Abstract
In the present study, we performed antibody-capture guanosine-5′-O-(3-[35S]thio)triphosphate ([35S]GTPγS) scintillation proximity assay (SPA), in which immuno-capture of Gα subunits following [35S]GTPγS binding was combined with SPA technology, in rat brain membranes. Preliminary experiments using a series of agonists and commercially available anti-Gα antibodies indicated the increase in specific [35S]GTPγS binding to Gαq determined with the anti-Gα antibody sc-393 and evoked by carbamylcholine chloride (CCh) was pharmacologically relevant. The experimental conditions were optimized as for the concentrations of GDP, MgCl2, and NaCl, the dilution of the anti-Gαq antibody, and membrane protein contents incubated. Under the optimized conditions, CCh-stimulated specific [35S]GTPγS binding to Gαq in a concentration-dependent and saturable manner with an EC50 of around 10 μM in all of the membranes prepared from rat hippocampus, cerebral cortex, and striatum. The maximum responses were varied according to the brain regions, with the rank order in magnitude of hippocampus > cerebral cortex > striatum. The addition of MT-7, a snake toxin with high selectivity for M1 over the other muscarinic acetylcholine receptors (mAChRs) (M2–M5), almost completely extinguished CCh-stimulated [35S]GTPγS binding to Gαq, even at a concentration as low as 1 nM. These results indicate that the functional coupling between M1 mAChR and Gαq can be investigated in rat native brain membranes by means of antibody-capture SPA/[35S]GTPγS binding assay. The assay developed in the present study would provide a useful strategy for investigation of possible pathophysiological alterations in neuropsychiatric disorders such as Alzheimer’s disease and schizophrenia as well as for drug discovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Classical [35S]GTPγS binding assay using filtration techniques has nowadays been widely used to assess functional coupling between metabotropic neurotransmitter receptors and heterotrimeric G proteins in membranes prepared from transfected cells as well as native brain membranes (Harrison and Traynor 2003). Although the research work with a use of this technique has provided us with invaluable results regarding physiological, pharmacological, and pathological functionality of G protein-mediated signal transduction stimulated by many metabotropic receptors, it is usually applicable preferentially to Gi/o proteins, through which the inhibitory receptors are negatively coupled with adenylyl cyclase, especially in native brain membranes. More recently, the antibody-capture [35S]GTPγS scintillation proximity assay (SPA), in which immuno-capture of Gα subunits following [35S]GTPγS binding is combined with the SPA technology (Kahl and Felder 2005), has been newly developed to investigate the specific interactions between several G protein-coupled receptors (GPCRs) and Gα subunits, even in native brain membranes (DeLapp 2004). Using the membranes prepared from rodent brain regions, functional activation of Gα subunits have been reported for Gαq/11, Gαi(1-3), and Gαo coupled with muscarinic acetylcholine receptors (mAChRs) in rat striatum (DeLapp et al. 1999), Gαq/11 coupled with M1 mAChR in mouse hippocampus and cortex (Porter et al. 2002), Gαo and Gαi3 in rat hippocampus and Gαi3 in rat anterior raphe coupled with 5-HT1A receptors (Mannoury la Cour et al. 2006), Gαs/olf and Gαq coupled with dopamine D1 receptors in rat striatum and cortex (Mannoury la Cour et al. 2007), Gαo coupled with 5-HT1A receptors in rat hippocampus (Martel et al. 2007), and Gαo and Gαi1/3 coupled with GABAB receptors in rat cortex, hippocampus, and cerebellum (Mannoury la Cour et al. 2008). This method has also been applied to human postmortem cerebral cortical membranes to investigate functional coupling between M1 mAChR and Gαq/11 (Salah-Uddin et al. 2008, 2009). Nevertheless, it has not been yet as popular as classical [35S]GTPγS binding assay using filtration techniques, and further studies appear necessary in order to reveal fundamental features of this newly developed assay.
In the present study, we have tried to establish the standardized SPA/[35S]GTPγS binding assay in rat brain membranes by using many commercially available anti-Gα antibodies for different Gα subclasses. Pharmacologically relevant response was obtained at least for mAChR-mediated Gαq in rat hippocampal and cerebral cortical membranes, and this functional interaction was probed in detail to gain the optimal experimental conditions.
Materials and methods
Materials
Antibody-capture SPA/[35S]GTPγS binding assay was performed on 96-well microplate, OptiPlate-96 (PerkinElmer; Waltham, MA, USA). [35S]GTPγS (NEG030H, 1,250 Ci/mmol) and anti-rabbit polyvinyltoluene (PVT) SPA scintillation beads, RPNQ0016, were also purchased from PerkinElmer. Carbamylcholine chloride (CCh), (−)-epinephrine, (−)-isoproterenol, dopamine (DA), serotonin (5-HT), (±)-baclofen, l-glutamate, (±)-2,5-dimethoxy-4-bromoamphetamine hydrochloride [(±)-DOB HCl], (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride [(±)-DOI HCl], GDP, and GTPγS were obtained from Sigma-Aldrich (St. Louis, MO, USA). Muscarinic toxin 7 (MT-7) was from Peptide Institute Inc. (Osaka, Japan). The following anti-Gα rabbit polyclonal antibodies for Gα subclasses (indicated in parentheses) were purchased from the indicated companies: sc-393 (Gαq, E-17), sc-392 (Gαq/11, C-19), sc-383 (Gαs/olf, C-18), sc-391 (Gαi-1, I-20), sc-7276 (Gαi-2, T-19), sc-262 (Gαi-3, C-10), and sc-387 (Gαo, K-20) from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA); SA-131 (Gαs), SA-232 (Gαq/11), SA-127 (Gαi-1), SA-128 (Gαi-1&2), SA-129 (Gαi-3), and SA-130 (Gαi-3&o) from Enzo Life Sciences Inc. (Plymouth Meeting, PA, USA); 21014 (Gαq) and 21006 (Gαi) from NewEast Biosciences (Malvern, PA, USA); and G6040 (Gαi) from Sigma-Aldrich. Non-ionic detergent Nonidet P40 was from Roche Diagnostics GmbH (Mannheim, Germany). Other chemicals used were of analytical grade.
Membrane preparation
The experimental protocols were reviewed and approved by the Animal Committee of Saitama Medical University, and the animal care and use procedures conformed to the European Community Guidelines for the use of Experimental Animals (86/609/EEC). Male Sprague–Dawley rats weighing 200–250 g were killed by decapitation and their brains were quickly removed. The cerebral cortex, hippocampus, and striatum dissected from each rat was homogenized in 5 ml of ice-cold TED buffer [5 mM Tris–HCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM dithiothreitol, pH 7.4] containing 10% (w/v) sucrose by 20 strokes with a motor-driven Teflon/glass tissue grinder. All the following centrifuge procedures were carried out at 4°C. Subsequent to centrifugation of the homogenate at 1,000 g for 10 min, the supernatant was decanted to another centrifuge tube. The pellet was vortexed in 5 ml of TED/sucrose buffer and centrifuged again at 1,000 g for 10 min. The combined supernatant (10 ml) was centrifuged at 9,000 g for 20 min and resuspended in 10 ml of TED buffer. After the same procedure was repeated, the homogenate was kept on ice for 30 min, followed by the final centrifugation at 35,000 g for 10 min. The resulting pellet was resuspended in 50 mM Tris–HCl buffer (pH 7.4) to produce the homogenate with a protein concentration ranging from 1.0 to 2.0 mg/ml. The homogenate was frozen quickly on fine-grained dry ice and stored at −80°C until use.
Antibody-capture SPA/[35S]GTPγS binding assay
The experimental protocol for antibody-capture SPA/[35S]GTPγS binding was essentially according to the report of Porter et al. (2002) with minor modifications. The brain membranes were thawed slowly on ice, and 50 μl aliquots were incubated in microplate wells with 25 μl of solutions containing various concentrations of an agonist at room temperature for 30 min. The assay buffer (25 μl) was then added to the each well, and the incubation was performed for 60 min in a volume of 100 μl of 50 mM Tris–HCl buffer (pH 7.4), which contained 0.5 nM [35S]GTPγS, 20 mM MgCl2, 100 mM NaCl, and 100 nM GDP, unless otherwise indicated. The membranes were then solubilized with 0.3% Nonidet P40 for 30 min, followed by a 60-min incubation with a rabbit-raised anti-Gα antibody generally at a dilution of 1:400. Then, anti-rabbit PVT SPA scintillation beads were added and incubated further at room temperature for 3 h. Finally, the microplate was centrifuged at 450 g (1,800 rpm) with a centrifuge CD-100R (Tomy Seiko; Tokyo, Japan) for 15 min and counted for 5 min per well by the microplate scintillation and luminescence counter, TopCount (Packard BioScience; Waltham, MA, USA). Non-specific binding was defined in the presence of 1 mM GTPγS, and subtracted from the total binding to calculate the specific [35S]GTPγS binding.
Data analysis
All results except for Fig. 1 were presented as the mean ± S.E.M. of the indicated number of separate experiments, each performed at least in duplicate. The concentration-dependent increase in the specific [35S]GTPγS binding by an agonist was expressed as percent over the basal unstimulated value, and analyzed by means of a non-linear regression method using GraphPad Prism (GraphPad Software, La Jolla, CA, USA), to produce the concentration eliciting the half-maximal effect (EC50) and the maximal percent increase (%Emax).
Stimulation of [35S]GTPγS binding to Gαq elicited by CCh at various concentrations of GDP in rat hippocampal membranes. Antibody-capture SPA/[35S]GTPγS binding assay was performed with anti-Gαq (E-17) (sc-393) in the absence (open circle) and presence (filled circle) of 1 mM CCh in rat hippocampal membranes in the presence of various concentrations of GDP. Each symbols represent cpm values determined in quadruplicate in one representative experiment, and the similar results were obtained in other two independent experiments. The non-specific binding defined in the presence of 1 mM unlabeled GTPγS was shown as a dotted line
Results
Preliminary and exploratory experiments were performed to detect pharmacologically relevant activation of Gα subclasses coupled with GPCRs in rat discrete brain regions, by the use of different kinds of agonists [CCh, (−)-epinephrine, (−)-isoproterenol, DA, 5-HT, (±)-baclofen, and l-glutamate] and commercially available anti-Gα antibodies (sc-393, sc-392, sc-383, sc-391, sc-7276, sc-262, sc-387, SA-131, SA-232, SA-127, SA-128, SA-129, SA-130, NewEast 21014, 21006, and G6040), in the presence of varied concentrations of GDP (not shown). Among a variety of combinations, CCh-stimulated Gαq determined with sc-393 appeared promising as a pharmacologically relevant response, which prompted us to investigate this interaction further in detail. As shown in Fig. 1, specific [35S]GTPγS binding in rat hippocampal membranes to Gαq determined with sc-393 was markedly augmented by the addition of 1 mM CCh in the presence of lower concentrations (<10 μM) of GDP. The addition of high concentrations of GDP lowered the specific [35S]GTPγS binding to Gαq and canceled the stimulatory effects of CCh on the specific binding. The similar results were also gained in the membranes prepared from cerebral cortex and striatum (not shown). Based on these results, the concentration of GDP was fixed at 100 nM for further experiments.
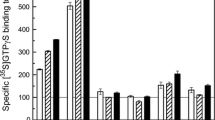
The addition of 1 mM CCh markedly augmented the [35S]GTPγS binding to Gαq determined with sc-393 in rat cerebral cortical membranes, whereas other commercially available anti-Gα antibodies did not appear to work effectively (Fig. 2). Although the stimulatory effect of CCh was significant for SA-232 (anti-Gαq/11), the signal/noise ratio for this response was apparently too small to characterize further. Antibody-capture SPA/[35S]GTPγS binding assay was also performed with NewEast 21014 (anti-Gαq), 21006 (anti-Gαi), and G6040 (anti-Gαi) in rat cerebral cortical membranes, with no stimulatory effects of CCh detected (not shown).
Effects of CCh on [35S]GTPγS binding to various subclasses of Gα in rat cerebral cortical membranes. Antibody-capture SPA/[35S]GTPγS binding assay was performed under the standard assay condition with different anti-Gα antibodies in the absence (open column) and presence (hatched column) of 1 mM CCh in rat cerebral cortical membranes. The non-specific binding (closed column) was defined in the presence of 1 mM unlabelled GTPγS. Values represent cpm means ± S.E.M. of four independent experiments, each performed in duplicate. *P < 0.05, ***P < 0.001, determined by two-tailed paired t-test
The effects of membrane protein contents added into the respective wells were then investigated (Fig. 3). Although the [35S]GTPγS binding to Gαq determined with sc-393 in rat cerebral cortical membranes was maximized with 2–3 μg protein/well, the stimulatory effects of CCh were negligible or unremarkable with membrane proteins up to these contents. It was necessary to use at least 8 μg of protein/well to obtain the maximal signal/noise ratio, and all of the following experiments were performed with the membranes of 10 μg protein/well. The similar results were also ascertained with the membranes prepared from hippocampus and striatum (not shown).
Effects of membrane protein contents on CCh-stimulated [35S]GTPγS binding to Gαq in rat cerebral cortical membranes. Antibody-capture SPA/[35S]GTPγS binding assay was performed under the standard assay condition with anti-Gαq (E-17) (sc-393) in the absence (open circle) and presence (filled circle) of 1 mM CCh in various amounts of rat cerebral cortical membranes. The non-specific binding (open triangle) was defined in the presence of 1 mM unlabelled GTPγS. Values represent cpm means ± S.E.M. of three independent experiments, each performed at least in duplicate
The dilution of anti-Gα antibody was one of the most important factors to optimize the assay conditions. As shown in Fig. 4, the stimulatory effect of CCh on specific [35S]GTPγS binding to Gαq determined with sc-393 in rat cerebral cortical membranes was obscure at a 1:6,400 dilution of the antibody, and maximized at 1:400 and 1:200 dilution. The standardized experiments were therefore performed with sc-393 at a 1:400 dilution.
Effects of dilution of anti-Gαq antibody on CCh-stimulated [35S]GTPγS binding in rat cerebral cortical membranes. Antibody-capture SPA/[35S]GTPγS binding assay was performed under the standard assay condition with anti-Gαq (E-17) (sc-393) at various dilutions in the absence (open circle) and presence (filled circle) of 1 mM CCh in rat cerebral cortical membranes. The non-specific binding (open triangle) was defined in the presence of 1 mM unlabelled GTPγS. Values represent cpm means ± S.E.M. of three independent experiments, each performed at least in duplicate
The effects of cations on CCh-stimulated [35S]GTPγS binding to Gαq in rat cerebral cortical membranes were shown in Fig. 5. When NaCl concentration was fixed at 100 mM, specific [35S]GTPγS binding to Gαq was stimulated by 1 mM CCh only in the presence of MgCl2, and the signal/basal ratio was maximized in the presence of 20–40 mM MgCl2. On the other hand, the effects of NaCl appeared less critical. When MgCl2 concentration was fixed at 20 mM, specific [35S]GTPγS binding to Gαq was stimulated significantly by 1 mM CCh even in the absence of NaCl. However, as both the unstimulated and CCh-stimulated [35S]GTPγS bindings were gradually suppressed by the addition of increasing concentrations of NaCl in parallel, the signal/basal ratio was optimized in the presence of higher concentrations of NaCl. Based on these results, MgCl2 at 20 mM and NaCl at 100 mM were included in the standardized assay buffer.
Effects of MgCl2 and NaCl on CCh-stimulated [35S]GTPγS binding to Gαq in rat cerebral cortical membranes. Antibody-capture SPA/[35S]GTPγS binding assay was performed with anti-Gαq (E-17) (sc-393) in the absence (open circle, open triangle) and presence (filled circle, filled triangle) of 1 mM CCh in rat cerebral cortical membranes under either of the following experimental conditions: in the presence of 100 mM NaCl and various concentrations of MgCl2 (open circle, filled circle), or in the presence of 20 mM MgCl2 and various concentrations of NaCl (open triangle, filled triangle). Non-specific binding was determined in the presence of 1 mM unlabelled GTPγS, which was subtracted from the total binding to define specific binding. Values represent cpm means ± S.E.M. of three experiments, each performed at least in duplicate
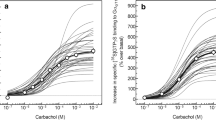
Under the optimized standard assay conditions determined as described above, the concentration-dependent increases in specific [35S]GTPγS binding to Gαq elicited by CCh were determined in the rat brain regions (Fig. 6). CCh-stimulated specific [35S]GTPγS binding to Gαq in a concentration-dependent and saturable manner in all regions with EC50 values of around 10 μM [10.2 μM (pEC50 = 4.99 ± 0.02, N = 49) in hippocampus, 11.4 μM (pEC50 = 4.94 ± 0.03, N = 16) in cerebral cortex, and 10.4 μM (pEC50 = 4.98 ± 0.39, N = 4) in striatum]. On the other hand, the %Emax values were varied according to the brain regions investigated, with the maximum (183.0 ± 6.0%) observed in hippocampal membranes, and followed by cerebral cortex (116.8 ± 8.9%) and then striatum (43.0 ± 2.2%).
CCh-stimulated [35S]GTPγS binding to Gαq in rat brain membranes. Antibody-capture SPA/[35S]GTPγS binding assay was performed with anti-Gαq (E-17) (sc-393) in the presence of increasing concentrations of CCh in membranes prepared from rat hippocampus (open circle), cerebral cortex (filled circle), and striatum (open triangle). Non-specific binding was determined in the presence of 1 mM unlabeled GTPγS, which was subtracted from the total binding to define specific binding. The increase in specific [35S]GTPγS binding by CCh was expressed as percentage over the unstimulated basal binding. Values represent means ± S.E.M. of 49 experiments for hippocampus, 16 for cerebral cortex, and four for striatum, each performed in duplicate
Finally, the effects of MT-7 on CCh-stimulated [35S]GTPγS binding to Gαq were investigated in rat hippocampal membranes. MT-7 (also named m1-toxin 1) is a 65-amino acid peptide toxin isolated from the venom of the green mamba Dendroaspis angusticeps and known as the most selective ligand currently available for the M1 mAChR subtype (Karlsson et al. 2000; Onali et al. 2005). As shown in Fig. 7, the addition of 0.1 nM MT-7 barely altered the concentration-dependent curve of CCh-stimulated [35S]GTPγS binding to Gαq (pEC50 = 4.87 ± 0.01, %Emax = 141.6 ± 6.8 in the absence of MT-7 (N = 3) vs. pEC50 = 4.74 ± 0.10, %Emax = 133.9 ± 21.6 in the presence of 0.1 nM MT-7 (N = 3)). On the other hand, CCh-stimulated [35S]GTPγS binding to Gαq was remarkably attenuated by the addition of MT-7 at 1 and 10 nM, and concentration-dependent non-linear regression curves were undeterminable due to too low responses in these cases.
Effects of MT-7 on CCh-stimulated [35S]GTPγS binding to Gαq in rat cerebral cortical membranes. Antibody-capture SPA/[35S]GTPγS binding assay was performed with anti-Gαq (E-17) (sc-393) in the presence of increasing concentrations of CCh in rat hippocampal membranes in the absence (open circle) and presence of 0.1 nM (filled circle), 1 nM (open triangle), and 10 nM (filled triangle) MT-7. Non-specific binding was determined in the presence of 1 mM unlabelled GTPγS, which was subtracted from the total binding to define specific binding. The increase in specific [35S]GTPγS binding by CCh was expressed as percentage over the unstimulated basal binding. Values represent means ± S.E.M. of three experiments, each performed in duplicate
Discussion
The main purpose of the present investigation was to establish antibody-capture SPA/[35S]GTPγS binding assay in native rat brain membranes for functional coupling between specific GPCRs and Gα subclasses, especially Gαs/olf and Gαq/11 rather than Gαi/o, because activation of Gαi/o coupled with many GPCRs has been preferentially detected by means of classical [35S]GTPγS binding assay using filtration techniques (Harrison and Traynor 2003; Strange 2010). In our own experience for instance, stimulation of Gαi/o coupled with 5-HT1A (Odagaki and Toyoshima 2005a, b), 5-HT1B (Odagaki and Toyoshima 2006a), dopamine D2 (Odagaki and Toyoshima 2006b), α2D-adrenergic (Odagaki and Toyoshima 2008), GABAB (Odagaki and Yamauchi 2004), and metabotropic glutamate (Odagaki et al. 2011) receptors has been successfully detected by using classical [35S]GTPγS binding assay in native membranes prepared from discrete rat brain regions.
Among the commercially available anti-Gα antibodies investigated in the present study, sc-393 from Santa Cruz Biotechnology Inc. (an affinity purified rabbit polyclonal antibody raised against a peptide mapping within the N-terminus of Gαq of mouse origin) was effective to obtain substantial signal/noise ratio subsequent to the stimulation of mAChRs by CCh. The choice of anti-Gα antibody is critical, and DeLapp et al. (1999) also described that they had tried a number of anti-Gαq/11 and anti-Gαi/o antibodies that did not allow demonstration of appreciable agonist-induced signals. Although CCh-stimulated [35S]GTPγS binding was also detected with SA-232 (anti-Gαq/11) in the present study, the signal/basal ratio in this case was disappointingly small for further characterization. Although Porter et al. (2002) succeeded to detect M1 mAChR-mediated [35S]GTPγS binding to Gαq/11 in mouse hippocampal and cortical membranes by using anti-Gαq/11 antibody, which was raised against C-terminal 19 amino acids common to both mouse Gαq and human Gαq/11, the equivalent anti-Gαq/11 antibody, sc-392, did not work in our study.
Besides CCh-stimulated [35S]GTPγS binding to Gαq determined with sc-393, we were aware that specific [35S]GTPγS bindings to some Gα subclasses were also enhanced by the addition of several agonists. However, we were uncertain as to whether these increases were pharmacologically relevant and derived from GPCR-mediated responses. For instance, specific [35S]GTPγS binding to Gαq determined with sc-393 or NewEast 21014 in rat cerebral cortical membranes was evidently stimulated by 5-HT in a concentration-dependent manner, but no saturability was obtained event at 10 mM. Furthermore, neither of selective 5-HT2 receptor agonists [(±)-DOB nor (±)-DOI] worked as an effective stimulant, contrary to the expectation that 5-HT-stimulated Gαq was mediated via 5-HT2 receptors. Although further sophisticated pharmacological investigations are needed for these responses, we intended to focus on the mAChR-mediated [35S]GTPγS binding to Gαq determined with sc-393 in the present study.
Based on the results concerning the effect of GDP concentrations on CCh-stimulated [35S]GTPγS binding to Gαq, GDP at 100 nM was included in the standard assay buffer. The similar results of preference of lower concentrations (<1 μM) of GDP for optimization of mAChR-mediated [35S]GTPγS binding to Gαq/11 were reported in rat striatal membranes (DeLapp et al. 1999) and in mouse hippocampal membranes (Porter et al. 2002). The preferred concentrations of GDP in these assays are in sharp contrast to those required for maximization of GPCR-mediated [35S]GTPγS binding to Gαi/o determined with classical [35S]GTPγS binding assay (20–30 μM), and the result is likely derived from the difference in molecular processes of G protein activation between Gαi/o and Gαq. In the case of Gαi/o subfamily, GPCR-mediated [35S]GTPγS binding becomes apparent in the presence of high concentrations of GDP, probably due to the suppression of [35S]GTPγS binding to non-heterotrimeric G proteins as well as to the reduction of the affinity of G proteins for GDP in the presence of an agonist (Strange 2010). On the other hand, the latter mechanism is unlikely for mAChR-mediated [35S]GTPγS binding to Gαq, since rightward shift of the inhibition curve of [35S]GTPγS binding by GDP induced by the addition of CCh has not been observed (Fig. 1). Low concentrations of GDP appeared sufficient to fill empty nucleotide binding sites on the non-heterotrimeric G proteins and/or Gαq to reduce the basal level of [35S]GTPγS binding, such that CCh-induced increase in [35S]GTPγS binding can be detected. Even in the absence of added exogenous GDP, a trace of endogenous GDP may be sufficient to work.
The results concerning the effects of protein contents on CCh-stimulated [35S]GTPγS binding to Gαq were entirely against expectations, and we have been unaware of such embarrassing relationship between the added protein contents and GPCR-mediated [35S]GTPγS binding to any Gα subclass. Since no stimulatory effects of CCh were observed with the protein contents lower than 1–2 μg/well, [35S]GTPγS might be bound specifically and preferentially to the GTP binding sites of non-heterotrimeric G proteins and/or heterotrimeric G proteins other than Gαq functionally coupled with mAChRs, if the added protein contents were insufficient. When the protein contents exceed the critical level, the [35S]GTPγS molecules bound to these sites should be transferred to the GTP binding sites of Gαq that are functionally coupled with mAChRs, with the stimulatory effects of CCh being progressively evident.
As with GPCR-mediated [35S]GTPγS binding assay using classical filtration technique (Harrison and Traynor 2003; Strange 2010), Mg2+ ions were absolutely required for observing agonist stimulation of [35S]GTPγS binding in the present study, indicating that the response certainly originates from heterotrimeric G proteins functionally coupled to GPCRs. The effects of Na+ ions on CCh-stimulated increase in [35S]GTPγS binding to Gαq were also similar to those observed for agonist-induced [35S]GTPγS binding to Gαi/o, though the extent of decreasing effects of the ion on [35S]GTPγS binding to Gαq was not so prominent as in the assay for Gαi/o subfamily. The results of antibody dilution study indicated that at least 1:400 dilution was necessary for sc-393 to obtain the maximum CCh-stimulated [35S]GTPγS binding to Gαq. As a result of these observations, the assay condition was optimized at long last, and the stimulatory effects of CCh on specific [35S]GTPγS binding to Gαq were successfully determined in the three discrete brain regions.
Although the EC50 values for CCh-stimulated specific [35S]GTPγS binding to Gαq were indistinguishable among the three brain regions, the %Emax values were considerably varied, with the rank order in magnitude of hippocampus > cerebral cortex > striatum. At least in hippocampal and cerebral cortical membranes, the signal/basal ratio appeared enough high for further pharmacological characterization, which is now under investigation in our laboratory. As for the mAChR subtype(s) involved in CCh-stimulated [35S]GTPγS binding to Gαq in the present study, mAChRs coupled to phosphoinositide hydrolysis and calcium mobilization via Gαq/11, i.e., M1, M3, and M5 receptors (Caulfield and Birdsall 1998; Felder et al. 2000; Eglen 2005) should be considered. Among them, M1 receptor is most likely implicated in CCh-stimulated [35S]GTPγS binding to Gαq, because this receptor subtype is the predominant mAChR in the CNS including the three areas examined in the present study (Levey 1993). In mouse hippocampal and cerebral cortical membranes, it was demonstrated that the stimulatory effect of oxotremorine-M on specific [35S]GTPγS binding to Gαq/11 was mediated entirely via M1 receptor subtype, but not via M3 receptor, by means of knockout mice lacking either mAChR (Porter et al. 2002). As the same method was unable to adopt in rats, the effects of MT-7 on CCh-stimulated [35S]GTPγS binding to Gαq were investigated in the present study. The CCh-stimulated [35S]GTPγS binding to Gαq was almost totally extinguished by the addition of the toxin at a concentration as low as 1 nM. As compared with limited selectivity of the commonly used conventional mAChR antagonists such as pirenzepine, MT-7 exhibits very high selectivity for M1 receptor (K i = 0.1 nM) over the other four mAChR subtypes (K i > 2,000 nM) (Karlsson et al. 2000), with the property as a non-competitive antagonist (Olianas et al. 2000; Bradley et al. 2003). The results obtained in the present study thus strongly suggest the principal involvement of M1 receptor in CCh-induced activation of Gαq determined by means of the antibody-capture SPA/[35S]GTPγS binding assay with ant-Gαq antibody sc-393.
In conclusion, the assay for detecting functional activation of Gαq principally coupled with M1 mAChR subtype in rat native brain membranes has been developed in the present investigation by means of antibody-capture SPA/[35S]GTPγS binding technique with a commercially available anti-Gαq antibody (sc-393). The choice of anti-Gα antibody appeared critical to succeed in detecting enough high signal/basal ratio of GPCR-mediated [35S]GTPγS binding, and careful optimization of experimental conditions was necessary to maximize the ratio for further pharmacological investigations. Although mAChR-mediated activation of Gαq was the focus in the present study, the antibody-capture SPA/[35S]GTPγS binding assay would be useful to investigate the functional activation of Gαs and Gαq/11 coupled with other GPCRs, e.g., β-adrenergic and 5-HT2 receptors. The mAChRs are widely distributed throughout the CNS, and involved in a number of fundamental physiological functions such as control of motor systems and modulation of cognition, learning and memory, and thus also in pathophysiological processes of a variety of neuropsychiatric disorders such as Alzheimer’s disease, schizophrenia, and Parkinson’s disease (Langmead et al. 2008; Scarr 2009). Although mAChRs have long-shown promise as potential targets for the treatment of these disorders, only limited success has been made so far due to the lack of highly selective drugs for each mAChR subtype. The assay developed in the present study provides a useful strategy for investigation of possible pathophysiological alterations in neuropsychiatric disorders as well as for drug discovery with relation to M1-Gαq interaction.
References
Bradley KN, Rowan EG, Harvey AL (2003) Effects of muscarinic toxins MT2 and MT7, from green mamba venom, on m1, m3 and m5 muscarinic receptors expressed in Chinese Hamster Ovary cells. Toxicon 41:207–215. doi:10.1016/S0041-0101(02)00278-7
Caulfield MP, Birdsall NJM (1998) International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50:279–290
DeLapp NW (2004) The antibody-capture [35S]GTPγS scintillation proximity assay: a powerful emerging technique for analysis of GPCR pharmacology. Trends Pharmacol Sci 25:400–401. doi:10.1016/j.tips.2004.06.003
DeLapp NW, McKinzie JH, Sawyer BD, Vandergriff A, Falcone J, McClure D, Felder CC (1999) Determination of [35S]guanosine-5′-O-(3-thio)triphosphate binding mediated by cholinergic muscarinic receptors in membranes from Chinese Hamster Ovary cells and rat striatum using an anti-G protein scintillation proximity assay. J Pharmacol Exp Ther 289:946–955
Eglen RM (2005) Muscarinic receptor subtype pharmacology and physiology. Prog Med Chem 43:105–136. doi:10.1016/S0079-6468(05)43004-0
Felder CC, Bymaster FP, Ward J, DeLapp N (2000) Therapeutic opportunities for muscarinic receptors in the central nervous system. J Med Chem 43:4333–4353. doi:10.1021/jm990607u
Harrison C, Traynor JR (2003) The [35S]GTPγS binding assay: approaches and applications in pharmacology. Life Sci 74:489–508. doi:10.1016/j.lfs.2003.07.005
Kahl SD and Felder CC (2005) Scintillation proximity assay. Curr Protoc Neurosci Chapter 7: Unit 7.15
Karlsson E, Jolkkonen M, Mulugeta E, Onali P, Adem A (2000) Snake toxins with high selectivity for subtypes of muscarinic acetylcholine receptors. Biochimie 82:793–806. doi:10.1016/S0300-9084(00)01176-7
Langmead CJ, Watson J, Reavill C (2008) Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther 117:232–243. doi:10.1016/j.pharmthera.2007.09.009
Levey AI (1993) Immunological localization of m1–m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci 52:441–448
Mannoury la Cour C, El Mestikawy S, Hanoun N, Hamon M, Lanfumey L (2006) Regional differences in the coupling of 5-hydroxytryptamine-1A receptors to G proteins in the rat brain. Mol Pharmacol 70:1013–1021. doi:10.1124/mol.106.022756
Mannoury la Cour C, Vidal S, Cussac PD, Millan MJ (2007) Dopamine D1 receptor coupling to Gs/olf and Gq in rat striatum and cortex: A scintillation proximity assay (SPA)/antibody-capture characterization of benzazepine agonists. Neuropharmacology 52:1003–1014. doi:10.1016/j.neuropharm.2006.10.021
Mannoury la Cour C, Herbelles C, Pasteau V, de Nanteuil G, Millan MJ (2008) Influence of positive allosteric modulators on GABAB receptor coupling in rat brain: a scintillation proximity assay characterization of G protein subtypes. J Neurochem 105:308–323. doi:10.1111/j.1471-4159.2007.05131x
Martel J-C, Ormière A-M, Leduc N, Assié M-B, Cussac D, Newman-Tancredi A (2007) Native rat hippocampal 5-HT1A receptors show constitutive activity. Mol Pharmacol 71:638–643. doi:10.1124/mol.106.029769
Odagaki Y, Toyoshima R (2005a) Detailed pharmacological characterization of 5-HT1A receptor-mediated [35S]GTPγS binding in rat hippocampal membranes. J Pharmacol Sci 98:66–76 (Erratum; J Pharmacol Sci 98:190, 2005)
Odagaki Y, Toyoshima R (2005b) 5-HT1A receptor-mediated G protein activation assessed by [35S]GTPγS binding in rat cerebral cortex. Eur J Pharmacol 521:49–58. doi:10.1016/j.ejphar.2005.07.018
Odagaki Y, Toyoshima R (2006a) 5-HT-stimulated [35S]guanosin-5′-O-(3-thio)triphosphate binding as an assay for functional activation of G proteins coupled with 5-HT1B receptors in rat striatal membranes. Naunyn-Schmiedebergs Arch Pharmacol 372:335–345. doi:10.1007/s00210-006-0041-x
Odagaki Y, Toyoshima R (2006b) Dopamine D2 receptor-mediated G protein activation assessed by agonist-stimulated [35S]guanosine 5′-O-(γ-thiotriphosphate) binding in rat striatal membranes. Prog Neuropsychopharmacol Biol Psychiatry 30:1304–1312. doi:10.1016/j.pnpbp.2006.05.007
Odagaki Y, Toyoshima R (2008) Pharmacological characterization of α2D-adrenergic receptor-mediated [35S]GTPγS binding in rat cerebral cortical membranes. Pharmacol Res 57:435–444. doi:10.1016/j.phrs.2008.04.006
Odagaki Y, Yamauchi T (2004) γ-Hydroxybutyric acid, unlike γ-aminobutyric acid, does not stimulate Gi/Go proteins in rat brain membranes. Basic Clin Pharmacol Toxicol 94:89–98
Odagaki Y, Kinoshita M, Toyoshima R (2011) Functional coupling between metabotropic glutamate receptors and G-proteins in rat cerebral cortex assessed by guanosine-5′-O-(3-[35S]thio)triphosphate ([35S]GTPγS) binding assay. Basic Clin Pharmacol Toxicol 109:175–185. doi:10.1111/j.1742-7843.2011.00705.x
Olianas MC, Maullu C, Adem A, Mulugeta E, Karlsson E, Onali P (2000) Inhibition of acetylcholine muscarinic M1 receptor function by the M1-selective ligand muscarinic toxin 7 (MT-7). Br J Pharmacol 131:447–452. doi:10.1038/sj.bjp.0703606
Onali P, Adem A, Karlsson E, Olianas MC (2005) The pharmacological action of MT-7. Life Sci 76:1547–1552. doi:10.1016/j.lfs.2004.10.029
Porter AC, Bymaster FP, DeLapp NW, Yamada M, Wess J, Hamilton SE, Nathanson NM, Felder CC (2002) M1 muscarinic receptor signaling in mouse hippocampus and cortex. Brain Res 944:82–89. doi:10.1016/S0006-8993(02)02721-X
Salah-Uddin H, Thomas DR, Davies CH, Hagan JJ, Wood MD, Watson JM, Challis RAJ (2008) Pharmacological assessment of M1 muscarinic acetylcholine receptor-Gq/11 protein coupling in membranes prepared from postmortem human brain tissue. J Pharmacol Exp Ther 325:869–874. doi:10.1124/jpet.108.137968
Salah-Uddin H, Scarr E, Pavey G, Harris K, Hagan JJ, Dean B, Challis RAJ, Watson JM (2009) Altered M1 muscarinic acetylcholine receptor (CHRM1)-Gαq/11 coupling in a schizophrenia endophenotype. Neuropsychopharmacology 34:2156–2166. doi:10.1038/npp.2009.41
Scarr E (2009) Muscarinic receptors in psychiatric disorders–Can we mimic ‘health’? Neurosignals 17:298–310. doi:10.1159/000231896
Strange PG (2010) Use of the GTPγS ([35S]GTPγS and Eu-GTPγS) binding assay for analysis of ligand potency and efficacy at G protein-coupled receptors. Br J Pharmacol 161:1238–1249. doi:10.1111/j.1476-5381.2010.00963.x
Acknowledgments
The authors thank Mr. Masakazu Kinoshita for his technical assistance. This work was supported by the grant for Research Work from the Saitama Medical University, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Odagaki, Y., Toyoshima, R. Muscarinic acetylcholine receptor-mediated activation of Gq in rat brain membranes determined by guanosine-5′-O-(3-[35S]thio)triphosphate ([35S]GTPγS) binding using an anti-G protein scintillation proximity assay. J Neural Transm 119, 525–532 (2012). https://doi.org/10.1007/s00702-011-0742-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-011-0742-2