, DAO was always detectable but showed considerable variation of abundance relative to total urinary protein without a clear circadian pattern (Fig. 5a). In the series of samples collected daily once in the morning over 72 days, there was also a wide variation of DAO abundance from nearly undetectable levels on days 13, 14, and 67 to relatively high levels on days 18, 24, 41, 42, 46, and 48 (Fig. 5b). Even in samples with high DAO levels no enzymatic activity was measurable due to the much lower sensitivity of the enzymatic assay. Further studies are in progress to clarify these variations in urinary DAO levels and to assess if this parameter might be diagnostically useful. In any case, this study demonstrates the feasibility of this type of analyses making use of the excellent sensitivity of the DAO antibodies.
Short- and long-term variations of DAO in human urine. Urine samples from one healthy volunteer were collected continuously for 4 days at the indicated times (panel a) and daily once in the morning for 72 days (panel b). For each sample, a volume containing 1 μg protein was separated on a 10 % SDS polyacrylamide gel, blotted onto a PVDF membrane and DAO was detected with HYB313-03 (1:2,000 in TBSTM) and horseradish peroxidase-conjugated anti-mouse immunoglobulins (1:1,500 in TBSTM), followed by ECL Plus substrate, and exposure to film for 5 min. Sizes of molecular weight markers (M) are given in kDa on the right and the migration position of monomeric DAO at 102 kDa is indicated by arrows on the left. Co, Co1, and Co2 are loading controls containing 100 ng, 200 ng, and 100 ng, respectively, of a human kidney homogenate
Discussion
Five monoclonal antibodies exhibiting a high specificity and sensitivity for human DAO were obtained by immunizing mice with DAO protein fragments expressed in vitro. All antibodies recognized a single protein band of 102 kDa corresponding to monomeric DAO on immunoblots of various tissues and did not bind to any other human protein in the samples analyzed. With these antibodies, it was possible to detect DAO with more than 100-fold higher sensitivity than with the most sensitive radioenzymatic assay in use (Schwelberger et al. 1995). Furthermore, these antibodies work excellent in immunohistochemical staining of human tissue sections and, thus, facilitate the sensitive and specific detection of the DAO protein at the cellular and subcellular level.
Using these antibodies, it was possible to confirm the presence of DAO in human tissues such as kidney, intestine and placenta where DAO mRNA and enzymatic analyses had revealed the expression of the enzyme earlier (Schwelberger 2004). The cellular and subcellular localization of DAO in human tissues found here perfectly agrees with earlier studies in pigs where DAO had been localized at the basolateral plasma membrane of proximal tubular epithelial cells in the kidney and resorptive enterocytes of intestinal villi (Schwelberger et al. 1998a). Besides, it was possible to detect DAO also in samples where the sensitivity of the enzymatic DAO assay was insufficient to demonstrate the presence of the enzyme such as the urine. Interestingly, DAO levels appear to exhibit considerable short- and long-term variation in human urine. The most likely source for urinary DAO is proximal tubular cells of the kidney where the enzyme is abundantly expressed. Further studies will have to elucidate the physiological and possible pathological importance of urinary DAO and if it could be an interesting diagnostic marker for kidney diseases affecting proximal tubules.
Besides demonstrating the presence of DAO in various human samples, the high sensitivity of the monoclonal antibodies for DAO also allowed to exclude the presence of significant amounts of the protein in other human samples such as liver tissue and blood serum. For the liver, this result is in accordance with mRNA analyses and activity measurements showing that DAO expression is not detectable in this organ in humans and most other mammals except for the guinea pig (Schwelberger 2004; Rajtar et al. 2006). In human blood serum or plasma, significant DAO activity was previously found only during pregnancy, where the enzyme appears to be released into the circulation from the placenta (Bardsley et al. 1974) and after application of the anticoagulant heparin that can release cellular DAO into the bloodstream (Hansson et al. 1966; Klocker et al. 2000). The failure to detect any DAO protein in serum samples on immunoblots with the DAO antibodies confirms the results of enzymatic assays showing that DAO activity is at the limits of detection in normal human blood plasma or serum (Klocker et al. 2000). Therefore, studies reporting a reduced plasma or serum DAO activity for certain human diseases should be taken with caution as the enzyme under consideration in these reports is most likely not DAO but another AOC family member called soluble vascular adhesion protein-1 (Abella et al. 2004; Stolen et al. 2004; Schwelberger 2007).
In conclusion, the five monoclonal antibodies described here represent excellent tools for the sensitive and specific detection of the human DAO protein allowing a comprehensive evaluation of the expression and cellular localization of the enzyme in man. Furthermore, these antibodies will facilitate a better understanding of the role of DAO in various metabolic pathways as well as sensitive analyses of disease-associated alterations of the enzyme.
References
Abella A, Garcia-Vicente S, Viguerie N, Ros-Baró A, Camps M, Palacín M, Zorzano A, Marti L (2004) Adipocytes release a soluble form of VAP-1/SSAO by a metalloprotease-dependent process and in a regulated manner. Diabetologia 47:429–438
Barbry P, Champe M, Chassande O, Munemitsu S, Champigny G, Lingueglia E, Maes P, Frelin C, Tartar A, Ullrich A, Lazdunski M (1990) Human kidney amiloride-binding protein: cDNA structure and functional expression. Proc Natl Acad Sci USA 87:7347–7351
Bardsley WG, Crabbe MJC, Scott IV (1974) The amine oxidases of human placenta and pregnancy plasma. Biochem J 139:169–181
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Chassande O, Renard S, Barbry P, Lazdunski M (1994) The human gene for diamine oxidase, an amiloride binding protein. Molecular cloning, sequencing, and characterization of the promoter. J Biol Chem 269:14484–14489
Hansson R, Holmberg CG, Tibbling S, Tryding N, Westling H, Wetterqvist H (1966) Heparin-induced diamine oxidase increase in human blood plasma. Acta Med Scand 180:533–536
Houen G (1999) Mammalian Cu-containing amine oxidases (CAOs): new methods of analysis, structural relationships, and possible functions. APMIS 107(Suppl 96):1–46
Klocker J, Drasche A, Sattler J, Bodner E, Schwelberger HG (2000) Postheparin plasma diamine oxidase activity and the anticoagulant effect of heparin. Inflamm Res 49(Suppl 1):S53–S54
Köhler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495–497
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
McGrath AP, Hilmer KM, Collyer CA, Shepard EM, Elmore BO, Brown DE, Dooley DM, Guss JM (2009) Structure and inhibition of human diamine oxidase. Biochemistry 48:9810–9822
Novotny WF, Chassande O, Baker M, Lazdunski M, Barbry P (1994) Diamine oxidase is the amiloride-binding protein and is inhibited by amiloride analogues. J Biol Chem 269:9921–9925
Rajtar S, Feurle J, Schwelberger HG, Irman-Florjanc T (2006) Expression of histamine degrading enzymes in guinea pig tissues. Inflamm Res 55(Suppl 1):S61–S62
Schwelberger HG (2004) Diamine oxidase (DAO) enzyme and gene. In: Falus A (ed) Histamine: biology and medical aspects. SpringMed Publishing, Budapest, pp 43–52
Schwelberger HG (2007) The origin of mammalian plasma amine oxidases. J Neural Transm 114:757–762
Schwelberger HG, Bodner E (1997) Purification and characterization of diamine oxidase from porcine kidney and intestine. Biochim Biophys Acta 1340:152–164
Schwelberger HG, Klocker J, Sattler J, Bodner E (1995) Determination of the activity of diamine oxidase in extremely small tissue samples. Inflamm Res 44(Suppl 1):S94–S95
Schwelberger HG, Hittmair A, Kohlwein SD (1998a) Analysis of tissue and subcellular localization of mammalian diamine oxidase by confocal laser scanning fluorescence microscopy. Inflamm Res 47(Suppl 1):S60–S61
Schwelberger HG, Stalzer B, Maier H, Bodner E (1998b) Expression and cellular localisation of diamine oxidase in the gastrointestinal tract of pigs. Inflamm Res 47(Suppl 1):S62–S63
Stolen CM, Yegutkin GG, Kurkijärvi R, Bono P, Alitalo K, Jalkanen S (2004) Origins of serum semicarbazide-sensitive amine oxidase. Circ Res 95:50–57
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Wilflingseder D, Sørensen BK, Houen G, Schwelberger HG (2002) Complex formation of human placental diamine oxidase. Inflamm Res 51(Suppl 1):S89–S90
Acknowledgments
Anne Mortensen and Dorthe Tange Olsen are thanked for excellent technical assistance. This work was supported by grants from the Austrian Science Fund and by COST Action BM0806.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwelberger, H.G., Feurle, J. & Houen, G. New tools for studying old questions: antibodies for human diamine oxidase. J Neural Transm 120, 1019–1026 (2013). https://doi.org/10.1007/s00702-012-0936-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-012-0936-2