Abstract
Central cholinergic dysfunction has been reported in patients with Parkinsonʼs disease (PD) and hallucinations by evaluating short latency afferent inhibition (SAI), a transcranial magnetic stimulation protocol which gives the possibility to test an inhibitory cholinergic circuit in the human brain. REM sleep behavior disorder (RBD) was also found to be associated with cognitive impairment in PD patients. The objective of the study was to assess the cholinergic function, as measured by SAI, in PD patients with RBD (PD-RBD) and PD patients without RBD (PD-nRBD). We applied the SAI technique in 10 PD-RBD patients, in 13 PD-nRBD patients and in 15 age-matched normal controls. All PD patients and control subjects also underwent a comprehensive battery of neuropsychological tests. Mean SAI was significantly reduced in PD-RBD patients when compared with PD-nRBD patients and controls. Neuropsychological examination showed mild cognitive impairment in 9 out of the 10 PD-RBD patients, and in 5 out of the 13 PD-nRBD. SAI values correlated positively with neuropsychological tests measuring episodic verbal memory, executive functions, visuoconstructional and visuoperceptual abilities. Similar to that previously reported in the idiopathic form of RBD, SAI abnormalities suggest a cholinergic dysfunction in PD patients who develop cognitive impairment, and present findings indicate that RBD is an important determinant of MCI in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid eye movement (REM) sleep behavior disorder (RBD) is a clinical condition characterized by an intermittent or complete loss of muscle atonia and an increase of phasic muscular activity during REM sleep, leading to complex nocturnal motor behaviors (Mahowald and Schenck 2000).
RBD affects mainly older men and may occur alone (idiopathic form) or in association with a variety of neurological disorders. In particular, RBD is frequently associated with synucleinopathies (Boeve et al. 2001; Iranzo et al. 2006; Postuma et al. 2009), including Parkinsonʼs disease (PD) (Schenck et al. 1996a; Comella et al. 1998; Gagnon et al. 2002a, b). RBD often precedes the clinical onset of the neurodegenerative disease.
The pathophysiological mechanisms of RBD are still matter of debate. Animal models studies (Jouvet and Delorme 1965; Hendricks et al. 1982) and neuropathological observations in humans (Uchiyama et al. 1995; Schenck et al. 1996b; Turner et al. 2000; Arnulf et al. 2000) suggest that some brainstem structures, in particular the ventral mesopontine junction, the pedunculopontine nucleus (PPN), the laterodorsal tegmental nucleus, the locus coeruleus (LC), and the peri-LC area, are involved in the pathogenesis of the disease. From PPN and LC originate the most numerous cholinergic and noradrenergic neurons within the brainstem reticular formation, respectively (Jones 2000). These pontine structures play a critical role in arousal, cortical activation, and cognitive functions (Berridge et al. 1993; Kleiner and Bringmann 1996; Steckler et al. 1994, Winn 1998; Aston-Jones et al. 1999).
A modulatory role of the dopaminergic system in behavioral arousal and alertness has been documented; however, abnormalities in dopaminergic neurotransmission are not sufficient to explain the occurrence of RBD and cognitive impairment in all PD patients.
There is evidence that a moderate cholinergic deficit is present in several cortical regions in patients with PD (Hilker et al. 2005). Moreover, poor performance of tasks assessing visuospatial and attentional/frontal lobe functions can be associated with cortical cholinergic denervation in patients with PD and dementia (Bohnen et al. 2006).
In vivo evaluation of some cholinergic circuits of the human brain has recently been introduced using a transcranial magnetic stimulation (TMS) protocol that may reveal information about the function of some cholinergic circuits in the human brain. This technique relies on short latency afferent inhibition (SAI) of the motor cortex (Tokimura et al. 2000). SAI is significantly reduced in cholinergic forms of dementia, such as AD and dementia with Lewy bodies (Di Lazzaro et al. 2002, 2004b, 2005a, 2007) and in AD patients it can be increased by ACh inhibitors (Di Lazzaro et al. 2005a), while SAI is normal in non-cholinergic forms of dementia, such as fronto-temporal dementia (Di Lazzaro et al. 2006). Recently, SAI was also found to be reduced in patients with amnestic mild cognitive impairment (MCI) in multiple domains (Nardone et al. 2012a) and in patients with idiopathic REM sleep behavior disorder (iRBD) (Nardone et al. 2012b). SAI has never been investigated in patients with PD and RBD compared with those without.
The aim of the present study was to evaluate whether there is a cholinergic dysfunction, as evaluated by means of SAI testing, in PD patients with RBD. We also examined the relationship between neurophysiological findings and the cognitive performances in neuropsychological tests.
Materials and methods
Patients
Ten PD patients with concomitant RBD (PD-RBD; 2 women, mean age 65.9 ± 6.5 years), 13 PD patients without RBD (PD-nRBD; 4 women, mean age 63.7 ± 6.4 years), and 15 male healthy control subjects (4 women, mean age 66.4 ± 7.0 years) were recruited for the study. Demographic and clinical features are shown in Table 1.
Subject underwent one-night polysomnography (PSG). The PD-RBD patients fulfilled standard clinical criteria for RBD (American Academy of Sleep Medicine 2005), and showed excessive phasic or tonic electromyography (EMG) activity during REM sleep defined as tonic chin EMG activity for more than 20 % of the total REM sleep episode (Gagnon et al. 2002a).
None of the patients or control subjects had a history of other neurological, psychiatric, or cardiovascular disorder. All patients completed the Unified Parkinsonʼs disease Rating Scale (UPDRS) part III (Fahn et al. 1987), and were screened for depression according to the Beck Depression Inventory (BDI)-II (Beck et al. 1996). In order to control for vigilance impairment, the four choice reaction time test (FCRTT), a sensitive tool measuring attentional decrement, was administered to each subject (Wilkinson and Houghton 1975; Glenville and Wilkinson 1979). Cut-off values of BDI-II and FCRTT were score of 14 and 1 s (upper reaction time), respectively.
All patients were treated with L-DOPA alone or in combination with a dopamine-agonist. An L-DOPA equivalent dose was calculated for each patient using a conversion formula (Williams-Gray et al. 2007; Rowe et al. 2008) and shown in Table 1. None of the subjects were treated with anticholinergic medication or other drugs known to influence the motor cortex excitability. In particular, none of the patients were treated with benzodiazepines before the examination.
Patients provided informed consent before participation in this study, which was performed according the Declaration of Helsinki and approved by the local Ethics Committee of the Christian Doppler Clinic.
Neuropsychological examination
The battery included two tests of global cognitive function: the MMSE and the dementia rating scale. Executive functions were assessed by the Trail Making test parts A and B (outcome measure = time in seconds), the Stroop color word test (outcome measure = time in seconds and number of errors on the interference condition), and the letter and semantic (fruit/vegetable, and animal items) fluency tests. Episodic verbal memory was assessed by the Rey auditory verbal learning test (outcome measure = total number of words in trials 1 to 5, immediate recall, delayed recall, and correct recognition). Visuospatial abilities were assessed by the copy of the Rey-Osterrieth complex figure and the block design subtest from the Wechsler Adult Intelligence Scale-III (adjusted score). Finally, visuoperceptual (visual exploration) abilities were assessed by the Bells test (outcome measure = number of omissions) (for reference and description of all tests, see Lezak et al. 2004).
The diagnosis of MCI was made according to the revised criteria of Petersen and Morris (2005), with a cut-off of 1.5 SD.
Transcranial magnetic stimulation
TMS was performed using a High-power Magstim 200 magnetic stimulator (Magstim Co., Whitland, Dyfed, UK) connected to a Bistim module throughout all measurements. A figure-of-eight coil with external loop diameters of 9 cm was held over the motor cortex at the optimum scalp position to elicit motor responses in the first dorsal interosseous (FDI) muscle. The dominant hemisphere was selected for stimulating patients and healthy subjects. The induced current flows in a postero-anterior direction. Motor evoked potentials (MEPs) were recorded via two 9-mm diameter Ag–AgCl electrodes with the active electrode applied over the motor point of the muscle and the reference on the metacarpophalangeal joint of the index finger. MEPs were amplified and filtered (bandwidth 3–3,000 Hz) by D150 amplifiers (Digitimer, Welwyn Garden City, Herfordshire, UK).
All patients were examined in the on state.
We evaluated the following TMS parameters: the resting motor threshold (RMT), the central motor conduction time (CMCT), the short latency intracortical inhibition (SICI), and intracortical facilitation (ICF) to paired TMS, and the SAI.
RMT was defined as the minimum stimulus intensity that produced a minimal motor evoked response (about 50 μV in 50 % of 10 trials) at rest. CMCT was calculated by subtracting the peripheral conduction time from spinal cord to muscles from the latency of responses evoked by cortical stimulation with the formula: MEP latency − (F latency + M latency – 1)/2 (Rossini et al. 1994).
SICI and ICF were studied using the technique of Kujirai et al. (1993). Two magnetic stimuli were given through the same stimulating coil over the motor cortex and the effect of the first (conditioning) stimulus on the second (test) stimulus was investigated. The intensity of the conditioning stimulus was set to 80 % RMT; the second (test) shock intensity was adjusted to evoke a MEP in relaxed FDI with an amplitude of approximately 1 mV, peak-to-peak.
The timing of the conditioning shock was altered in relation to the test shock. Inhibitory interstimulus intervals (ISIs) of 2, 3, and 5 ms and facilitatory ISIs of 7, 10, and 20 ms were investigated. Ten stimuli were delivered at each ISI also for test stimulus and single MEP. For these recordings, muscle relaxation is very important and the subject was given audiovisual feedback at high gain to assist in maintaining complete relaxation. The presentation of conditioned and unconditioned trials was randomised. The amplitude of the conditioned EMG responses was expressed as the percentage of the amplitude of the test EMG responses. The amplitudes of the conditioned responses were averaged obtaining grand mean amplitudes of the three inhibitory and of the three facilitatory ISIs.
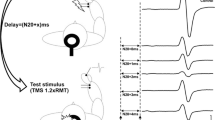
SAI was studied using the recently described technique (Tokimura et al. 2000). Conditioning stimuli were single pulses (200 μs) of electrical stimulation (with the cathode positioned proximally), applied through bipolar electrodes to the median nerve at the wrist. The intensity of the conditioning stimuli was set at just over motor threshold for evoking a visible twitch of the thenar muscles. The intensity of the test cortical magnetic shock was adjusted to evoke a muscle response in relaxed FDI with an amplitude of approximately 1 mV peak-to-peak. The conditioning stimulus to the peripheral nerve preceded the test magnetic cortical stimulus. ISIs were determined relative to the latency of the N20 component of the somatosensory evoked potential evoked by stimulation of the median nerve. In right-handed subjects, the active electrode for recording the N20 potential was attached 3 cm posterior to C3 (10–20 system), and the reference was 3 cm posterior to C4 (vice-versa for left-handed subjects). Five hundred responses were averaged to identify the latency of N20 peak. ISIs from the latency of the N20 component plus 2 ms to the latency of the N20 component plus 8 ms were investigated in steps of 1 ms.
Eight stimuli were delivered at each ISI also for test stimulus and single MEP. We calculated the average of the MEP obtained after cortical magnetic stimulation alone, and the average of the MEP obtained by conditioning cortical magnetic stimulus with a peripheral stimulus to the median nerve at the wrist at the seven different ISIs studied. The amplitude of the conditioned MEP was expressed as a percentage of the amplitude of the test MEP. The percentage inhibition of the conditioned responses at the seven different ISIs was averaged to obtain a grand mean. Subjects were given audio–visual feedback at high gain to assist in maintaining complete relaxation.
In order to clarify a possible spinal or peripheral contribution on the motor cortex excitability parameters, supramaximal stimulation (0.2 ms square-wave constant current pulses) of the ulnar nerve at the wrist was used to assess spinal and peripheral motor excitability. While FDI was relaxed, the peak-to-peak amplitude of F waves (average, 20 trials) and CMAP (maximum, 3 trials) were determined. We identified the F waves according to the criteria reported by the International Federation of Clinical Neurophysiology as responses that are variable in their latency, amplitude and configuration, but that occur grouped with a consistent range of latency (Kimura et al. 1994).
Statistical analysis
The demographic and clinical data, the neurophysiological parameters (SAI, RMT, SICI, ICF, CMCT, CMAP and F-wave), and all the neuropsychological measures were analysed by means of an ANOVA for repeated measures. If the ANOVA reveals a significant group effect, groups were compared by means of Tukey HSD post hoc comparisons.
To evaluate the relationship between the electrophysiological parameters and the examined neuropsychological measures, a partial correlation analysis controlling for the variables age and educational level was carried out. This analysis was conducted for patient and control groups separately.
P value <0.01 was taken as the significance threshold for all tests.
To illustrate on a single-subject level the possible deviation from the mean values of the control data, we also calculated in all patients individual z-scores for all neuropsychological measures and SAI.
Results
The neurophysiological data are summarized in the Table 2.
MEPs in healthy controls were inhibited when the median nerve stimulus was given before TMS of the cerebral cortex at an interval corresponding to the N20 latency plus 2 ms to the N20 latency plus 8 ms. The amount of inhibition over this period was significantly smaller in PD-RBD patients than in PD-nRBD patients and control subjects, as confirmed by a significant ANOVA group effect for SAI (F (2, 35) = 30.65, P < 0.001) and post hoc Tukey-HSD group comparisons (see Table 2). ANOVA group effects were not found for any other neurophysiological measures in either of the patient groups (Fs < 1, ps > 0.74).
In both patient groups, the other TMS parameters did not differ significantly from the controls (ps > 0.01 for all tests).
9 out of the 10 PD-RBD patients and five out of the 13 PD-nRBD patients were diagnosed as having MCI (see Table 3). Compared with control subjects, these PD patients performed significantly worse on the neuropsychological tests measuring global cognition, episodic verbal memory, executive functions, and visuoconstructional and visuoperceptual abilities.
SAI was abnormal in the PD-RBD patients with impaired cognitive performances.
Partial correlation analyses controlling for age and educational level for each group separately revealed that almost all examined neuropsychological test scores highly correlated with SAI in PD-RBD patients and less highly in the PD-nRBD patients. Correlations are shown in Table 4. No significant correlations were found for control subjects. Significant correlations were not found between disease duration and SAI in either of the patient groups. Only a borderline significant correlation between RBD disease duration and SAI was observed (r (6) = 0.66, P = 0.076), indicating a trend towards reduced inhibition with higher disease duration.
Discussion
We report a significant reduction in SAI amount in PD-RBD patients compared with PD patients without RBD. Similar SAI abnormalities have been recently described in PD patients with visual hallucination (Manganelli et al. 2009). Notably, four patients with RBD and none of the patients with RBD reported visual hallucinations. There is evidence that visual hallucinations in PD can be due to cholinergic dysfunction. Therefore, our observations suggest a cholinergic system imbalance also in PD patients with RBD.
In fact, SAI is thought to be related to central cholinergic activity because in normal subjects it can be reduced or abolished by intravenous injection of the muscarinic antagonist scopolamine (Di Lazzaro et al. 2000) or modulated by ACh (Di Lazzaro et al. 2005a; Fujiki et al. 2006). However, it is still unclear if other neurotransmitters/neuromodulators are involved in the regulation of SAI. Indeed, SAI is influenced by GABAergic drugs such as some benzodiazepines in healthy subjects (Di Lazzaro et al. 2005b, c), and by dopaminergic drugs in patients with PD (Sailer et al. 2003), while quetiapine, an antagonist at multiple neurotransmitter receptors in the brain such as serotonin 5HT1A and 5HT2, dopamine D1 and D2, histamine, and adrenergic α1 and α2 receptors, does not modify SAI in healthy subjects (Di Lazzaro et al. 2005b). On the other hand, it has been reported that dopamine modifies SAI in AD (Martorana et al. 2009). Therefore, it should be considered that other neurotransmitters such as dopamine may be able to modulate cortical cholinergic function in AD patients. Interestingly, RMT [which is thought to reflect both neuronal membrane excitability and non-NMDA receptors glutamatergic neurotransmission (Ziemann et al. 1996; Di Lazzaro et al. 2003)], as well as SICI [which is considered to reflect mostly the GABAA-mediated intracortical inhibitory interactions (Kujirai et al. 1993)] and ICF [which is believed to reflect intracortical excitatory neurotransmission, which is largely mediated by NMDA receptors (Ziemann et al. 2008)] did not differ significantly between either group of patients and the control group.
SAI abnormalities, the presence of RBD, and neuropsychological results strongly support the hypothesis of cholinergic dysfunction in some patients with PD, who will probably develop dementia. Our findings are thus consistent with a recent perspective study (Postuma et al. 2012), showing an evolution toward dementia in PD patients with RBD and not in those without RBD.
In PD patients, SAI has been found to be normal or slightly increased in off condition and reduced more on the affected side with drug administration (Sailer et al. 2003). SAI of drug-free PD patients has been found to be increased on the affected side (Di Lazzaro et al. 2004a), and these results have been confirmed in 10 patients with off-condition PD (Nardone et al. 2005). A denervation hypersensitivity of muscarinic receptors has been proposed to explain the enhanced SAI in patients with PD (Di Lazzaro et al. 2004a; Nardone et al. 2005). A possible explanation of our results is that RBD—expression of the brainstem cholinergic circuit involvement—can modify the equilibrium of the whole cholinergic system or the balance between it and other neurotransmitter systems. The appearance of decreased SAI values could be the signal that, in some patients with PD, the frontal cortical cholinergic deficit cannot be compensated by denervation hypersensitivity of muscarinic receptors or other feedback control (Di Lazzaro et al. 2004a; Nardone et al. 2005).
The findings of the present study are also in agreement with previous neuropsychological studies demonstrating that cognitive impairment in patients with PD is closely related to the presence of RBD (Gagnon et al. 2002a; Vendette et al. 2007). Moreover, waking EEG spectral analysis performed in non-demented PD patients showed EEG slowing only in patients with concomitant RBD, and not in patients with PD without RBD (Gagnon et al. 2004). Association between waking EEG slowing and cognitive impairment has been reported previously in patients with mild or severe cognitive disturbances (Prichep et al. 1994; Babiloni et al. 2006).
On the other hand, several studies have demonstrated neuropsychological impairment (Ferini-Strambi et al. 2004; Massicotte-Marquez et al. 2008; Terzaghi et al. 2008) and EEG slowing (Fantini et al. 2003) even in patients with the idiopathic form of RBD. Interestingly, mean SAI was also found to be reduced in patients with iRBD (Nardone et al. 2012b).
Many studies have demonstrated that MCI is present in patients with PD at various stages of their illness (Green et al. 2002; Emre 2003; Janvin et al. 2003, 2006; Foltynie et al. 2004; Muslimovic et al. 2005). Deficits in executive function tasks requiring the integrity of the frontostriatal pathway, episodic memory impairment, as well as visuospatial and visuoperceptual dysfunctions have often been reported in PD (Helkala et al. 1988; Levin et al. 1991; Green et al. 2002; Emre 2003; Janvin et al. 2003; Foltynie et al. 2004; Muslimovic et al. 2005).
Poor performance of tasks assessing visuospatial and attentional/frontal lobe functions has been associated with cortical cholinergic denervation in PD and Parkinsonian dementia (Bohnen et al. 2006). In our previous study, we found that iRBD patients show impairments in verbal memory and executive functions (Nardone et al. 2012a) while, in line with previous studies (Gagnon et al. 2002a; Vendette et al. 2007), in PD-RBD patients lower performances in visuospatial and visuocontructional tests were also detected.
Neuropathologic and brain imaging studies performed in PD with cognitive impairment and in patients with RBD showed common neural alterations in several brainstem nuclei (i.e., substantia nigra, PPN, LC-subcoeruleus complex, and raphe nucleus) and anomalies in their corresponding neurotransmitters (i.e., dopaminergic, cholinergic, noradrenergic, and serotonergic systems) (Emre 2003; Iranzo et al. 2006; Braak et al. 2005). All of these brainstem structures have diffuse projections to the cerebral cortex and perturbations of these neural networks may explain the presence of cognitive deficits in patients with PD-RBD. In particular, the PPN is an important part of a network for maintaining attention, and may control attentional processes through its direct projections to the forebrain cholinergic system or indirectly through activation of thalamocortical projection (Perry et al. 1999; Inglis et al. 2001).
Non-demented patients with PD have a moderate cholinergic dysfunction and patients with PD-associated dementia present with a severe cholinergic deficit in various cortical regions (Hilker et al. 2005).
Therefore, the core feature of cognitive impairment in PD patients with RBD, as well as in patients with iRBD, seems to be related to a widespread central cholinergic dysfunction.
In conclusion, our SAI findings and neuropsychological results support the hypothesis of cholinergic dysfunction in some patients with PD, who will probably develop a dementia, and raise the possibility that the presence of RBD may indicate increased risk of cognitive impairment in patients with PD. Longitudinal studies of the patients are required to verify whether SAI abnormalities can predict a future severe cognitive decline.
References
American Academy of Sleep Medixine, Task Forse Chair; Hauri PJ, Chairman (2005) The international classification of sleep disorders: diagnostic and coding manual, 2nd edn. American Academy of Sleep Medicine, Westchester
Arnulf I, Bonnet AM, Damier P, Bejjani BP, Seilhean D, Derenne JP, Agid Y (2000) Hallucinations, REM sleep, and Parkinsonʼs disease: a medical hypothesis. Neurology 55:281–288
Aston-Jones G, Rajkowski J, Cohen J (1999) Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry 46:1309–1320
Babiloni C, Binetti G, Cassetta E, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Frisoni G, Hirata K, Lanuzza B, Miniussi C, Moretti DV, Nobili F, Rodriguez G, Romani GL, Salinari S, Rossini PM (2006) Sources of cortical rhythms change as a function of cognitive impairment in pathological aging: a multicenter study. Clin Neurophysiol 117:252–268
Beck AT, Steer RA, Brown GK (1996) The Beck depression inventory, 2nd edn. The Psychological Corporation, San Antonio
Berridge CW, Page ME, Valentino RJ, Foote SL (1993) Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience 55:381–393
Boeve BF, Silber MH, Ferman TJ, Lucas JA, Parisi JE (2001) Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord 16:622–630
Bohnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti BJ, Constantine GM, Mathis ChA, Davis JG, Moore RY, Dekosky ST (2006) Cognitive correlates of cortical cholinergic denervation in Parkinsonʼs disease and Parkinsonian dementia. J Neurol 253:242–247
Braak H, Rüb U, Jansen Steur EN, Del Tredici K, de Vos RA (2005) Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 64:1404–1410
Comella CL, Nardine TM, Diederich NJ, Stebbins GT (1998) Sleep-related violence, injury, and REM sleep behavior disorder in Parkinsonʼs disease. Neurology 51:526–529
Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, Tonali P, Rothwell JC (2000) Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res 135:455–461
Di Lazzaro V, Oliviero A, Tonali PA, Marra C, Daniele A, Profice P, Saturno E, Pilato F, Masullo C, Rothwell JC (2002) Noninvasive in vivo assessment of cholinergic cortical circuits in AD using transcranial magnetic stimulation. Neurology 59:392–397
Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Pilato F, Zito G, Dileone M, Nicoletti R, Pasqualetti P, Tonali PA (2003) Ketamine increases human motor cortex excitability to transcranial magnetic stimulation. J Physiol 547:485–496
Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Bentivoglio AR, Tonali A (2004a) Normal or enhanced short-latency afferent inhibition in Parkinsonʼs disease? Brain 127:E8
Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, Daniele A, Ghirlanda S, Gainotti G, Tonali PA (2004b) Motor cortex excitability to transcranial magnetic stimulation in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 75:555–559
Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, Ghirlanda S, Ranieri F, Gainotti G, Tonali (2005a) Neurophysiological predictors of long term response to AChE inhibitors in AD patients. J Neurol Neurosurg Psychiatry 76:1064–1069
Di Lazzaro V, Oliviero A, Saturno E, Di leone M, Pilato F, Nardone R, Ranieri F, Musumeci G, Fiorilla T, Tonali P (2005b) Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. J Physiol 564:661–668
Di Lazzaro V, Pilato F, Dileone M, Tonali PA, Ziemann U (2005c) Dissociated effects of diazepam and lorazepam on short-latency afferent inhibition. J Physiol 569:315–323
Di Lazzaro V, Pilato F, Dileone M, Saturno E, Oliviero A, Marra C, Daniele A, Ranieri F, Gainotti G, Tonali PA (2006) In vivo cholinergic circuit evaluation in frontotemporal and Alzheimer dementias. Neurology 66:1111–1113
Di Lazzaro V, Pilato F, Dileone M, Saturno E, Profice P, Marra C, Daniele A, Ranieri F, Quaranta D, Gainotti G, Tonali PA (2007) Functional evaluation of cerebral cortex in dementia with Lewy bodies. Neuroimage 37(2):422–429
Emre M (2003) Dementia associated with Parkinsonʼs disease. Lancet Neurol 2:229–237
Fahn S, Elton RL, members of the UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale (1987) In: Fahn S, Marsden CD, Calne DB, Liebermann A (eds) Recent developments in Parkinson’s disease. Macmillan Health Care Information, Florham Park, pp 153–163
Fantini ML, Gagnon JF, Petit D, Rompré S, Décary A, Carrier J, Montplaisir J (2003) Slowing of electroencephalogram in rapid eye movement sleep behavior disorder. Ann Neurol 53(6):774–780
Ferini-Strambi L, Di Gioia MR, Castronovo V, Oldani A, Zucconi M, Cappa SF (2004) Neuropsychological assessment in idiopathic REM sleep behavior disorder (RBD): does the idiopathic form of RBD really exist? Neurology 62(1):41–45
Foltynie T, Brayne CE, Robbins TW, Barker RA (2004) The cognitive ability of an incident cohort of Parkinsonʼs patients in the UK. The CamPaIGN study. Brain 127:550–560
Fujiki M, Hikawa T, Abe T, Ishii K, Kobayashi H (2006) Reduced short latency afferent inhibition in diffuse axonal injury patients with memory impairment. Neurosci Lett 405:226–230
Gagnon JF, Bedard MA, Fantini ML, Petit D, Panisset M, Rompré S, Carrier J, Montplaisir J (2002a) REM sleep behavior disorder and REM sleep without atonia in Parkinsonʼs disease. Neurology 59:585–589
Gagnon JF, Montplaisir J, Bédard MA (2002b) Les troubles du sommeil paradoxal dans la maladie de Parkinson. Rev Neurol 158:135–152
Gagnon JF, Fantini ML, Bedard MA, Petit D, Carrier J, Rompré S, Décary A, Panisset M, Montplaisir J (2004) Association between waking EEG slowing and REM sleep behavior disorder in PD without dementia. Neurology 62:401–406
Glenville M, Wilkinson RT (1979) Portable devices for measuring performance in the field: the effects of sleep deprivation and might shift on the performance of computer operators. Ergonomics 22:927–933
Green J, McDonald WM, Vitek JL, Evatt M, Freeman A, Haber M, Bakay RA, Triche S, Sirockman B, DeLong MR (2002) Cognitive impairments in advanced PD without dementia. Neurology 59:1320–1324
Helkala EL, Laulumaa V, Soininen H, Riekkinen PJ (1988) Recall and recognition memory in patients with Alzheimerʼs and Parkinsonʼs diseases. Ann Neurol 24:214–217
Hendricks JC, Morrison AR, Mann GL (1982) Different behaviors during paradoxical sleep without atonia depend on pontine lesion site. Brain Res 239:81–105
Hilker R, Thomas AV, Klein JC, Weisenbach S, Kalbe E, Burghaus L, Jacobs AH, Herholz K, Heiss WD (2005) Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology 65:1716–1722
Inglis WL, Olmstead MC, Robbins TW (2001) Selective deficits in attentional performance on the 5-choice serial reaction time task following pedunculopontine tegmental nucleus lesions. Behav Brain Res 123:117–131
Iranzo A, Molinuevo JL, Santamaria J, Serradell M, Martí MJ, Valldeoriola F, Tolosa E (2006) Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol 5:572–577
Janvin C, Aarsland D, Larsen JP, Hugdahl K (2003) Neuropsychological profile of patients with Parkinsonʼs disease without dementia. Dement Geriatr Cogn Disord 15:126–131
Janvin CC, Larsen JP, Aarsland D, Hugdahl K (2006) Subtypes of mild cognitive impairment in Parkinson’s disease. Mov Disord 21(9):1343–1349
Jones BE (2000) Basic mechanisms of sleep–wake states. In: Kryger MH, Roth T, Dement WC (eds) Principles and practice of sleep medicine, 3rd edn. Saunders, Philadelphia, pp 134–154
Jouvet M, Delorme F (1965) Locus coeruleus et sommeil paradoxal. C R Soc Biol 159:895–899
Kleiner S, Bringmann A (1996) Nucleus basalis magnocellularis and pedunculopontine tegmental nucleus: control of the slow EEG waves in rats. Arch Ital Biol 134:153–167
Kimura J, Daube J, Burke D, Hallett M, Cruccu G, Ongerboer de Visser BW, Yanagisawa N, Shimamura M, Rothwell J (1994) Human reflexes and late responses. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 40:393–403
Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD (1993) Corticocortical inhibition in human motor cortex. J Physiol 471:501–519
Levin BE, Llabre MM, Reisman S, Weiner WJ, Sanchez-Ramos J, Singer C, Brown MC (1991) Visuospatial impairment in Parkinsonʼs disease. Neurology 41:365–369
Lezak MD, Howieson DB, Loring DW (2004) Neuropsychological assessment, 4th edn. Oxford University Press, New York
Mahowald MW, Schenck CH (2000) REM sleep parasomnias. In: Kryger MH, Roth T, Dement WC (eds) Principles and practice of sleep medicine, 3rd edn. Saunders, Philadelphia, pp 724–741
Manganelli F, Vitale C, Santangelo G, Pisciotta C, Iodice R, Cozzolino A, Dubbioso R, Picillo M, Barone P, Santoro L (2009) Functional involvement of central cholinergic circuits and visual hallucinations in Parkinsonʼs disease. Brain 132(Pt 9):2350–2355
Martorana A, Mori F, Esposito Z, Kusayanagi H, Monteleone F, Codecà C, Sancesario G, Bernardi G, Koch G (2009) Dopamine modulates cholinergic cortical excitability in Alzheimerʼs disease patients. Neuropsychopharmacology 34(10):1328–2323
Massicotte-Marquez J, Décary A, Gagnon JF, Vendette M, Mathieu A, Postuma RB, Carrier J, Montplaisir J (2008) Executive dysfunction and memory impairment in idiopathic REM sleep behavior disorder. Neurology 70(15):1250–1257
Muslimovic D, Post B, Speelman JD, Schmand B (2005) Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 65:1239–1245
Nardone R, Florio I, Lochner P, Tezzon F (2005) Cholinergic cortical circuits in Parkinsonʼs disease and in progressive supranuclear palsy: a transcranial magnetic stimulation study. Exp Brain Res 163:128–131
Nardone R, Bergmann J, Christova M, Caleri F, Tezzon F, Ladurner G, Trinka E, Golaszewski S (2012a) Short latency afferent inhibition differs among the subtypes of mild cognitive impairment. J Neural Transm 119(4):463–471
Nardone R, Bergmann J, Kunz A, Christova M, Brigo F, Tezzon F, Trinka E, Golaszewski S (2012b) Cortical afferent inhibition is reduced in patients with idiopathic REM sleep behavior disorder and cognitive impairment. Sleep Med 13(7):919–925
Perry E, Walker M, Grace J, Perry R (1999) Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci 22:273–280
Postuma RB, Gagnon JF, Vendette M, Montplaisir JY (2009) Idiopathic REM sleep behavior disorder in the transition to denerative disease. Mov Disord 24(15):2225–2232
Postuma RB, Bertrand JA, Montplaisir J, Desjardins C, Vendette M, Rios Romenets S, Panisset M, Gagnon JF (2012) Rapid eye movement sleep behavior disorder and risk of dementia in Parkinsonʼs diesease: a prospective study. Mov Disord 27(6):720–726
Petersen RC, Morris JC (2005) Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol 62(7):1160–1163
Prichep LS, John ER, Ferris SH, Reisberg B, Almas M, Alper K, Cancro R (1994) Quantitative EEG correlates of cognitive deterioration in the elderly. Neurobiol Aging 15:85–90
Rossini PM, Barker T, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, Maertens de Noordhout AL, Marsden CD, Murray NMF, Rothwell JC, Swash M, Tomberg C (1994) Non invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application: report of IFCN committee. Electroenceph Clin Neurophysiol 91:79–92
Rowe JB, Hughes L, Ghosh BC, Eckstein D, Williams-Gray CH, Fallon S, Barker RA, Owen AM (2008) Parkinson’s disease and dopaminergic therapy—differential effects on movement, reward and cognition. Brain 131(Pt 8):2094–2105
Sailer A, Molnar GF, Paradiso G, Gunraj CA, Lang AE, Chen R (2003) Short and long latency afferent inhibition in Parkinson’s disease. Brain 26:1883–1894
Schenck CH, Bundlie SR, Mahowald MW (1996a) Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology 46:388–393
Schenck CH, Garcia-Rill E, Skinner RD, Anderson ML, Mahowald MW (1996b) A case of REM sleep behavior disorder with autopsy-confirmed Alzheimerʼs disease: post-mortem brain stem histochemical analyses. Biol Psychiatry 40:422–425
Steckler T, Inglis W, Winn P, Sahgal A (1994) The pedunculopontine tegmental nucleus: a role in cognitive processes? Brain Res Rev 19:298–318
Terzaghi M, Sinforiani E, Zucchella C, Zambrelli E, Pasotti C, Rustioni V, Manni R (2008) Cognitive performance in REM sleep behaviour disorder: a possible early marker of neurodegenerative disease? Sleep Med 9(4):343–351
Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC (2000) Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol 523:503–513
Turner RS, D’Amato CJ, Chervin RD, Blaivas M (2000) The pathology of REM sleep behavior disorder with comorbid Lewy body dementia. Neurology 55:1730–1732
Uchiyama M, Isse K, Tanaka K, Yokota N, Hamamoto M, Aida S, Ito Y, Yoshimura M, Okawa M (1995) Incidental Lewy body disease in a patient with REM sleep behavior disorder. Neurology 45:709–712
Vendette M, Gagnon JF, Décary A, Massicotte-Marquez J, Postuma RB, Doyon J, Panisset M, Montplaisir J (2007) REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology 69(19):1843–1849
Wilkinson RT, Houghton D (1975) Portable four-choise reaction timewith magnetic tape memory. Behav Res Methods Instum 7:441–446
Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA (2007) Catechol O-methyltransferease Vall58Met genotyper influences frontoparietal activity during planning in patients with Parkinson’s disease. J Neurosci 27(18):4832–4838
Winn P (1998) Frontal syndrome as a consequence of lesions in the pedunculopontine tegmental nucleus: a short theoretical review. Brain Res Bull 47:551–563
Ziemann U, Lonnecker S, Steinhoff BH, Paulus W (1996) Effects of antiepileptics drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol 40:367–378
Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, Siebner HR, Classen J, Cohen LG, Rothwell JC (2008) Consensus: motor cortex plasticity protocols. Brain Stimul 1:164–182
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nardone, R., Bergmann, J., Brigo, F. et al. Functional evaluation of central cholinergic circuits in patients with Parkinson’s disease and REM sleep behavior disorder: a TMS study. J Neural Transm 120, 413–422 (2013). https://doi.org/10.1007/s00702-012-0888-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-012-0888-6